Issue Archive
Table of Contents
BLOOD COMMENTARIES
PLENARY PAPER
A novel mouse model of hemoglobin SC disease reveals mechanisms underlying beneficial effects of hydroxyurea
Patients with sickle cell hemoglobin C (HbSC) disease experience significant morbidity. In this Plenary Paper, Setayesh and colleagues present a new mouse model that exhibits hallmark features of human HbSC disease. The authors studied the effects of hydroxyurea and show significant abrogation of sickling independent of the observed modest rise in fetal Hb (HbF), in agreement with a recent reported trial that demonstrated clinical benefit regardless of HbF increase. The model’s ability to replicate genotype-specific clinical manifestations and therapeutic responses makes it a valuable tool for mechanistic and preclinical studies in HbSC.
REVIEW ARTICLE
Metabolic pathways in deep vein thrombosis: a new frontier for therapeutic intervention
There is increasing evidence that deep vein thrombosis (DVT) is an inflammation-driven process involving endothelial cells, neutrophils, and platelets. Metabolic reprogramming of these cells in response to stasis, hypoxemia, and inflammatory stimuli can trigger DVT. In this review, Budnik and colleagues describe the role of metabolic reprogramming on DVT formation and discuss how targeting these cellular pathways initiating DVT may identify novel treatments that attenuate thrombosis without disrupting hemostasis.
HOW I TREAT
How I treat Wiskott-Aldrich syndrome
Wiskott-Aldrich syndrome (WAS) is a rare X-linked disorder with highly variable clinical presentation. In this How I Treat article, Vallée and coauthors provide 2 case-based vignettes that outline the current management of WAS, propose the use of genotype to predict severity and guide therapeutic interventions, and discuss recent results of hematopoietic stem cell transplant and gene therapy for the disease.
CLINICAL TRIALS AND OBSERVATIONS
Isatuximab, carfilzomib, lenalidomide, and dexamethasone induction in newly diagnosed myeloma: analysis of the MIDAS trial
Clinical Trials & Observations
Current standard initial treatment regimens for transplant-eligible patients with multiple myeloma do not use measurable residual disease (MRD) status to guide therapy. Perrot and colleagues present preliminary results of the MIDAS study showing that approximately two-thirds of newly diagnosed patients can achieve MRD negativity after induction with 6 cycles of isatuximab, carfilzomib, lenalidomide, and dexamethasone. Although longer follow-up is needed, these promising results set the stage for the second portion of the study where MRD-informed treatment deintensification will be tested.
HEMATOPOIESIS AND STEM CELLS
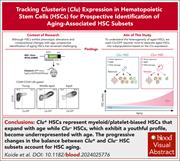
Tracking clusterin expression in hematopoietic stem cells reveals their heterogeneous composition across the life span
Aging skews hematopoiesis toward myeloid and platelet lineages while reducing lymphoid output, but tracking and identifying the aging-associated hematopoietic stem cells (HSCs) subsets is challenging. In this study, Koide and colleagues demonstrate that clusterin (Clu) expression reliably marks a subset of myeloid/platelet-biased HSCs that expand with age in Clu–green fluorescent protein (GFP) reporter mice. Clu-GFP mice represent a promising tool to identify megakaryocyte-biased HSCs and to study megakaryopoiesis in young and aged mice.
IMMUNOBIOLOGY AND IMMUNOTHERAPY
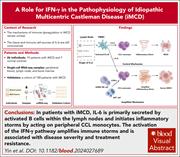
IFN-γ promotes the progression of iMCD by activating inflammatory monocytes
Idiopathic multicentric Castleman disease (iMCD) is a rare life-threatening chronic inflammatory disorder with generalized lymphadenopathy and cytokine storm with high levels of interleukin-6 (IL-6). In this study, Yin and colleagues performed single-cell RNA sequencing analysis of peripheral blood and lymph node samples from patients with iMCD. The authors found that IL-6 was primarily expressed in B cells from lymph nodes and that specific natural killer and natural killer T cells amplified monocyte proinflammatory activity via interferon gamma (IFN-γ) secretion, highlighting potential novel mechanisms in iMCD.
LYMPHOID NEOPLASIA
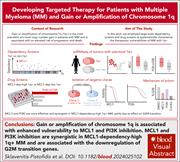
Large-scale dependency and drug screens to characterize the therapeutic vulnerabilities of multiple myeloma with 1q+
One of the most common chromosomal alterations in multiple myeloma (MM) is amplification of chromosome 1q (1q+), which is associated with worse outcomes. Sklavenitis-Pistofidis et al report the use of large-scale genetic dependency and drug screens in MM cell lines to identify myeloid cell leukemia-1 and phosphatidylinositol 3-kinase as specific therapeutic vulnerabilities for MM with 1q+. These findings not only provide potential new targets for MM therapies but could have implications beyond MM because of the high frequency of this genetic alteration in cancer.
Loss of BCL7A permits IRF4 transcriptional activity and cellular growth in multiple myeloma
IRF4 is a transcription factor with an oncogenic role in several malignancies including multiple myeloma (MM). Chakraborty et al performed whole genome sequencing of MM samples, revealing new insights into MM biology by uncovering a novel mechanism of control of IRF4. The authors found that >60% of patients exhibited noncoding mutations in the BCL7A gene that led to loss of expression, with BCL7A acting as a tumor suppressor through interactions with IRF4 that impaired IRF4’s ability to bind DNA and orchestrate its usual downstream transcriptional program. Loss of BCL7A therefore allows IRF4 activity to proceed unchecked, driving myeloma cell proliferation.
MYELOID NEOPLASIA
Cell-autonomous dysregulation of interferon signaling drives clonal expansion of SRSF2-mutant MDS stem/progenitor cells
Brief Report
Previous studies have shown that inflammatory and innate immune signaling play a critical role in promoting myelodysplastic syndrome (MDS) through the differential effects of cytokines on malignant cells relative to normal cells, but the mechanisms remain unclear. In this Brief Report, Takashima and colleagues show that reduced STAT1 abundance in SRSF2-mutant MDS cells confers protection against interferon (IFN)-driven cell suppression relative to normal cells. Importantly, the authors demonstrate that treatment with the proteasome inhibitor bortezomib in vitro increased STAT1 abundance and sensitized SRSF2-mutant cells to IFN, providing a potential rationale for using bortezomib therapy in poor prognosis SRSF2-mutated MDS.
LETTER TO BLOOD
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
Confocal image of retinal vasculature of a humanized mouse with sickle cell hemoglobin C (HbSC) disease, showing abnormal vascular growth, leakage, and “comma”-shaped distal vessels following fluorescein isothiocyanate–dextran injection, resembling the classic retinopathy seen in patients with HbSC disease. See the article by Setayesh et al on page 13.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals









HbSC gets its mouse model 75 years after discovery