Key Points
The MIRCA is a reproducible assay with readouts that correlate to SCD severity.
We propose using MIRCA to measure RBC deformability at clinic visits to monitor SCD complication risk and need for therapeutic optimization.
Visual Abstract
In sickle cell disease (SCD), red blood cells (RBCs) are poorly deformable, even under normoxia (NOI). With deoxygenation, deformability of sickle RBCs is further reduced due to polymerization of hemoglobin S (HbS). Rigid, poorly deformable sickle RBCs block microvasculature, causing ischemia, pain, and organ damage. The microfluidic impedance red cell assay (MIRCA) can mechanically measure RBC deformability, providing occlusion index under NOI or hypoxia (HOI) as readouts. We analyzed RBCs from 68 adult and 34 pediatric patients with SCD using the MIRCA. Higher HOI and NOI values were positively associated with markers of inflammation, hemolysis, RBC density, older age, and severe SCD genotypes (homozygous HbSS or HbS β0-thalassemia. Each 1% higher NOI across individuals was associated with a 6.3% higher incidence of acute complications per year. Individuals with chronic complications in the past year had a 3.1% higher median NOI than those without chronic complications. Individuals on chronic transfusion therapy exhibit a subpopulation of poorly deformable RBCs captured by the MIRCA but not by a commercially available device that also measures RBC deformability, the laser assisted optical rotational cell analyzer (LoRRca). In vitro addition of voxelotor or osivelotor to samples from individuals on chronic transfusion therapy improved the deformability of these endogenous RBCs. Longitudinally collected NOI and HOI values in individuals with HbSS were stable, with a median percent point change of 13.3% and 15.7%, respectively. MIRCA can be used in combination with clinical laboratory tests to monitor RBC deformability as a biomarker of clinical status at routine clinic visits and included in clinical trials of disease-modifying agents.
Introduction
SCD
Sickle cell disease (SCD) is a group of inherited hemoglobinopathies caused by a point mutation in the beta-globin gene, forming abnormal sickle hemoglobin (HbS).1 HbS polymerizes when exposed to low-oxygen conditions, making red blood cells (RBCs) more rigid and poorly deformable.2 The RBCs of individuals with SCD exhibit more endothelial adhesion events; both adhesion and poor deformability3 contribute to microvascular occlusion.4 These occlusions trap more blood cells, leading to more sickling and resulting in a “vicious cycle” of sickling.5,6 This sickling cycle leads to vaso-occlusive events (VOE) in the microvasculature, acute chest syndrome (ACS) in the lungs, and avascular necrosis in osseous tissue, and it is associated with systemic end-organ damage and premature mortality.7
RBC deformability is a crucial therapeutic target to produce clinical benefit in individuals with SCD and can be improved with transfusion therapy or hydroxyurea (HU). RBC transfusions suppress %HbS by diluting recipient RBCs with healthy donor RBCs, and HU induces fetal hemoglobin (HbF) production, with variable distribution within the RBC. Although low %HbS is protective on a population level, HbS levels do not always correlate with clinical severity.8,9 Some individuals with low HbS value may still have frequent disease complications, and others with higher %HbS value may be relatively healthy. Other novel targeted SCD therapies include antisickling agents, P-selectin inhibitors, and pyruvate kinase activators. The antisickling agents voxelotor10 (Oxybryta, Pfizer) and osivelotor11 stabilize the hemoglobin molecule in its oxygenated conformation to increase the ratio of oxygenated to deoxygenated HbS and inhibit polymerization. Pyruvate kinase activators improve RBC membrane health, sickling tendency, and deformability by reducing 2,3-diphosphoglycerate to improve hemoglobin oxygen affinity.12 P-selectin inhibitors such as crizanlizumab work by blocking p-selectin–mediated RBC adhesion to the endothelial wall.13
Allogeneic hematopoietic stem cell transplant and US Food and Drug Administration–approved gene therapy strategies may be curative; engrafted stem cells can alter the host hemoglobin profile to a milder phenotype.14,15 However, abnormal rheological characteristics such as poor deformability and high percent dense RBCs (%DRBCs) may persist after engraftment, which are associated with higher odds of SCD clinical complications.16,17 Devices such as the microfluidic impedance red cell assay (MIRCA) can help assess the degree of RBC normalization achieved by various gene therapy strategies.
RBC functional phenotype and clinical trials
Individuals with SCD have variable RBC function even while on therapy. We have previously determined that individuals with SCD may not be severe in all rheological domains.18 Unfortunately, the failure to include RBC rheology tests as part of clinical trials and indications for novel SCD therapies seeking US Food and Drug Administration approval has resulted in a poor understanding of their effects on RBC function. Because many SCD therapeutics directly or indirectly target specific components of abnormal RBC rheology, it is crucial to know the RBC function of the subject. For example, clinical trials with crizanlizumab may have benefited from RBC adhesion testing, permitting subgroup analysis and identification of individuals likely to benefit from this therapy. However, rheological testing is becoming more common: A current Afimune phase 2 clinical trial assessing the efficacy of the investigational fatty acid derivative drug epeleuton includes extent of RBC adhesion to laminin as an inclusion criterion.19 RBC function tests can provide insight into clinical severity, monitor therapeutic improvement, improve RBC functional phenotype subgroup analyses, and contribute to a more comprehensive understanding of adverse events.
Assays that measure RBC deformability
MIRCA is a deformability assay that measures the ability of RBC to squeeze between the gaps within a micropillar array.20,21 MIRCA (Figure 1) calculates the total chip occlusion index (OI) after a 10-minute sample perfusion, which can be measured under normoxia (NOI) or with sodium metabisulfite–induced hypoxia (HOI).22
MIRCA device, assay schematic, and readouts. (A) The current MIRCA system setup includes 2 FlowEZ pressure pumps with a Link module for automated pressure control, a control circuit that interfaces with 2 MIRCA chips for signal acquisition and processing, and a 7-inch touchscreen display. (B) Illustration of the MIRCA assay steps, in which isolated RBCs are either incubated in PBS (under NOI) or sodium metabisulfite (MBS) (under HOI) and perfused through a micropillar network in which poorly deformable RBCs occlude. (C) Brightfield images revealing the difference in occlusion levels for a typical HbSS sample under NOI and HOI in the 3 μm array. Arrows illustrate RBCs stuck in the micropillars with more RBCs in HOI compared with NOI. (D) Time course of occlusion indices obtained through the MIRCA device for a typical HbSS sample in NOI and HOI during sample perfusion.
MIRCA device, assay schematic, and readouts. (A) The current MIRCA system setup includes 2 FlowEZ pressure pumps with a Link module for automated pressure control, a control circuit that interfaces with 2 MIRCA chips for signal acquisition and processing, and a 7-inch touchscreen display. (B) Illustration of the MIRCA assay steps, in which isolated RBCs are either incubated in PBS (under NOI) or sodium metabisulfite (MBS) (under HOI) and perfused through a micropillar network in which poorly deformable RBCs occlude. (C) Brightfield images revealing the difference in occlusion levels for a typical HbSS sample under NOI and HOI in the 3 μm array. Arrows illustrate RBCs stuck in the micropillars with more RBCs in HOI compared with NOI. (D) Time course of occlusion indices obtained through the MIRCA device for a typical HbSS sample in NOI and HOI during sample perfusion.
Ektacytometry is another technique that can measure bulk RBC deformability.23 Standard RBC amounts are added to a viscous solution of polyvinylpyrrolidone, which is then loaded into the space between a stationary bob and a rotating cup. The ratio of the difference and sum of the length and width of the laser diffraction pattern forms as RBCs deform through shear stress as the cup rotates is calculated as the sample elongation index (EI). In oxygen-gradient ektacytometry (laser assisted optical rotational cell analyzer [LoRRca], RR Mechatronics), EI can also be measured as a function of oxygen tension, as nitrogen gas induces deoxygenation within the sample. Therefore, RBC deformability can be assessed at NOI and HOI, represented as EI maximum (EI Max) and minimum (EI Min), respectively. LoRRca also provides the point of sickling (PoS) for a sample, a unique biomarker not shared by MIRCA which reflects the partial pressure of oxygen where HbS sickling, or the first drop in EI, begins. LoRRca readouts are associated with clinical outcomes in SCD and can track treatment-associated improvements to RBC deformability.24
Clinical need
LoRRca (Warwick, RI) oxygen-gradient ektacytometry is the most widely used RBC deformability assay in SCD, and it is used in clinical trials for novel second-line therapeutics. Although LoRRca measures of deformability correlate with several clinical complications in SCD, including painful VOE, ACS, and stroke, the lack of device-to-device agreement of values of the LoRRca, its cost, and difficulty of use seems to be insurmountable obstacles to its widespread use.25
In contrast, the MIRCA is a small, portable device with excellent reproducibility of readouts between chips, allowing a universal range of values between devices and centers,21 overcoming the biggest obstacle to the LoRRca. We have previously revealed that MIRCA and LoRRca biomarkers have similar correlations to laboratory parameters associated with disease severity.26-28 The MIRCA is cheaper ($80 000 vs ∼$10 000) and measures RBC deformability in a more physiological manner. Because of the strengths the MIRCA has as a tool in SCD management, here we report its clinical validation and propose its potential use within routine SCD patient care.
Methods
Peripheral blood samples from adult and pediatric individuals with SCD and healthy controls were collected in EDTA under an Emory University institutional review board–approved protocol. Samples were stored at 4°C for at most 48 hours. Whole blood samples were analyzed on a Siemens ADVIA 2120i Hematology analyzer (Munich, Germany) to generate complete blood count data. Standard RBC amounts in whole blood, determined by complete blood count, were added to polyvinylpyrrolidone for analysis with oxygen-gradient ektacytometry.
MIRCA devices were fabricated at Case Western Reserve University with soft-lithography techniques and shipped dry to Emory University.20 MIRCA devices were perfused with filtered 70% ethanol, 1× phosphate-buffered saline (PBS), and 3% (weight-to-volume ratio) bovine serum albumin before an overnight 4°C incubation to block nonspecific RBC adhesion. Chips were then equilibrated to room temperature and perfused with 1× PBS using a Fluigent FlowEZ LineUp microfluidic flow controller (Paris, France) to account for any volumetric changes.
Whole blood samples were washed, and pelleted RBCs were resuspended to 20% (volume-to-volume ratio) in 1× PBS (NOI) or 1.5% (weight-to-volume ratio) sodium metabisulfite in 1× PBS (HOI) to run on MIRCA. Baseline OI measurements were collected by running sample matrix through the device before a 10-minute sample perfusion. To test the impact of antisickling agents on deformability, RBCs were treated with 1.66 mM voxelotor or osivelotor dissolved in dimethyl sulfoxide (DMSO) or with DMSO alone, incubated at 25°C for 1 hour, and then analyzed with MIRCA.
Two physicians extracted the clinical data from the electronic medical records (Epic). Transfusion history, date, and frequency of ACS and VOE in the past year, clinical and silent stroke data, and secondary diagnoses (eg, priapism, splenic infarction, kidney injury, retinopathy, chronic pain) were obtained using International Classification of Diseases 9/10 codes and manual review of discharge records. Recent history of transfusion was defined as any acute or chronic transfusion within 100 days before sample collection and/or HbA >0, which ensured identification of transfusions received at other hospitals. A minimum chronic transfusion duration of 3 months was selected to permit suppression of endogenous stress erythropoiesis and subsequent decreases in HbF levels.29,30 Demographic data were also collected. Maximum tolerated HU dose (MTD) was defined as HU dosage ≥35 mg/kg per day or an absolute neutrophil count (ANC) <4 × 103/μL.31
StataNow version 18.5 and GraphPad Prism version 10.2.1 were used to analyze data. Nonparametric Mann-Whitney U or Wilcoxon signed rank test was used to determine significant differences between groups. Clinical complications were analyzed using negative binomial or logistic regression models. An adjusted P < .05 was considered significant.
Results
A total of 110 peripheral blood samples were taken from healthy controls and individuals with SCD between July 2022 and August 2024. Overall, 37 individuals were <18 years of age and 62 individuals were female. Samples were taken from individuals with the following genotypes: 79 HbSS, 8 HbSβ0-thalassemia, 12 HbSC, 2 HbSβ+-thalassemia, 1 HbSE, and 8 HbAA controls. In addition, 27 individuals had a history of transfusion. The median patient age was 25 years (range, 6-78 years). A subject demographic breakdown (Table 1) and a histogram plotting the age distribution between genotypes (supplemental Figure 3) are provided.
Patient demographic information
| Genotype . | N . | Female . | Age <18 years . | Recent transfusion . | HU . |
|---|---|---|---|---|---|
| HbAA | 8 | 4 | 3 | N/A | N/A |
| HbSS | 79 | 44 | 26 | 23 | 54 |
| HbSβ0 | 8 | 4 | 3 | 1 | 7 |
| HbSC | 12 | 9 | 4 | 2 | 2 |
| HbSβ+ | 2 | 0 | 0 | 1 | 1 |
| HbSE | 1 | 1 | 1 | 0 | 0 |
| Genotype . | N . | Female . | Age <18 years . | Recent transfusion . | HU . |
|---|---|---|---|---|---|
| HbAA | 8 | 4 | 3 | N/A | N/A |
| HbSS | 79 | 44 | 26 | 23 | 54 |
| HbSβ0 | 8 | 4 | 3 | 1 | 7 |
| HbSC | 12 | 9 | 4 | 2 | 2 |
| HbSβ+ | 2 | 0 | 0 | 1 | 1 |
| HbSE | 1 | 1 | 1 | 0 | 0 |
Demographic information of all individuals sampled during the course of this study is provided. Any history of recent transfusion is defined as any chronic or acute transfusion within 100 days before sample collection.
N/A, not applicable.
Higher NOI and HOI were associated with more severe clinical phenotype
Individuals with SCD (HbSC/HbSβ+/HbSE) had 6% lower NOI and 18.2% lower HOI values (P = .002 and .001, Figure 2A-B), than individuals with sickle cell anemia (HbSS/HbSβ0). NOI and HOI values were 2.64% and 10.75% higher in adults compared with children (P = .04 and .004, respectively) (Figure 2C-D). When the analysis was restricted to HbSS/HbSβ0 individuals, NOI was not associated with age groups (Figure 2C, P = .26), but HOI was still associated with age groups (Figure 2D, P = .01). OIs were not associated with sex (supplemental Figure 2).
MIRCA readouts are associated with clinical indicators of disease severity. Median NOI (A) and HOI (B) values were higher by 2.64% (P = .04) and 10.75% (P = .004) in 62 and 44 adults compared with 41 and 21 children, respectively. Median NOI (C) and HOI (D) values were lower by 6% (P = .002) and 18.2% (P = .001) in 11 and 7 HbSC/HbSβ+/HbSE individuals compared with 87 and 57 HbSS/HbSβ0 individuals, respectively. (E) Twenty-two individuals on MTD of HU (dosage >35 mg/kg per d ay or <4 × 103 ANC/μL) had HOI values lower by 6.9% compared with 19 individuals not on MTD (P = .04). No significant difference (P = .85) was observed in the median HOI of individuals (n = 23) not on HU and those on HU but not HU MTD. (F) NOIs from 62 individuals who developed chronic SCD complications (priapism, splenic infarction, kidney injury, retinopathy, chronic pain) within 1 year before sample collection were found to have 3.1% higher NOI values compared with 45 individuals who did not have any SCD complication (P = .015). In panels A-F, all comparisons were performed using a Mann-Whitney U test to compare sample medians. (G) The margins plot of NOI and cumulative incidence rate of acute complications (VOE + ACS) within the past year reveals that 1 unit increase in NOI was associated with 6.3% high risk of acute complications (P = .025; negative binomial model). ∗P ≤ .05; ∗∗P ≤ .01. ns, not significant.
MIRCA readouts are associated with clinical indicators of disease severity. Median NOI (A) and HOI (B) values were higher by 2.64% (P = .04) and 10.75% (P = .004) in 62 and 44 adults compared with 41 and 21 children, respectively. Median NOI (C) and HOI (D) values were lower by 6% (P = .002) and 18.2% (P = .001) in 11 and 7 HbSC/HbSβ+/HbSE individuals compared with 87 and 57 HbSS/HbSβ0 individuals, respectively. (E) Twenty-two individuals on MTD of HU (dosage >35 mg/kg per d ay or <4 × 103 ANC/μL) had HOI values lower by 6.9% compared with 19 individuals not on MTD (P = .04). No significant difference (P = .85) was observed in the median HOI of individuals (n = 23) not on HU and those on HU but not HU MTD. (F) NOIs from 62 individuals who developed chronic SCD complications (priapism, splenic infarction, kidney injury, retinopathy, chronic pain) within 1 year before sample collection were found to have 3.1% higher NOI values compared with 45 individuals who did not have any SCD complication (P = .015). In panels A-F, all comparisons were performed using a Mann-Whitney U test to compare sample medians. (G) The margins plot of NOI and cumulative incidence rate of acute complications (VOE + ACS) within the past year reveals that 1 unit increase in NOI was associated with 6.3% high risk of acute complications (P = .025; negative binomial model). ∗P ≤ .05; ∗∗P ≤ .01. ns, not significant.
Of 102 patients with SCD, 64 were on HU, and 39 of those patients were on HU MTD. HOI values were 6.9% lower in patients on MTD (Figure 2E, P = .04). The median HOI of individuals on HU but not at MTD and individuals not on HU was comparable (P = .85). These associations did not change when the analysis was restricted to HbSS/HbSβ0 individuals. NOI was not associated with MTD status. OIs were not associated with transfusion status or time between sample collection and transfusion date.
Each 1% higher NOI across individuals with SCD was associated with 6.3% higher risk of ACS + VOE in the previous year (P = .025, negative binomial model; Figure 2G). However, the association was lost when adjusted for HbF and age. Patients with occlusion-related SCD complications (priapism, splenic infarction, kidney injury, retinopathy, chronic pain) had 0.25% higher median NOI compared with those who did not have SCD complications after adjusting for genotype and DRBC (P = .02, linear mixed model; Figure 2F) but were 3.1% higher without covariate adjustment. Age, %HbF, ANC, and absolute reticulocyte count (ARC) were not retained in the final model as predictors as they had a P value of >.05.
HOI was not associated with ACS or VOE (P = .5). OIs were not associated with silent stroke (P = .65), clinical stroke (P = .88), or avascular necrosis (P = .4).
Higher MIRCA values were associated with abnormal traditional laboratory parameters associated with SCD
Scatterplots of MIRCA and LoRRca readouts (Figure 3A-D) reveal nonlinear relation of HbS with all biomarkers. OIs worsen as HbS levels drop below 40%, whereas LoRRca values remain stable or improve. Samples with HbS between 40% and 89% have higher OIs, and samples with HbS above 90% have a greater rise in OI with higher %HbS. The association of HOI and HbS (P = .001) could be because of lower HbF values in this group compared with the patients with HbS between 40 and 80 (median HbF = 0% [range, 0-9.3] vs 12.2% [range, 6.5-20], P = .013). The median OIs of individuals with <40% HbS and those with HbS >90% without HU optimization (Figure 3E-F) are not significantly different.
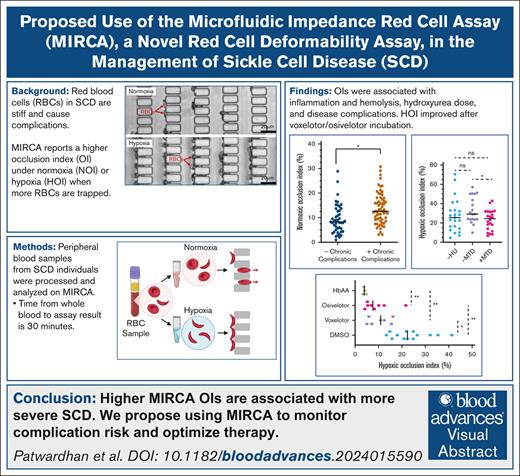
MIRCA readouts capture low red cell deformability in individuals on chronic transfusion therapy, which can be improved with the in vitro addition of osivelotor or voxelotor. (A-D) A nonlinear relationship of HbS% with MIRCA (A-B) and LoRRca (C-D) readouts. NOI and HOI values worsen, EI max values remain stable, and EI Min values improve as HbS values decrease below 40% after blood transfusion. The statistically significant association of HOI and HbS (P = .001) could be due to lower HbF values in this group compared with the patients with HbS between 40 and 80 (median HbF = 0% [range, 0-9.3] vs 12.2% [range, 6.5-20], P = .013). (E-F) No significant difference in the median NOI (E) and HOI (F) of individuals with HbS <40% vs those with HbS >90% and not on HU MTD (P = .3 and P = .3, respectively). To test the impact of in vitro addition of antisickling agents on deformability, RBCs from 12 individuals with HbSS were treated with 1.66 mM osivelotor (n = 8) or voxelotor (n = 6) (G) dissolved in DMSO or with DMSO alone, incubated at 25°C for 1 hour and resuspended in 1.5% MBS in 1× PBS and run on the MIRCA. Osivelotor- and voxelotor-treated samples were found to have 17.3% and 10.8% lower HOI values compared with DMSO-treated controls (P = .008 and .03, respectively, Wilcoxon signed rank test). However, osivelotor and voxelotor sample HOI values were still elevated by 3.7% and 7.0% compared with 4 healthy controls (P = .008 and .006, respectively, Mann-Whitney U test). ∗P ≤ .05; ∗∗P ≤ .01. ns, not significant.
MIRCA readouts capture low red cell deformability in individuals on chronic transfusion therapy, which can be improved with the in vitro addition of osivelotor or voxelotor. (A-D) A nonlinear relationship of HbS% with MIRCA (A-B) and LoRRca (C-D) readouts. NOI and HOI values worsen, EI max values remain stable, and EI Min values improve as HbS values decrease below 40% after blood transfusion. The statistically significant association of HOI and HbS (P = .001) could be due to lower HbF values in this group compared with the patients with HbS between 40 and 80 (median HbF = 0% [range, 0-9.3] vs 12.2% [range, 6.5-20], P = .013). (E-F) No significant difference in the median NOI (E) and HOI (F) of individuals with HbS <40% vs those with HbS >90% and not on HU MTD (P = .3 and P = .3, respectively). To test the impact of in vitro addition of antisickling agents on deformability, RBCs from 12 individuals with HbSS were treated with 1.66 mM osivelotor (n = 8) or voxelotor (n = 6) (G) dissolved in DMSO or with DMSO alone, incubated at 25°C for 1 hour and resuspended in 1.5% MBS in 1× PBS and run on the MIRCA. Osivelotor- and voxelotor-treated samples were found to have 17.3% and 10.8% lower HOI values compared with DMSO-treated controls (P = .008 and .03, respectively, Wilcoxon signed rank test). However, osivelotor and voxelotor sample HOI values were still elevated by 3.7% and 7.0% compared with 4 healthy controls (P = .008 and .006, respectively, Mann-Whitney U test). ∗P ≤ .05; ∗∗P ≤ .01. ns, not significant.
Margin plots in Figure 4 reveal the association of OIs with ARC, ANC, Hb, and DRBCs in mixed multivariate linear models, including genotype, age, and transfusion status. Each 1% gain in NOI across individuals was associated with 5.68 × 106/μL higher ARC (Figure 4A, P = .02), 0.1 gm% lower hemoglobin (Figure 4D, P = .001), and 0.33% higher dense RBCs (Figure 4F, P < .001). Each 1% gain in HOI was associated with 6.59 × 106/μL higher ARC (Figure 4B, P < .001), .088 × 103/μL higher ANC (Figure 4C, P < .001), 0.04 gm% lower hemoglobin (Figure 4E, P = .05), and 0.1% higher DRBC (Figure 4G, P = .04). Mean corpuscular volume (MCV) was associated with HOI in a univariate analysis (supplemental Figure 5), but not after adjusting for covariates. NOI was not associated with MCV.
Low RBC deformability under NOI and HOI is associated with laboratory markers of severe clinical phenotype. An increase of 1% in NOI is associated with 5.68 × 106/μL higher reticulocyte count (P = .02, A), 0.1 gm% lower hemoglobin (P = .001, C), and 0.33% higher dense RBCs (P < .001, F). One percent increase in HOI is associated with 6.59 × 106/μL higher ARC (P < .001, B), 0.04% lower hemoglobin (P = .05, E), 88/μL higher ANC (P < .001, C), and 0.1% higher dense RBCs (P = .04, G). (H) MIRCA NOI and HOI readouts were collected for a subset of individuals with SCD (n = 91) also run on LoRRca oxygen-gradient ektacytometry. Statistically significant standardized effect sizes of the association between MIRCA and LoRRca biomarkers with traditional laboratory markers of SCD severity (Hb, g/dL), ARC (109/μL), ANC (103/μL), DRBC (%) are reported after controlling for age, genotype, and transfusion status. The standardized effect sizes reflect 1 SD change in MIRCA or LoRRca values with 1 SD increase in traditional laboratory markers.
Low RBC deformability under NOI and HOI is associated with laboratory markers of severe clinical phenotype. An increase of 1% in NOI is associated with 5.68 × 106/μL higher reticulocyte count (P = .02, A), 0.1 gm% lower hemoglobin (P = .001, C), and 0.33% higher dense RBCs (P < .001, F). One percent increase in HOI is associated with 6.59 × 106/μL higher ARC (P < .001, B), 0.04% lower hemoglobin (P = .05, E), 88/μL higher ANC (P < .001, C), and 0.1% higher dense RBCs (P = .04, G). (H) MIRCA NOI and HOI readouts were collected for a subset of individuals with SCD (n = 91) also run on LoRRca oxygen-gradient ektacytometry. Statistically significant standardized effect sizes of the association between MIRCA and LoRRca biomarkers with traditional laboratory markers of SCD severity (Hb, g/dL), ARC (109/μL), ANC (103/μL), DRBC (%) are reported after controlling for age, genotype, and transfusion status. The standardized effect sizes reflect 1 SD change in MIRCA or LoRRca values with 1 SD increase in traditional laboratory markers.
In a subset of patient samples run on both MIRCA and LoRRca (n = 91), multivariate linear mixed models were used to assess associations between device biomarkers and conventional laboratory parameters (Figure 4H). MIRCA readouts were more strongly associated with hemoglobin compared with EI Max; 1 standard deviation (SD) higher NOI and EI Max values were associated with 0.34 SD (P = .001) lower and 0.26 SD (P = .003) higher Hb values, respectively. HOI had a weaker association with DRBC compared with EI Min; 1 SD higher HOI and EI Min values were associated with 0.16 SD (P = .04) higher and 0.48 SD (P < .001) lower DRBC values, respectively. HbF values were not associated with OIs, whereas LoRRca readouts were associated in univariate and multivariate analyses.
MIRCA can measure improvements to RBC deformability with in vitro osivelotor and voxelotor treatment
Eight paired HbSS samples, 7 of which were recently transfused, were treated with osivelotor dissolved in DMSO or DMSO alone before measuring HOI (Figure 3G). Eight paired HbSS samples, 5 of which were recently transfused, were also treated with voxelotor dissolved in DMSO or DMSO alone.
Median HOI after drug incubation compared with DMSO control was 14.6% (P = .008) lower with osivelotor and 9.8% lower with voxelotor (P = .016). However, compared with HbAA controls, osivelotor and voxelotor treatment samples still had 3.6% (P = .008) and 7% (P = .006) higher median HOI, respectively. The median EI Min of 6 voxelotor-treated, recently transfused HbSS samples was 0.011 higher compared with DMSO control (supplemental Figure 1A), but this difference was not statistically significant (P = .44).
MIRCA longitudinal sample analysis and similarities to LoRRca
Intrapatient variability of samples collected from steady-state individuals with HbSS at 2 time points was calculated as the median percent point change of NOI and HOI values between the first and second collection and was 13.3% (interquartile range [IQR]: 6.7%-20.1%) and 15.7% (IQR: 11.7%-28.3%), respectively (Figure 5A-B). Four individuals (Figure 5C) had an acute event within 30 days after sample collection with a 101% median percent increase in NOI values compared with their previous value (P = .12). Median percent change of EI Max and that of EI Min in another 25 HbSS steady-state patients (Figure 5E-F) were found to be 15.7% (IQR: 6.2%-19.1%) and 82.1% (IQR: 31.1%-171.7%), respectively. Furthermore, a 1-sample t test comparing the mean of the intrapatient difference in biomarker values between time points found no significant deviation from 0 for all MIRCA and LoRRca biomarkers: NOI (P = .32), HOI (P = .09), EI Max (P = .15), and EI Min (P = .61).
MIRCA is a reproducible assay. (A-C) MIRCA OIs for individuals with HbSS were recorded longitudinally at routine clinic visits between November 2022 and July 2024. The NOI (n = 6) (A) and HOI (n = 5) (B) of steady-state individuals remained stable over time (P = .44, P = .13, Wilcoxon signed rank test) with a median percent point change of 13.3% and 15.7%, respectively. Four individuals (C) were not at steady state, defined as having had an acute VOE or ACS episode within the past 30 days, and were analyzed separately. The median NOI value of individuals with recent history of acute crisis rose by 101% compared with initial baseline measurement and median NOI increased by 8.6% (P = .12, Wilcoxon signed rank test). (D) CVs from comparable biomarkers (NOI vs EI Max and HOI vs EI Min) were compared using a nonparametric Mann-Whitney U test. Although NOI and EI Max were not significantly different in terms of reproducibility (P = .13), EI Min was found to have a 33.9% higher CV compared with HOI (P = .0095). Raw values used to calculate CVs can be found in supplemental Table 1. LoRRca EI Max (E) and EI Min (F) values in another 25 HbSS steady-state individuals were also recorded longitudinally at routine clinic visits, with median percent point changes of 15.7% and 82.1%, respectively. However, the intrapatient difference between MIRCA and LoRRca biomarker values between sample collection time was not significantly different from 0 using a 1-sample t test: NOI (P = .32), HOI (P = .09), EI Max (P = .15), and EI Min (P = .61). ∗∗P ≤ .01. ns, not significant.
MIRCA is a reproducible assay. (A-C) MIRCA OIs for individuals with HbSS were recorded longitudinally at routine clinic visits between November 2022 and July 2024. The NOI (n = 6) (A) and HOI (n = 5) (B) of steady-state individuals remained stable over time (P = .44, P = .13, Wilcoxon signed rank test) with a median percent point change of 13.3% and 15.7%, respectively. Four individuals (C) were not at steady state, defined as having had an acute VOE or ACS episode within the past 30 days, and were analyzed separately. The median NOI value of individuals with recent history of acute crisis rose by 101% compared with initial baseline measurement and median NOI increased by 8.6% (P = .12, Wilcoxon signed rank test). (D) CVs from comparable biomarkers (NOI vs EI Max and HOI vs EI Min) were compared using a nonparametric Mann-Whitney U test. Although NOI and EI Max were not significantly different in terms of reproducibility (P = .13), EI Min was found to have a 33.9% higher CV compared with HOI (P = .0095). Raw values used to calculate CVs can be found in supplemental Table 1. LoRRca EI Max (E) and EI Min (F) values in another 25 HbSS steady-state individuals were also recorded longitudinally at routine clinic visits, with median percent point changes of 15.7% and 82.1%, respectively. However, the intrapatient difference between MIRCA and LoRRca biomarker values between sample collection time was not significantly different from 0 using a 1-sample t test: NOI (P = .32), HOI (P = .09), EI Max (P = .15), and EI Min (P = .61). ∗∗P ≤ .01. ns, not significant.
When the same HbSS samples were run on 3 MIRCA and LoRRca devices (Figure 5D), the median coefficient of variance (CV) of HOI was 33.95% lower compared with EI Min (P = .0095). CVs for EI Max and NOI were both below 10% and comparable (P = .97). Raw values used to calculate CVs can be found in supplemental Table 1. MIRCA and LoRRca values are also closely correlated (Figure 7).
Discussion
MIRCA readouts are associated with traditional markers of disease severity
MIRCA readouts were associated with traditional laboratory markers of clinical severity after controlling for age, genotype, and MTD status. Higher NOI values were associated with markers of hemolysis (ARC, Hb) and abnormal RBC density (Figure 4). Higher HOI values were associated with markers of hemolysis (ARC, Hb), abnormal RBC density, and inflammation (ANC).
OIs were higher in adults with SCD compared with children with SCD and were higher in individuals with HbSS/HbSβ0 genotypes compared with milder SCD genotypes (Figure 2A-D). MIRCA can capture differences in RBC deformability from the progressive nature of SCD and across more severe genotypes. HOI may be more sensitive to differences in RBC deformability associated with age and genotype, with greater median differences between subgroups compared with NOI.
MIRCA NOI is associated with clinically severe disease
Higher NOI values were associated with a higher incidence of acute events (Figure 2G), where each NOI increase of 1% across individuals was associated with 6.3% higher rate of acute VOE or ACS within the past year (P = .025). The association between NOI and acute events trends toward significance after controlling for age and %HbF; an increase of each 1% NOI across individuals was associated with 3.7% higher rate of acute complications (P = .09).
Higher NOI values were associated with SCD-related chronic complications. Individuals with chronic complications before sample collection had 3.1% higher NOI values compared with those without (P = .015, Figure 2F). This association remained significant after adjusting for covariates.
Our study was not designed to analyze prospective data; validation of the associations between MIRCA values and SCD complications using prospective longitudinal data is in progress. In addition, the assessment of the relationship between MIRCA values and silent stroke is limited because many subjects did not have a recent MRI performed; it is, therefore, possible that an association between MIRCA values and silent stroke risks exists but was not detected.
MIRCA can aid clinicians in therapy optimization
Our cohort included 64 subjects (63%) on HU, 39 of which were HU MTD. HOI was significantly lower in the HU MTD group compared with those not on MTD (Figure 2E), demonstrating that HOI can be used to monitor patients on HU. We, then, performed a Fisher exact test to analyze the association of MTD status with chronic SCD complications and found no association (P = .11). It is well established that HU MTD status is associated with a lower incidence of chronic complications.32-34 Potentially due to the short, 1-year follow-up, MTD status was not associated with chronic complications in our study. Despite the short follow-up period, MIRCA HOI captured differences in RBC deformability between individuals on MTD of HU. These results suggest that HOI may be used to identify high-risk patients after HU optimization, which may benefit from second-line therapies before the onset of complications, as a complementary assay to clinical laboratory panels.
A nonlinear relationship of %HbS with MIRCA (Figure 3A-B) was found alongside a subpopulation of poorly deformable cells even at very low %HbS typical of red cell exchange, the most aggressive transfusion regimen.35 These poorly deformable, endogenous RBCs also have low %HbF. The median OIs of this subpopulation were not significantly different compared with individuals with high HbS and unoptimized HU dose (Figure 3E-F). It is unlikely that donor-derived stiff RBCs or %DRBCs contribute to high OI as the proportion of these abnormal cells relative to the total donor RBC population is below 1%, even while in prolonged storage (supplemental Figure 4A-B). However, we cannot rule out a role for poor-quality recipient cells in VOE and organ damage; a possible use of MIRCA and LoRRca is in assessing the quality of donor RBC, particularly for very ill or vulnerable patients.
In vitro addition of osivelotor and voxelotor improved the population of RBCs with poor deformability present in transfused individuals (Figure 3G). Improving RBC deformability after in vitro treatment may ameliorate microvascular occlusion mediated by abnormal RBC deformability. Although we observed a small median increase of 0.011 (P = .44) in EI Min after voxelotor treatment (supplemental Figure 1A), the median DMSO-control sample EI Min was already elevated (0.333). Because EI Min measures average RBC deformability, the high EI Min in these samples is likely due to a larger presence of healthy donor RBCs compared with HbSS RBCs.
It is possible that the drastic improvement to HOI after incubation could be a combination of (1) antisickling drugs modifying endogenous HbSS RBCs and (2) the ability of MIRCA to preferentially capture recipient HbSS RBCs while allowing donor HbAA RBCs to flow through unobstructed. MIRCA may allow clinicians to characterize patients’ endogenous RBC function even while on transfusion therapy, a feature helpful in assessing the benefit of combined drug and transfusion therapy.
However, although modified RBCs had improved RBC deformability, median HOI values were still elevated compared with healthy controls. Prolonged in vivo treatment with osivelotor or voxelotor may produce further improvements in the RBC over the immediate drug effects of in vitro addition.
Longitudinal stability of MIRCA values and comparison with LoRRca
MIRCA OI values for individuals with HbSS from samples obtained at steady state (in usual state of health, on stable therapies at scheduled clinic visits) across sample collection time points are stable (Figure 5A-B). There was no significant change in the median NOI and HOI values of these individuals with a low median percent point change of 13.3% and 15.7%, respectively. Although the sample size is limited, these results suggest that individuals without changes to their disease state have minimal fluctuation in their MIRCA values.
However, for the 4 individuals who experienced an acute event (Figure 5C) before second sample collection, the average percent change in NOI was 101%. This difference was not statistically significant (P = .12) as the sample size was small and the study was not designed to capture acute complications.
MIRCA NOI and HOI have similar correlations to laboratory parameters associated with disease severity: Hb, ANC, ARC, and %DRBC, when compared with their respective LoRRca biomarker EI Max and EI Min (Figure 4). When comparing CVs between MIRCA and LoRRca readouts, although EI Max and NOI CVs were both similar and below 20%, EI Min had 33.95% higher CV compared with HOI. The median CV of LoRRca PoS was also low at 14%. Our data suggest that MIRCA HOI readouts could be more consistent and reproducible compared with LoRRca EI Min.
The proposed use of MIRCA
Although LoRRca oxygenscan is more widespread in use and has undergone some clinical validation, it may be challenging to use clinically for SCD because of its cost and poor reproducibility. The rapid, reproducible nature of MIRCA and its associations with disease severity allows for its widespread use as a clinical laboratory test at SCD centers. We, therefore, present a model algorithm that considers the patient clinical status alongside OI (Figure 6). However, applying this algorithm in SCD centers remains a future direction that requires further clinical validation.
Proposed use of MIRCA as a clinical tool in the management of SCD. Patient care algorithms are categorized as asymptomatic without treatment (A), with treatment (B), and symptomatic on treatment (C). In all cases, it is recommended to define an individual’s MIRCA OI baseline. For asymptomatic individuals, an increase in MIRCA OI during follow-up is recommended as an indicator to start a first-line therapy (A) or to optimize HU after starting therapy (B). For symptomatic individuals (C), stable or rising MIRCA OIs may indicate a second-line therapy beyond HU or CTF. For dropping OIs, other red cell function tests may be prioritized to understand and target the underlying cause behind symptomatic disease (eg, adhesion microfluidics for increased red cell adhesion, viscometry, or hematocrit/viscosity ratio for high viscosity). Regardless of patient status, individuals should have OIs monitored at routine clinic visits and during acute crises.
Proposed use of MIRCA as a clinical tool in the management of SCD. Patient care algorithms are categorized as asymptomatic without treatment (A), with treatment (B), and symptomatic on treatment (C). In all cases, it is recommended to define an individual’s MIRCA OI baseline. For asymptomatic individuals, an increase in MIRCA OI during follow-up is recommended as an indicator to start a first-line therapy (A) or to optimize HU after starting therapy (B). For symptomatic individuals (C), stable or rising MIRCA OIs may indicate a second-line therapy beyond HU or CTF. For dropping OIs, other red cell function tests may be prioritized to understand and target the underlying cause behind symptomatic disease (eg, adhesion microfluidics for increased red cell adhesion, viscometry, or hematocrit/viscosity ratio for high viscosity). Regardless of patient status, individuals should have OIs monitored at routine clinic visits and during acute crises.
MIRCA OI and LoRRca EI values closely correlate. Scatterplots are colored by SCD genotype, and associations were tested with Spearman correlation coefficients (rs). (A) MIRCA NOI and LoRRca EI Max share a fairly negative linear association (rs = −0.45; P < .0001). (B) MIRCA HOI and LoRRca EI Min share a poorly negative linear association (rs = −0.28; P = .046).
MIRCA OI and LoRRca EI values closely correlate. Scatterplots are colored by SCD genotype, and associations were tested with Spearman correlation coefficients (rs). (A) MIRCA NOI and LoRRca EI Max share a fairly negative linear association (rs = −0.45; P < .0001). (B) MIRCA HOI and LoRRca EI Min share a poorly negative linear association (rs = −0.28; P = .046).
Asymptomatic patients should have baseline OIs collected at routine clinic visits to monitor worsened readouts associated with disease complications. We report that each 1% increase in NOI is associated with a 6.3% higher risk of acute complications (Figure 2G). Each 4% rise in NOI is associated with an ∼25% higher VOE or ACS rate per year, a cutoff used in clinical practice to adjust or add another line of treatment. This rise could indicate starting HU, transfusions/phlebotomy, or another second agent for individuals without current treatment or considering second-line therapy for patients already on HU (Figure 6A-B).
OI values from symptomatic individuals on treatment should be checked while in an acute setting (Figure 6C); if rising or stable, their current therapy should be optimized, and a second-line agent should be considered. If dropping, other RBC function tests should be considered as abnormal RBC deformability may not drive the acute status of the patient.
MIRCA readouts may be used as an enrollment criterion or as a secondary end point in clinical trials. For example, baseline-elevated OIs could help trials target individuals with poor deformability who may benefit from novel targeted therapies that address that defect. Secondary end point could include OIs to capture therapy-driven improvements to RBC deformability.
In conclusion, MIRCA readouts are rapid, reproducible, and comparable to existing measures of RBC deformability. NOI and HOI are associated with laboratory parameters of disease severity and disease complications. MIRCA measures subpopulations of RBCs with poor deformability in chronic transfusion therapy recipients, which can be improved with in vitro incubation with antisickling agents. Although the MIRCA requires further clinical validation before its widespread adoption, its lower cost and portable nature make it well suited for use in low- and middle-income countries, especially where SCD is more prevalent. Future studies aim to prospectively test MIRCA readouts to determine patient risk of acute events, to explore the role of MIRCA in assessing the safety and efficacy of chronic transfusion therapy, and to investigate the use of MIRCA in choosing and monitoring second-line therapies.
Acknowledgments
This work is supported by the Case-Coulter Translational Research Partnership program at Case Western Reserve University, Atlanta Center for Microsystems Engineered Point-of-Care Technologies under Award No. U54EB027690, National Heart, Lung, and Blood Institute under Award No. R56HL165946, and National Institutes of Health Office of the Director under Award No. U01AI176469.
Authorship
Contribution: A.A.P., A. Pasupuleti, S.O., C.A.D., E.E., J.W., and J.J.Y. performed the research; A.A.P. and A. Patel analyzed the results and made the figures; A.A.P., S.O., C.A.D., and Z.S. contributed to study methodology and edited the manuscript; S.O., C.A.D., and Z.S. contributed vital new analytical tools; A. Patel, M.A.S., P.M., U.A.G., and V.A.S. designed the research and edited the manuscript; and A.A.P. wrote the manuscript.
Conflict-of-interest disclosure: U.A.G. and Case Western Reserve University have financial interests in Hemex Health Inc and BioChip Labs Inc. M.A.S., U.A.G., P.M., and Case Western Reserve University have financial interests in XaTek Inc and BioChip Labs Inc. U.A.G. has financial interests in DxNow Inc. P.M. has financial interests in Haima Therapeutics LLC. Financial interests include licensed intellectual property, stock ownership, research funding, employment, consulting fees, and royalties during this study. The competing interests of Case Western Reserve University employees are overseen and managed by the Conflict of Interests Committee according to a Conflict-of-Interest Management Plan. The remaining authors declare no competing financial interests.
Correspondence: Vivien A. Sheehan, Department of Pediatrics, Emory University School of Medicine, 1760 Haygood Dr, Atlanta, GA 30322; email: vivien.sheehan@emory.edu.
References
Author notes
Presented in abstract form at the 66th annual meeting of the American Society of Hematology, San Diego, CA, 7 December 2024.
Data are available on request from the corresponding author, Akshay A. Patwardhan (apatwa6@emory.edu).
The full-text version of this article contains a data supplement.



![MIRCA readouts capture low red cell deformability in individuals on chronic transfusion therapy, which can be improved with the in vitro addition of osivelotor or voxelotor. (A-D) A nonlinear relationship of HbS% with MIRCA (A-B) and LoRRca (C-D) readouts. NOI and HOI values worsen, EI max values remain stable, and EI Min values improve as HbS values decrease below 40% after blood transfusion. The statistically significant association of HOI and HbS (P = .001) could be due to lower HbF values in this group compared with the patients with HbS between 40 and 80 (median HbF = 0% [range, 0-9.3] vs 12.2% [range, 6.5-20], P = .013). (E-F) No significant difference in the median NOI (E) and HOI (F) of individuals with HbS <40% vs those with HbS >90% and not on HU MTD (P = .3 and P = .3, respectively). To test the impact of in vitro addition of antisickling agents on deformability, RBCs from 12 individuals with HbSS were treated with 1.66 mM osivelotor (n = 8) or voxelotor (n = 6) (G) dissolved in DMSO or with DMSO alone, incubated at 25°C for 1 hour and resuspended in 1.5% MBS in 1× PBS and run on the MIRCA. Osivelotor- and voxelotor-treated samples were found to have 17.3% and 10.8% lower HOI values compared with DMSO-treated controls (P = .008 and .03, respectively, Wilcoxon signed rank test). However, osivelotor and voxelotor sample HOI values were still elevated by 3.7% and 7.0% compared with 4 healthy controls (P = .008 and .006, respectively, Mann-Whitney U test). ∗P ≤ .05; ∗∗P ≤ .01. ns, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/15/10.1182_bloodadvances.2024015590/2/m_blooda_adv-2024-015590-gr3.jpeg?Expires=1764963700&Signature=m4qkmxox8VW14dm8BlqDU5aM1gY0WgZN6nsn3AxOWRwm6zoWuoOIMFuBTHBtlVyScNb7TgLBmaHV01Pjf6x7pyMO6Wjk7UETRUSkvuMEmZ-aalMrFlYlPUuVHQALdcu1~570QIYAyoDN7NO3qjwTfnh9E28V6uMF~tFgYi9NAQ6k6ZVMOSwtpRm2yhuJm5yACN2oAaFsN9idiYTRt3YZF6tGcmwMPVZIH~5MiANpWkZGU7VoLj0kD0lE5~jv7sYico0eaH5LWPEFXWbVdxE8V-WyvIUH1AQBJuk9PV8MmbuFMcW-qTHDqLgi0ApdoYqaXS1u-Z-jfV3lOvIVhxqmaQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



