Key Points
FVIII is constitutively made as multiple partially disulfide-bonded states.
Patient-derived anti-FVIII antibodies bind preferentially to a subset of FVIII disulfide-bonded states.
Visual Abstract
Hemophilia A is a chronic life-threatening condition caused by the deficiency or dysfunction of plasma coagulation factor VIII (FVIII) and commonly managed by prophylaxis with regular infusion of FVIII protein. A major obstacle to FVIII replacement therapy is the generation of alloantibodies that diminish efficacy. Disulfide bonds link pairs of cysteine residues in proteins and, in several proteins, have been found to be only partially formed in the mature proteins. FVIII contains 8 disulfide bonds and their redox state in human blood and recombinant FVIII was determined using differential cysteine alkylation and mass spectrometry. All 8 disulfide bonds were found to be unformed in ∼10% to ∼70% of molecules of FVIII populations, which suggested a conformational flexibility that could favor the binding of certain ligands to subsets of FVIII with more or less formed disulfide bonds. To test this hypothesis, the binding of a panel of 5 patient-derived anti-FVIII antibodies to the population of FVIII disulfide-bonded states was evaluated. All 5 antibodies bound preferentially to FVIII states in which 2 or 3 of the 8 disulfides are significantly more unformed: C1918-C1922 in the A3 domain, C2040-C2188 in the C1 domain, and C2193-C2345 in the C2 domain. Disulfide bond mutagenesis experiments and molecular dynamics simulations indicate that this subset of FVIII states has long-range conformational dynamism that favors antidrug antibody binding. These findings will assist efforts to engineer an FVIII molecule that is less prone to neutralization by antidrug antibodies and has general implications for autoimmune conditions and antibody drug efficacy.
Introduction
Hemophilia A is a chronic life-threatening condition that confers a major clinical, psychologic, and economic burden on patients and caregivers.1,2 It is caused by the deficiency or dysfunction of plasma coagulation factor VIII (FVIII) owing to mutations of the F8 gene encoding this protein. Decreased FVIII expression or activity results in reduced thrombin generation and fibrin clot formation leading to bleeding episodes, most commonly into the joints (hemarthrosis), which in turn leads to chronic pain, joint deformity, reduced mobility, and increased mortality. Approximately a million males (17.1 cases per 100 000 males) are estimated to have hemophilia A.3,4 Current therapies include administration of replacement FVIII protein, a bispecific antibody that mimics FVIII or FVIII gene replacement delivered via liver tropic adeno-associated viral vectors.
Most patients with hemophilia A are treated with exogenously derived FVIII concentrates to increase blood FVIII levels sufficiently to minimize bleeding episodes and reduce the occurrence of hemarthrosis and subsequent joint disease.5,6 A major obstacle to FVIII replacement therapy is the generation of alloantibodies (known as inhibitors) that diminish efficacy. Approximately one-third of patients with severe hemophilia A and a smaller fraction of patients with mild or moderate hemophilia A develop neutralizing anti-FVIII antibodies that render the drug nonfunctional.7,8 Most of the antibodies bind to FVIII epitopes essential for activity that are conserved among different FVIII products, and therefore, the development of anti-FVIII antibodies to 1 product generally precludes further use of other FVIII products.
Disulfide bonds link the sulfur atoms of pairs of cysteine amino acids in proteins. These covalent bonds are concentrated in membrane and secreted proteins, although they are also found in proteins in the cytosol and nucleus. Discovered 60 years ago,9 disulfide bonds were long assumed to be fully formed and inert in the mature native protein, yet research over the last 2 decades has demonstrated that this is not the case. A subset of disulfide bonds called the allosteric disulfides10-12 control the function of the native protein when cleaved by different factors.13,14 Recent investigations have surprisingly demonstrated that disulfide bonds are also not fully formed in native proteins.15 Several proteins are produced and function as multiple partially disulfide-bonded states.16-19 This is not because the bonds are cleaved in the mature protein, but rather they do not form during maturation of the protein.
FVIII contains 8 disulfide bonds that are unformed in up to 70% of molecules of FVIII populations. A panel of 5 patient-derived anti-FVIII antibodies bound preferentially to FVIII states in which 2 or 3 of the 8 disulfides are significantly more unformed. Mutagenesis and structural studies indicate that this subset of FVIII states has structure-wide conformational dynamism that facilitates anti-FVIII antibody binding.
Methods
FVIII proteins and antibodies
Recombinant full-length human FVIII, Advate, was obtained from Takeda. Plasma-derived human FVIII was immunoprecipitated from Factane (LFB Biopharmaceuticals), a product derived from cryoprecipitated plasma, using polyclonal anti–von Willebrand factor antibodies (Dako). The FVIII-neutralizing monoclonal antibodies KM33 and KM4120 and LE2E9, BO2C11, and BOIIB221,22 were from author C.H.C. (Medicines & Healthcare Products Regulatory Agency Science Campus, Hertfordshire, United Kingdom),23 and the monovalent Fab Fc versions of BO2C11 and KM33 antibodies were from author S.L.-D. (Paris, France). For reference, the recombinant nomenclature for the antibodies is KM33 (NB33), KM41 (NB41), LE2E9 (NB2E9), BO2C11 (NB2C11), and BOIIB2 (NB11B2).
Quantification of the redox states of FVIII disulfide bonds
Plasma and recombinant FVIII
Recombinant full-length human FVIII (Advate, 7.8 μg) or plasma-derived human FVIII immunoprecipitated from Factane (300 IU) was incubated with 5 mM 2-iodo-N-phenylacetamide containing 12C atoms in the benzene ring (12C-IPA) in phosphate-buffered saline containing 10% dimethyl sulfoxide for 1 hour at 22°C in the dark to alkylate unpaired Cys thiols in the proteins. NuPAGE lithium dodecyl sulfate sample buffer (Life Technologies) containing 5 mM 12C-IPA was incubated with the proteins for 30 minutes at 60°C. This second labeling step in the presence of sodium dodecyl sulfate (SDS) facilitates access to buried thiols to ensure that their alkylation is complete. The proteins were resolved on SDS–polyacrylamide gel electrophoresis and stained with colloidal Coomassie (Sigma), and the FVIII bands excised, destained, dried, incubated with 40 mM dithiothreitol, and washed.24 The gel slices were incubated with 5 mM IPA containing 13C atoms in the benzene ring (13C-IPA) for 1 hour at 22°C in the dark to alkylate the disulfide bond–derived Cys, washed, and dried before digestion of proteins with 12.5 ng/μL of trypsin (Promega) in 25 mM NH4CO2 and 10 mM CaCl2 overnight at 25°C. Peptides were eluted from the slices with 5% formic acid and 50% acetonitrile. Liquid chromatography, mass spectrometry, and data analysis were performed as described.25 Cys labeled with 12C-IPA or 13C-IPA has a mass of 133.05276 or 139.07289, respectively. Cys-containing FVIII peptides were analyzed. The different redox states of the Cys residues were quantified from the relative ion abundance (area under the curve [AUC]) of peptides labeled with 12C-IPA and/or 13C-IPA. Matching 12C-IPA– and 13C-IPA–labeled peptides with AUC values <100 000 were not included in the analysis. The fraction of the bond unformed is calculated using the relationship: 12C-IPA AUC/(12C-IPA AUC + 13C-IPA AUC). The data were searched for peptides containing free Cys thiols, and these were not detected or comprised <2% of the peptide population, which indicates that alkylation of unpaired Cys residues by 12C-IPA or 13C-IPA was complete in the proteins.
Recombinant FVIII binding to anti-FVIII antibodies
Advate (7.8 μg, 148 nM) was incubated with antidrug antibodies (3 μg, 57 nM) in phosphate-buffered saline in a total volume of 0.35 mL. After 1 hour at 22°C on a rotating wheel, the reaction was incubated with 5 mM 12C-IPA in phosphate-buffered saline containing 10% dimethyl sulfoxide for 1 hour at 22°C in the dark to alkylate unpaired Cys thiols in the proteins. The antibodies were collected with 1 mg protein G Dynabeads (Life Technologies) on a rotating wheel for 0.5 hours at 22°C and the supernatant removed and concentrated with a spin column (3 kDa molecular weight cutoff) for 40 minutes to 40 μL. The concentrated supernatants and beads were incubated with NuPAGE lithium dodecyl sulfate sample buffer containing 5 mM 12C-IPA for 30 minutes at 60°C. The supernatants were resolved on SDS–polyacrylamide gel electrophoresis and the FVIII band processed as described earlier for FVIII proteins alone.
Other methods are provided in the supplemental Methods.
Results
FVIII is constitutively produced as multiple partially disulfide-bonded states
Eight disulfide bonds have been structurally defined in X-ray structures of FVIII (C172-C198 and C267-C348 in the A1 domain, C547-C573 and C649-C730 in the A2 domain, C1851-C1877 and C1918-C1922 in the A3 domain, and C2040-C2188 in the C1 domain and C2193-C2345 in the C2 domain).26-28 The redox state of the disulfide cysteine thiols in plasma FVIII immunoprecipitated from plasma concentrate (Factane) and full-length recombinant FVIII (Advate) was determined using differential cysteine alkylation with a pair of isotopic IPA alkylators (12C-IPA and 13C-IPA) and mass spectrometry16,17,24 (supplemental Figures 1 and 2). The abundance of the different redox states of the cysteines was determined from the relative abundance of cysteine-containing peptides labeled with 12C-IPA and/or 13C-IPA (supplemental Tables 1 and 2). The results are expressed as the fraction of the FVIII disulfide that is unformed in the population of FVIII molecules.
All 8 disulfide bonds were found to exist in unformed states to different extents. The redox state of 7 of the disulfide bonds in plasma FVIII and all 8 disulfides in recombinant FVIII were determined (Figure 1A). The bonds were unformed in ∼10% to ∼70% of molecules of the populations (Figure 1B-C). For instance, the A3 domain C1918-C1922 bond is unformed in ∼70% (plasma-derived FVIII) or ∼50% (recombinant FVIII) of molecules of the FVIII samples. Recombinant FVIII made by cultured cells has comparable disulfide status as the plasma-derived protein, indicating that the bonds are not cleaved in FVIII following secretion into blood but rather do not form during maturation of the protein in the cell. This same phenomenon has been reported in other plasma proteins, including fibrin(ogen),16 α2-macroglobulin,16 and von Willebrand factor,18 and the platelet αIIbβ3 integrin receptor.17
FVIII is constitutively produced as multiple partially disulfide-bonded states. (A) Ribbon representation of the AlphaFold structure of human VIII lacking the unstructured B domain that does not contain disulfide bonds. The domains (A1, A2, A3, C1, and C2) are color coded, and the 8 disulfide bonds are shown as yellow spheres and residue numbers indicated (UniProt P00451 numbering). (B) Incidence of unformed disulfide bonds in human plasma FVIII (n = 3 preparations of cryoprecipitated plasma, Factane) and (C) recombinant FVIII (n = 3 FVIII preparations). For example, the A3 domain C1918-C1922 bond is unformed in ∼70% (plasma) or ∼50% (recombinant) of molecules of the FVIII samples. The C2193-C2345 disulfide cysteines were not able to be resolved in the plasma sample. The bars and errors are mean ± standard deviation (SD).
FVIII is constitutively produced as multiple partially disulfide-bonded states. (A) Ribbon representation of the AlphaFold structure of human VIII lacking the unstructured B domain that does not contain disulfide bonds. The domains (A1, A2, A3, C1, and C2) are color coded, and the 8 disulfide bonds are shown as yellow spheres and residue numbers indicated (UniProt P00451 numbering). (B) Incidence of unformed disulfide bonds in human plasma FVIII (n = 3 preparations of cryoprecipitated plasma, Factane) and (C) recombinant FVIII (n = 3 FVIII preparations). For example, the A3 domain C1918-C1922 bond is unformed in ∼70% (plasma) or ∼50% (recombinant) of molecules of the FVIII samples. The C2193-C2345 disulfide cysteines were not able to be resolved in the plasma sample. The bars and errors are mean ± standard deviation (SD).
Independent validation of these findings is found in different crystal structures of FVIII. The A3 domain C1918-C1922 disulfide is presumed to be unformed in 3 different crystal structures, Protein Data Bank identifiers 6mf2,26 7kwo27 and 7kbt,28 given that neither Cys nor only C1918 of the potential bond is resolved in the structures. In addition, the C2 domain C2193-C2345 disulfide is reported to be missing in the 7kbt28 structure, where the distance between the C2193 and C2345 sulfur atoms is 3.18 Å that is substantially longer than the equilibrium S-S bond length of 2.04 Å29 (supplemental Figure 3).
The mean formed fraction for the individual FVIII disulfides represents the probability that the bond is formed in the population of molecules19 (Table 1). For the general case for any number of disulfides,19 the probability that a given number (n) of disulfide bonds will be formed in a protein, assuming there is no special dependence in the formation of bonds, is P(n) = 2–n. For the 8 disulfide bonds in FVIII, the probability that all bonds are formed from this relationship is very small at 0.004. The probability of a state containing any number of bonds is the product of the probabilities for each of the bonds.19 For the 8 experimental values of mean formed state (Table 1), this equates to a probability of 0.098. Given that the unconditional value of 0.004 is ∼25 times smaller than the experimentally determined value of 0.098, this indicates that disulfide bond formation in FVIII is conditional and the total number of states is likely far fewer than the maximum possible (28 = 256). The experimental value of 0.098 also indicates that only ∼10% of FVIII is predicted to contain all 8 formed disulfide bonds.
FVIII disulfide state and disulfide bond conformation
| FVIII disulfide . | Fraction formed . | Conformation . |
|---|---|---|
| C172-C198 | 0.93 | –LHhook |
| C267-C348 | 0.63 | –LHhook |
| C547-C573 | 0.76 | –RHstaple |
| C649-C730 | 0.77 | ±RHstaple |
| C1851-C1877 | 0.81 | –LHstaple |
| C1918-C1922 | 0.55 | n.d.∗ |
| C2040-C2188 | 0.80 | ±RHhook |
| C2193-C2345 | 0.81 | ±RHhook |
| FVIII disulfide . | Fraction formed . | Conformation . |
|---|---|---|
| C172-C198 | 0.93 | –LHhook |
| C267-C348 | 0.63 | –LHhook |
| C547-C573 | 0.76 | –RHstaple |
| C649-C730 | 0.77 | ±RHstaple |
| C1851-C1877 | 0.81 | –LHstaple |
| C1918-C1922 | 0.55 | n.d.∗ |
| C2040-C2188 | 0.80 | ±RHhook |
| C2193-C2345 | 0.81 | ±RHhook |
The disulfide conformations were determined using the Disulfide Bond Analysis tool30 and Protein Data Bank identifier 7k66 structure of FVIII.31 The fraction formed are the mean values of 3 recombinant FVIII preparations (data from Figure 1C).
n.d., not determined.
This bond is not present in the 7k66 FVIII structure.
Disulfide bonds adopt 20 different conformations defined by the geometry of the 5 dihedral angles that describe the cystine residue.10 Three of the 20 disulfide conformations, the –RHstaple, ±RHhook, and –LHhook bonds, are often associated with allosteric disulfide bonds.10,12,29 It is noteworthy that 3 of the 8 FVIII disulfides, the 2 bonds in the A1 domain and 1 of the 2 bonds in the A2 domain, have “allosteric” conformations (Table 1), which may contribute to the disulfide lability of the protein.
Different disulfide-bonded states of FVIII could confer a conformational flexibility that favors binding of certain ligands to subsets of the protein with more or less formed disulfide bonds. To test this hypothesis, we evaluated the binding of a panel of 5 FVIII-neutralizing antibodies representative of those found in inhibitor patients to the population of FVIII disulfide-bonded states.
Patient anti-FVIII drug antibodies bind preferentially to a subset of FVIII disulfide-bonded states
Antibodies were chosen that cover a range of FVIII epitopes and biochemical and biophysical properties23 (Figure 2A). The BOIIB232 antibody recognizes an epitope in the A2 domain (residues 379-456), KM4120,33 an epitope in the A3 domain (R1803-K1818), LE2E921,34 and KM3335,36 epitopes in the C1 domain (E2066, R2150, and residues 2091-2104 and 2157-2162, respectively), and BO2C1134,37 an epitope in the C2 domain (F2196, N2198, M2199, F2200, R2215, and R2220; Table 2; Figure 2A).
Patient anti-FVIII drug antibodies bind preferentially to a subset of FVIII disulfide-bonded states. (A) Ribbon representation of the AlphaFold structure of human FVIII lacking the unstructured B domain. The domains to which a panel of 5 patient-derived anti-FVIII drug antibodies bind (BOIIB2, KM41, LE2E9, KM33, and BO2C11) are shown. The residues that encompass the epitopes for the antibodies are shown as dots (see Table 1 for residue numbers) and the disulfide bond cysteines as yellow spheres. (B) Incidence of unformed disulfide bonds in the bound vs unbound FVIII (n = 3 experiments). The mean values for the starting (total) FVIII are shown as green crosses. Errors are mean ± SD. The patient anti-FVIII antibodies bound preferentially to FVIII covalent states in which 3 of the 8 disulfides are significantly more unformed (∗P < .05; ∗∗P < .01; ∗∗∗P < .001).
Patient anti-FVIII drug antibodies bind preferentially to a subset of FVIII disulfide-bonded states. (A) Ribbon representation of the AlphaFold structure of human FVIII lacking the unstructured B domain. The domains to which a panel of 5 patient-derived anti-FVIII drug antibodies bind (BOIIB2, KM41, LE2E9, KM33, and BO2C11) are shown. The residues that encompass the epitopes for the antibodies are shown as dots (see Table 1 for residue numbers) and the disulfide bond cysteines as yellow spheres. (B) Incidence of unformed disulfide bonds in the bound vs unbound FVIII (n = 3 experiments). The mean values for the starting (total) FVIII are shown as green crosses. Errors are mean ± SD. The patient anti-FVIII antibodies bound preferentially to FVIII covalent states in which 3 of the 8 disulfides are significantly more unformed (∗P < .05; ∗∗P < .01; ∗∗∗P < .001).
Features of the recombinant IgG4 anti-FVIII drug antibodies employed in the study
| Antibody . | Binding domain . | Epitope (UniProt P00451 numbering) . | References . |
|---|---|---|---|
| BO2C11 | C2 | F2215, N2217, M2218, F2219, R2234, and R2239 | 34,37 |
| LE2E9 | C1 | E2085, R2169 | 21,34 |
| KM33 | C1 | Residues 2110-2123 and 2176-2181 | 35,36 |
| KM41 | A3 | R1822-K1837 | 20,33 |
| BOIIB2 | A2 | Residues 398-475 | 32 |
The patient anti-FVIII antibodies bound preferentially to FVIII covalent states in which 2 or 3 of the 8 disulfides are significantly more unformed: C1918-C1922 in the A3 domain, C2040-C2188 in the C1 domain, and C2193-C2345 in the C2 domain (Figure 2B). The antibodies are selecting a subset of FVIII states given that there was enrichment in states with unformed disulfides bound to antibody and corresponding depletion of those states in the unbound fraction (Figure 2B). In addition, incubation of FVIII with molar excess of the antibodies does not change the state of the FVIII disulfide bonds (not shown), which indicates that the antibodies are not directly reducing disulfides in FVIII.
The bivalency of antibodies is critical for their effector functions in many systems. For instance, bivalent binding to pathogens can markedly promote their neutralization, whereas antibody activation of cells often relies on bivalent cross-linking of plasma membrane antigens. The importance of the bivalency of the FVIII-neutralizing antibodies for selective binding to the subset of FVIII covalent states was tested using monovalent Fab fragments of the antibodies.
Antibody bivalency is not required for selective binding to a subset of FVIII disulfide-bonded states
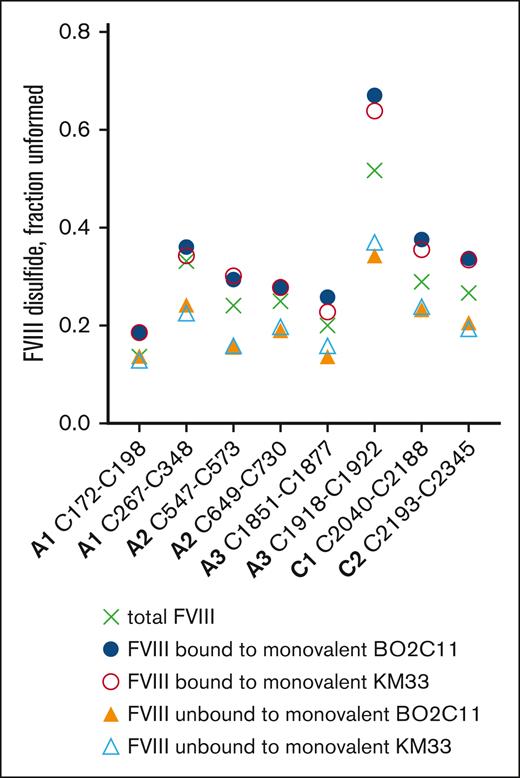
The monovalent versions of anti-FVIII antibodies BO2C11 and KM33 were coupled to magnetic beads, incubated with recombinant FVIII, and the incidence of unformed disulfide bonds in the bound vs free FVIII determined. The monovalent antibodies bound preferentially to FVIII covalent states where the same 3 disulfides are more unformed (C1918-C1922, C2040-C2188, and C2193-C2345; Figure 3). This result indicates that antibody bivalency is not required for the selective recognition of FVIII epitopes.
Antibody bivalency is not required for selective binding to a subset of FVIII disulfide-bonded states. The monovalent Fab Fc versions of antibodies, BO2C11 and KM33, were coupled to magnetic beads, incubated with recombinant FVIII and the bound and unbound FVIII fractions collected. Incidence of unformed disulfide bonds in the bound vs unbound FVIII (n = 1 experiment). The mean values for the starting (total) FVIII are shown as crosses.
Antibody bivalency is not required for selective binding to a subset of FVIII disulfide-bonded states. The monovalent Fab Fc versions of antibodies, BO2C11 and KM33, were coupled to magnetic beads, incubated with recombinant FVIII and the bound and unbound FVIII fractions collected. Incidence of unformed disulfide bonds in the bound vs unbound FVIII (n = 1 experiment). The mean values for the starting (total) FVIII are shown as crosses.
These findings suggest that the antigenicity of FVIII is influenced by the state of the disulfide bonds in the native molecule, in particular 3 of the 8 bonds. To probe the structural reasons for the selective binding of the antibodies, FVIII mutant proteins lacking the 3 relevant disulfide bonds were produced and analyzed for antibody binding.
Ablation of the A3 and C1 domain FVIII disulfide bonds influence binding of anti-FVIII drug antibodies
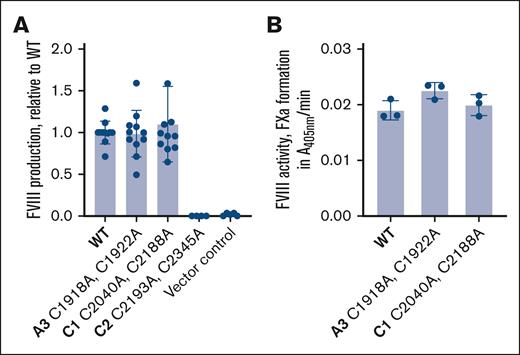
Wild-type (WT), C1918A,C1922A, C2040A,C2188A, or C2193A,C2345A disulfide mutant human B domain–deleted FVIII complementary DNA was transfected into Flp-In 293 cells and FVIII protein produced and secreted into serum-free conditioned medium was analyzed. WT, C1918A,C1922A, and C2040A,C2188A FVIII protein was produced at comparable levels by the human embryonic kidney cells, whereas the C2193A,C2345A disulfide mutant protein was not produced (Figure 4A). Ablation of the C2193-C2345 disulfide bond presumably results in a misfolded protein that is targeted for degradation. To determine the activity of FVIII proteins, they were added to FVIII-depleted plasma and potentiation of FXa formation measured. WT, C1918A,C1922A, and C2040A,C2188A FVIII protein had comparable cofactor activity (Figure 4B).
Production and activity of FVIII disulfide bond mutants. (A) WT, C1918A,C1922A, C2040A,C2188A, or C2193A,C2345A disulfide mutant human B domain–deleted FVIII protein secreted by Flp-InTM 293 cells. The data are from 4 to 11 independent experiments and bars and errors are mean ± SD. (B) Activity of WT, C1918A,C1922A, and C2040A,C2188A FVIII protein in FVIII-depleted plasma measured by the formation of FXa. The data are from 3 independent experiments and bars and errors are mean ± SD.
Production and activity of FVIII disulfide bond mutants. (A) WT, C1918A,C1922A, C2040A,C2188A, or C2193A,C2345A disulfide mutant human B domain–deleted FVIII protein secreted by Flp-InTM 293 cells. The data are from 4 to 11 independent experiments and bars and errors are mean ± SD. (B) Activity of WT, C1918A,C1922A, and C2040A,C2188A FVIII protein in FVIII-depleted plasma measured by the formation of FXa. The data are from 3 independent experiments and bars and errors are mean ± SD.
The binding of a panel of 4 anti-FVIII drug antibodies to WT, C1918A,C1922A, and C2040A,C2188A FVIII was measured using a sandwich enzyme-linked immunosorbent assay in which the antibodies were absorbed to the wells. The bound vs total FVIII binding curves were fit to a rectangular hyperbola to obtain apparent dissociation constant for the interaction. Ablation of the A3 domain C1918-C1922 disulfide bond increased affinity of all 4 anti-FVIII antibodies, whereas ablation of the C1 domain C2040-C2188 disulfide bond increased affinity for the BO2C11 and BOIIB2 antibodies and reduced affinity for the LE2E9 and KM41 antibodies (Figure 5). These results imply that the absence of the C1918A,C1922A and C2040A,C2188A disulfide bonds affects both the conformations of the domains in which the bonds reside and neighboring and more distant domains as well, which is in accordance with the selective binding of all the antibodies to an FVIII subset of molecules with more unformed C1918A,C1922A and C2040A,C2188A disulfide bonds (Figure 2B).
Ablation of FVIII C1 and A3 disulfide bonds changes affinity for patient anti-FVIII drug antibodies. (A) Ribbon representation of the AlphaFold structure of human VIII lacking the unstructured B domain. The domains to which a panel of 4 patient-derived anti-FVIII drug antibodies bind (BOIIB2, KM41, LE2E9, and BO2C11) are shown. The residues that encompass the epitopes for the antibodies are shown as dots (see Table 1 for residue numbers) and the disulfide bond cysteines as yellow spheres. The red spheres are the cysteines that have been replaced with alanine. (B) Apparent Kd for binding of 4 patient-derived antidrug antibodies (BOIIB2, KM41, LE2E9, and BO2C11) to WT, C1918A,C1922A, and C2040A,C2188A FVIII protein. The data are from 3 independent experiments and bars and errors are mean ± SD. An ordinary 1-way analysis of variance was used to compare groups (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). Kd, dissociation constant.
Ablation of FVIII C1 and A3 disulfide bonds changes affinity for patient anti-FVIII drug antibodies. (A) Ribbon representation of the AlphaFold structure of human VIII lacking the unstructured B domain. The domains to which a panel of 4 patient-derived anti-FVIII drug antibodies bind (BOIIB2, KM41, LE2E9, and BO2C11) are shown. The residues that encompass the epitopes for the antibodies are shown as dots (see Table 1 for residue numbers) and the disulfide bond cysteines as yellow spheres. The red spheres are the cysteines that have been replaced with alanine. (B) Apparent Kd for binding of 4 patient-derived antidrug antibodies (BOIIB2, KM41, LE2E9, and BO2C11) to WT, C1918A,C1922A, and C2040A,C2188A FVIII protein. The data are from 3 independent experiments and bars and errors are mean ± SD. An ordinary 1-way analysis of variance was used to compare groups (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001). Kd, dissociation constant.
The findings mentioned earlier indicate that FVIII states with unformed C1918-C1922 and C2040-C2188 disulfide bonds have a conformational dynamism that encompasses large areas of the protein, in some cases distal to the site of the disulfide bond itself. To probe this dynamism, molecular dynamics simulations of FVIII with unformed C1918-C1922, C2040-C2188, or C2193-C2345 disulfide bonds were performed.
Structural reasons for selective binding of anti-FVIII drug antibodies to a selective subset of FVIII molecules
Molecular dynamics simulations were performed on an AlphaFold-generated B domain–deleted construct of FVIII, which incorporates several surface loops and acidic regions that are not observed in existing FVIII X-ray crystal structures. Each unformed disulfide bond state was subjected to independent 250-nanosecond simulations in duplicate and subsequently analyzed for structural and dynamic changes. In all cases of unformed disulfide bond FVIII constructs in the simulation, each domain (A1-A3, C1-C2) did not have backbone root mean square deviations that changed to a statistically significant extent (supplemental Table 3). In comparison with a WT simulation, the root mean square deviation for each unformed disulfide structure deviated between 0.79-fold and 1.7-fold. In addition, analysis of interdomain contacts for each simulation indicate that the number of contacts between domains also did not change significantly (between 0.73-fold and 1.4-fold) except for a minor increase in contacts between the C1 and C2 domain in the C2193-C2345 unformed disulfide bond simulation (a 2.2-fold increase; supplemental Table 4).
Although the overall structure of FVIII and its interdomain contacts remain largely conserved and unchanged at the end of all simulations, backbone fluctuations deviate significantly throughout the FVIII structure for simulations with various unformed disulfide bonds, as measured by backbone root mean square fluctuations (RMSFs). Compared with WT, the C1918-C1922 simulation demonstrates large fluctuations in both the A3 and C1 domains, with a minor number of residues having increased fluctuations in the A1 and A2 domains (Figure 6A, supplemental Figure 4). For the C2040-C2188 simulation, we observed a minor number of residues with increases in RMSF in the C1 domain and 2 loops within the A2 domain (400-410, 598-599), which are proximal to the BOIIB2 putative epitope (Figure 6B; supplemental Figure 5). Finally, the simulation for the unformed C2193-C2345 disulfide bond yielded large regions in the A3 and C1 domains with increases in RMSF, whereas a surprisingly low number of RMSF increases were observed within the C2 domain, where the disulfide bond resides (Figure 6C; supplemental Figure 6).
Ablation of disulfide bonds in FVIII causes changes in RMSF of backbone residues. Fold change in backbone RMSF for unformed C1918-C1922 (A), unformed C2040-C2188 (B), and unformed C2193-C2345 (C) disulfide bonds in FVIII (AlphaFold identifier: AF-P00451-F1).
Ablation of disulfide bonds in FVIII causes changes in RMSF of backbone residues. Fold change in backbone RMSF for unformed C1918-C1922 (A), unformed C2040-C2188 (B), and unformed C2193-C2345 (C) disulfide bonds in FVIII (AlphaFold identifier: AF-P00451-F1).
To contrast simulations between FVIII and FVIII antibody inhibitor structures, we performed similar molecular dynamics simulations with BO2C11 in complex with FVIII and compared it with simulations for the C1918-C1922 unformed disulfide bond. When we compared simulations for the unformed C1918-C1922 with WT when both are bound to BO2C11, no substantive changes were observed, except for increases in RMSF at residues 332, 1695, and 2003 (supplemental Figure 7). However, when we compared the BO2C11-bound C1918-C1922 simulation with apo FVIII WT, we observed increases in RMSF at the C1-C2 connection, toward the core of the A3 domain, and in various loops in the A1 domain leading into A2 (supplemental Figure 8). Finally, as an additional control simulation, we compared the BO2C11-bound C1918-C1922 unformed disulfide bond simulation with the same construct without BO2C11 (supplemental Figure 9) and only observed decreases in RMSF at the BO2C11 epitope within the C2 domain. The comparison of RMSF of FVIII WT with FVIII bound to BO2C11 is shown in supplemental Figure 10.
Discussion
All 8 FVIII disulfide bonds in both native plasma and recombinant protein are unformed to some extent, and analysis of the probabilities of disulfide bond formation indicates that their formation is conditional. The conditional nature of disulfide formation in FVIII is not known, although the experimental probabilities of formation allow us to speculate. For example, given that the A1 domain C172-C198 bond is almost always formed (fraction formed is 0.93), it is likely that the formation of ≥1 other disulfides is dependent on the C172-C198 bond being formed. In support of this hypothesis, ablation of the C172-C198 bond in FVIII by replacing both Cys with Ala or Val resulted in no secretion of FVIII into the medium of transfected cells (data not shown). In addition, mutation of either C172 or C198 in humans leads to severe hemophilia A (European Association for Haemophilia and Allied Disorders database). Ablation of the A3 domain C1918-C1922 or C2 domain C2040-C2188 disulfide bonds, in contrast, did not affect the production of the recombinant protein.
Disulfide bonds are motifs that constrain the polypeptide backbone and reduce the conformational variations of the tertiary and quaternary protein structure. Therefore, the absence of ≥1of these constraints in FVIII was expected to result in a population of FVIII molecules with a wide variety of conformational forms. It has been repeatedly observed that several of the anti-FVIII antibody inhibitor epitopes are largely conformationally defined,31,37-39 as opposed to being defined by the linear display of peptide epitopes. This prompted us to assess the binding of a panel of patient-derived anti-FVIII inhibitor antibodies. Indeed, the antibodies bound preferentially to FVIII states in which 2 or 3 of the 8 disulfides are significantly more unformed: C1918-C1922 in the A3 domain, C2040-C2188 in the C1 domain, and C2193-C2345 in the C2 domain. In addition, this selective binding was not dependent on antibody bivalency of the immunoglobulin G4 (IgG4) molecules. It is of interest that Fab exchange in IgG4 can result in bispecific antibodies that are functionally monovalent, although bispecific anti-FVIII IgG4 molecules have not been described in patients with hemophilia A with inhibitors.
An unexpected finding is that all 5 antibodies, which bind to 4 different FVIII domains, demonstrated the same general preference for FVIII disulfide-bonded states. For example, BO2C11 antibody that binds to the C2 domain preferentially bound the same subset of FVIII molecules as BOIIB2 antibody that binds to the A2 domain on the opposite face of FVIII. This result was supported by disulfide mutagenesis studies. Genetic ablation of the FVIII A3 domain C1918-C1922 disulfide bond increased the affinity of all anti-FVIII inhibitor antibodies tested, whereas ablation of the C1 domain C2040-C2188 disulfide bond increased the affinity for the BO2C11 and BOIIB2 antibodies and reduced the affinity for the LE2E9 and KM41 antibodies. These observations imply that the subset of FVIII states with more unformed C1918-C1922, C2040-C2188, and C2193-C2345 disulfide bonds have a conformational dynamic that involves areas of the protein distant from the disulfides. This was supported by molecular dynamics studies.
Backbone fluctuations deviated significantly throughout the FVIII structure when the C1918-C1922, C2040-C2188, or C2193-C2345 disulfide bonds were unformed. This long-range dynamic behavior is consistent with the nature of the inhibitor antibody binding we have observed. Previous structural data have shown that FVIII has an innate dynamic behavior that can elicit alternative conformations for the C2 domain,26 which are also present in multiple structures of FVIII bound to antibody inhibitors.28,31 Moreover, previous in vitro data also suggest that antibodies with neighboring, separate epitopes display cooperative binding and inhibition activities.38,40 These results may also suggest that an increase in dynamic behavior for the conserved folded regions of FVIII (residing in the core A and C domains) may have a large-scale impact on the accessibility of epitopes that may be otherwise shielded by the large, ill-defined, and heavily glycosylated B domain. Although the structure of the B domain is currently an intractable pursuit, AlphaFold models illustrate that it likely lacks any conformational definition with loops that surround the FVIII core structure. Taken together, FVIII antigenicity correlates with dynamic behavior.
Our results imply that the antigenicity of FVIII is influenced by the state of its disulfide bonds and suggest that it may be possible to engineer the disulfide bonds in FVIII to diminish neutralization by antidrug antibodies. For example, the diselenide bond is more stable than the disulfide bond,41 so replacement of 1 or both disulfide cysteines with selenocysteines to produce Cys-Sec or Sec-Sec bonds is predicted to change the distribution of FVIII structural isoforms by promoting the formation of the bonds. For example, our results suggest that an FVIII molecule with intact Sec1918-Sec1922, Sec2040-Sec2188, and Sec2193-Sec2345 diselenide bonds will be less antigenic.
Several proteins have now been shown to be constitutively produced as multiple partially disulfide-bonded states,15-18 and the indications are that this is the norm rather than the exception. The finding that antibodies can selectively bind subsets of antigen disulfide-bonded states has widespread implications for the development of autoantibodies in general and antibody therapies. For instance, the distribution of disulfide-bonded states of certain proteins in some individuals may be altered owing to genetic or environmental factors and predispose to the development of autoantibodies. For example, patients with acquired hemophilia A may have an altered distribution of FVIII disulfide-bonded states that changes the immunogenicity of the molecule. There is experimental evidence for this scenario for a self-antigen. Disruption of disulfide bonding in the self-antigen influenced the selective display of class II-restricted dominant and subdominant T-cell epitopes.42 It will be of interest to determine whether the different disulfide-bonded states of FVIII influence antigen processing and epitope selection. In contrast, an antibody drug may bind better to some disulfide-bonded states of its antigen than others, and thus, complete inhibition of function may be difficult to achieve. In addition, as for autoantibodies, variable antibody drug efficacy is anticipated if the balance of disulfide-bonded states of the target antigen varies in the population.
Acknowledgments
Anton 2 computer time was provided by the Pittsburgh Supercomputing Center (PSC) through grant R01GM116961 from the National Institutes of Health (NIH), National Institute of General Medical Sciences. The Anton 2 machine at PSC was generously made available by D.E. Shaw Research.
This study was funded by the National Health and Medical Research Council of Australia (2026631) and a Senior Researcher Grant from the New South Wales Cardiovascular Research Capacity Program (P.J.H.), INSERM, Centre National de la Recherche Scientifique, Sorbonne Université, Université Paris Cité and Agence National de la Recherche (ANR-18-CE17-0010-02, number 18181LL, Exfiltrins [S.L.-D.]), and NIH/National Heart, Lung, and Blood Institute (award numbers R15HL135658 and U54HL141981 [P.C.S. Jr]).
Authorship
Contribution: D.B., A.E.P., and N.G.A. performed research and analyzed data; C.M., C.H.C., A.M., and S.L.-D. contributed vital new reagents or analytical tools; P.C.S. Jr designed research and wrote the manuscript; and P.J.H. conceived the study, designed research, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philip J. Hogg, Centenary Institute, Locked Bag No. 6, Newtown, Sydney 2042 NSW, Australia; email: p.hogg@centenary.org.au.
References
Author notes
D.B. and A.E.P. are joint first authors.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (available at https://doi.org/10.1093/nar/gkab1038) partner repository (data set identifier PXD060297).
All other relevant data are available from authors, Philip Hogg (p.hogg@centenary.org.au) and P. Clint Spiegel (spiegep@wwu.edu).
The full-text version of this article contains a data supplement.