In this issue of Blood Advances, Magenau et al1 evaluated the safety and preliminary efficacy of the programmed cell death protein 1 (PD-1) checkpoint inhibitor pembrolizumab for the treatment of patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) with disease relapse after an allogeneic hematopoietic stem cell transplant (HCT) in a phase 1b trial. These patients represent a population with a high unmet need for novel therapies and for whom standard treatment with chemotherapy, followed by donor lymphocyte infusion (DLI) or a second HCT, has limited efficacy and a risk for significant toxicity.2 Targeting the PD-1/programmed death ligand 1 (PD-L1) immune checkpoint pathway to treat disease relapse after HCT has led to some responses but also has been associated with a significant incidence of graft-versus-host disease (GVHD).3-5 Its inhibition in the context of early relapse after HCT, including in patients who are still on immune suppression at the time of relapse, has not been formally studied.
The primary end points of the trial included both a safety evaluation and an evaluation of the objective response rate. Among 16 patients enrolled in the trial, the median time to relapse was 5.5 months. Pembrolizumab was administered at a flat dose of 200 mg IV every 21 days for up to 4 cycles, and responding patients were eligible for maintenance therapy. The median time to the initial dose of pembrolizumab was 18.5 days, reflecting the time required to taper immune suppression. The median number of administered pembrolizumab cycles was 1 with malignancy progression and immunologic toxicity being the main reasons for treatment discontinuation. Three of the 16 patients attained complete response (CR) on day +35, and 3 of 16 patients were in CR on day +77 following pembrolizumab monotherapy. Mixed CD3+ chimerism before therapy was associated with the attainment of an objective response. Among patients who attained CR, the median response time was 20 months with eventual relapse manifesting in the extramedullary setting. A total of 11 of the 16 patients developed severe adverse events (SAEs) with 2 patients experiencing immune-related SAEs and 9 experiencing a flare of GVHD. Among the latter participants, 6 of 9 experienced grade 3 to 4 acute GVHD and 5 of 9 were steroid-refractory. The 1-year overall survival and event-free survival were 37.5% (6/16) and 31.3% (5/16), respectively, and the 1-year GVHD-free relapse-free survival (GRFS) was 18.75% (3/16).
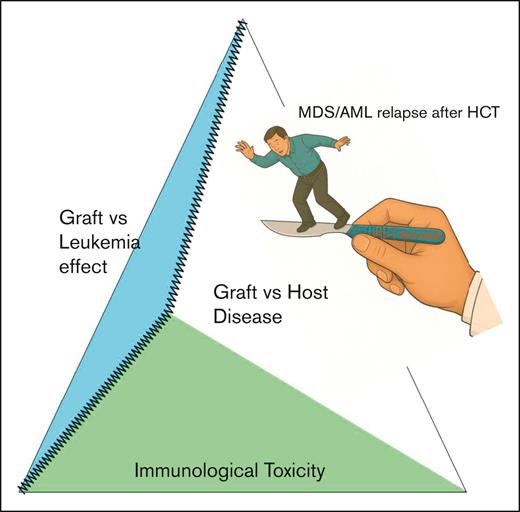
The results of the study highlight the challenge of attempting to dissect the graft-versus-leukemia effect from immunologic toxicity in patients with HCT. With respect to the former, responses to checkpoint inhibition alone remain modest, and although some patients did achieve sustained remission, eventual relapse did occur. The authors evaluated some potential biomarkers of treatment response, including the presence of mixed CD3 chimerism before therapy, but the findings from the small number of patients require confirmation in larger studies. What is clear from the data is that the potential benefit of PD-1 checkpoint inhibition for the treatment of MDS/AML relapse after HCT must be balanced against the risk of significant immunologic toxicity. In clinical practice, immune suppression taper usually precedes the addition of immunotherapy, and patients who develop a GVHD flare following the taper do not proceed with DLI until the GVHD is definitively controlled.2 A GVHD flare following taper of immune suppression was not used as a clinical marker predictive of immunologic toxicity in this study and may explain the high incidence of grade 3 to 4 GVHD.
Interestingly, the presence of PD-L1 expression on leukemia blasts did not correlate with the response to pembrolizumab, unlike in the lymphoma context,4 and suggests that this biomarker may have limited interpretability in myeloid blasts. This observation, coupled with the modest response rate to pembrolizumab monotherapy, suggests that myeloid blasts implement alternative mechanisms of immune evasion beyond the PD-1/PD-L1 pathway. Combination therapies with checkpoint inhibition may potentially overcome these other pathways, but these must address the double-edged nature of treatment intolerance of this therapy in patients who have undergone HCT (see figure).
The outcome data of this study are consistent with other trials that aimed to augment T-cell activity after HCT using checkpoint inhibition. In an earlier phase 1 trial that evaluated checkpoint inhibition with ipilimumab, the responses were primarily observed in extramedullary disease sites with fewer responses recorded in the bone marrow.6 A multicenter phase 1 trial of decitabine + ipilimumab in patients with relapsed/refractory MDS/AML both before and after HCT showed a 20% objective response in the latter group with 28% developing GVHD and 16% developing other immunologic AEs.7 A phase 1 trial of ipilimumab combined with regulatory T-cell–depleted DLI demonstrated an early CR of 48%, but the incidence of immunologic toxicity was high.8 Another prospective multicenter phase 1 trial of nivolumab for the treatment of relapsed hematologic malignancy after HCT yielded an objective response of 32%, but the response was proportionally lower in myeloid malignancies (21%) than in lymphoma (44%), and there was a 39% incidence of acute/chronic GVHD that was fatal in some cases.9
The limited efficacy of augmenting the T-cell–mediated effects following HCT may reflect the biology of relapse in this context, namely that relapse represents the ability of the disease to overcome sustained immunologic pressure. Increasing the immune repertoire with standard of care DLI in patients with AML relapse after HCT who first attained CR with other therapy, led to a 2-year leukemia-free survival of 38%,10 suggesting perhaps that myeloid disease that relapses after HCT uses T-cell–evading mechanisms that can be overcome in only about a third of patients. The late relapses that occur despite initial responses to immunotherapy with pembrolizumab in this phase 1 trial suggest a persistent reservoir of disease that evaded the enhanced T-cell effects. For the patients whose myeloid disease persistently evades T cells, alternative treatment modalities may be needed, including combination therapies, such as with antibodies, cytokines, or other cell therapies like natural killer cells, among others.
In summary, Magenau et al performed a phase 1b clinical trial that evaluated the safety and feasibility of the use of pembrolizumab for the treatment of MDS/AML relapse after HCT. Although a subset of patients did show sustained long-term responses, all patients eventually had either disease relapse and/or severe immunologic toxicity, including grade 3 to 4 GVHD. Checkpoint inhibition following HCT must be used cautiously, and future studies should aim to optimize the efficacy while limiting toxicity.
Conflict-of-interest disclosure: The authors declare no competing financial interests.