Key Points
Clec12a is a functionally important downstream gene activated by the NUP98::NSD1 oncogene.
Clec12a is required for leukemogenesis driven by the NUP98::NSD1 and NRASG12D oncogenes.
Visual Abstract
NUP98::NSD1 is one of the most recurring nucleoporin 98 (NUP98) fusions in acute myeloid leukemia (AML). NUP98::NSD1 positive AML is often associated with adverse outcomes and poor response to conventional treatments. However, limited studies have been done to identify new potential targets to develop better treatment approaches. The C-type lectin domain family 12 member A (CLEC12A) is a cell surface receptor that is differentially expressed in leukemic stem cells compared with healthy hematopoietic stem cells. We found a strong CLEC12A overexpression in both NUP98::NSD1 patients and murine AML cells transformed with the NUP98::NSD1 fusion oncogene. To understand the role of Clec12a in NUP98::NSD1 AML, we depleted Clec12a expression in NUP98::NSD1+NRASG12D-immortalized cells using the CRISPR/Cas9 approach. NUP98::NSD1+NRASG12D/Clec12a knockout cells had higher apoptosis levels and lower colony numbers in vitro compared with NUP98::NSD1+NRASG12D/Clec12a wild-type cells. Importantly, the deletion of Clec12a significantly reduced leukemic engraftment and prolonged survival of the NUP98::NSD1+NRASG12D murine model. Our data suggest to further explore CLEC12A as a potential target for the treatment of NUP98::NSD1 AML.
Introduction
Acute myeloid leukemia (AML) is a malignancy of the white blood cells (WBCs) that results from mutations and chromosomal aberrations in hematopoietic stem and progenitor cells.1,2 The NUP98::NSD1 fusion is more frequently detected in pediatric AML3 and characterized by a normal karyotype and a high WBC count.3,4 This fusion is produced as a result of a translocation between the nucleoporin 98 (NUP98) gene on chromosome 11 and the nuclear receptor-binding SET domain protein 1 (NSD1) gene on chromosome 5.5 In AML, the NUP98::NSD1 fusion is usually associated with mutations in FLT3, NRAS, MYC, and WT1.3,6,7,NUP98::NSD1 leukemia is also associated with upregulation of expression of HOX genes.3,8 Patients with NUP98::NSD1+ AML have an adverse clinical outcome and were found to be less responsive to treatment,3,9,10 highlighting the need to identify new druggable targets. Although NUP98::NSD1 is recognized as a diagnostic entity by the International Consensus Classification11 and World Health Organization12 classifications from 2022 with poor prognosis, patients are classified in the intermediate-risk group according to the European LeukemiaNet 202213 classification.
In our previous study, we found that Clec12a is upregulated in NUP98::NSD1-immortalized mouse bone marrow (BM) cells, and we speculated that Clec12a upregulation might contribute to NUP98::NSD1-mediated leukemogenesis.14
The cell surface receptor C-type lectin domain family 12, member A (CLEC12A) is a member of C-type lectin receptors, which have been primarily associated with recognizing carbohydrate ligands.15 The immunoreceptor tyrosine-based inhibition motif in the cytoplasmic tail of CLEC12A recruits phosphatases such as SHP1 and SHP2 upon receptor activation and modulates cellular functions.16 Tissue expression analysis revealed that the CLEC12A transcript is predominantly expressed in peripheral blood (PB) mononuclear cells and spleen.17 Furthermore, messenger RNA and protein level expression analyses of different cell types showed that CLEC12A was differentially expressed in myeloid cells, such as monocytes, granulocytes, and dendritic cells, compared with other mature hematopoietic cells.17-19
Expression of CLEC12A in the hematopoietic stem and progenitor cell compartment of healthy BM has been studied extensively. CLEC12A has high expression in the granulocyte-macrophage progenitor compartment, medium expression in common myeloid progenitor, and low expression in megakaryocyte-erythroid progenitor compartment of healthy BM donors.20 In addition, the CLEC12A+ subset had nonerythroid colony growth, indicating differential expression of CLEC12A in myeloid progenitors.20 However, negligible expression of CLEC12A was demonstrated in the healthy hematopoietic stem cell (HSC)/progenitor compartment (CD34+CD38–) of normal BM.21,22
Unlike HSCs, leukemia stem cells (LSCs; CD34+CD38– compartment) express CLEC12A in most patients with AML, suggesting a specific requirement for this protein in LSCs.21 It was previously demonstrated that other stem cell markers such as CD33 and CD123 are not specific to LSCs, which may increase the importance of CLEC12A as a stem cell marker.23 Owing to its consistent expression at both diagnosis and relapse, it has been suggested as a measurable residual disease marker.24-27 Moreover, CD34+ cells of patients with chronic myeloid leukemia and myelodysplastic syndrome demonstrated CLEC12A expression.17,28,29 Additionally, AML cells expressing CLEC12A were also linked to chemoresistance.30
Although CLEC12A expression across different hematopoietic compartments in both healthy BM and AML has been well studied, its role in AML progression remains unclear. We hypothesized that Clec12a is a downstream target of NUP98::NSD1 that mediates the oncogenic signal of this fusion gene and evaluated its function in a murine model of NUP98::NSD1 and NRASG12D.
Materials and methods
Cloning
The NUP98::NSD1 plasmid was received from the University of Veterinary Medicine Vienna, Austria, as described in Schmoellerl et al,31 and the NRASG12D plasmid was purchased from Addgene. The NUP98::NSD1 fusion gene and NRASG12D were further cloned into retroviral expression vector MSCV-IRES-GFP and MSCV-IRES-BFP, respectively. Single-guide RNAs (sgRNAs) against Clec12a and a control sgRNA against lacZ were cloned into the Cas9-expressing lentiviral vector L40C-CRISPR.EFS.dTomato (catalog no. 89392; Addgene). CRISPR-Cas9 sgRNAs were designed using the CCTop selection tool. The list of sgRNAs is provided in supplemental Table 1. The cloned gene and sgRNA sequences were verified by Sanger sequencing.
Mice and viral infections of primary mouse BM cells
Female C57BL/6J mice (6-8 weeks old) were purchased from Charles River, Sulzfeld, Germany, and kept in pathogen-free conditions at the central animal laboratory of Hannover Medical School. All animal experiments were executed with permission of the Lower Saxony State Office for Consumer Protection, Oldenburg, Germany. BM cells from mice were harvested after 4 days of 5-fluorouracil treatment at a dose of 150 mg/kg. Cells were cultured in Dulbecco’s modified Eagle medium (STEMCELL Technologies, Cologne, Germany) supplemented with 15% fetal bovine serum (Sigma-Aldrich, Munich, Germany), 10 ng/mL human interleukin-6 (IL-6), 6 ng/mL murine IL-3, and 20 ng/mL murine stem cell factor (all from PeproTech, Hamburg, Germany). Viral supernatant from transfection of retrovirus-producing Phoenix packaging cells with retroviral plasmids was used to transduce prestimulated BM cells. Lentiviral particles were produced by transient transfection of 293 LX cells using Lipofectamine 3000 (Thermo Fisher Scientific GmbH, Bremen, Germany). The CRISPR vector, pMD2.G, and pPAX2 were cotransfected, and the viral supernatant was used to transfect immortalized murine cell lines. Transduced cells were sorted based on dTomato reporter expression.
Proliferation assay
Cells were sorted 3 days before initiating in vitro proliferation assays, and experiments were executed using 3 independently derived Clec12a knockout cell lines and 2 lacZ control cell lines. Sorted cells were cultured in medium conditions as described previously. For in vitro growth and proliferation assays, sorted cells were counted using a Beckman Coulter CytoFLEX S analyzer (Beckman Coulter, Fullerton, CA), and cells were seeded in triplicate from each independent cell line at a concentration of 100 000 cells per mL of media. Cells were harvested and counted every 48 to 72 hours using a Beckman Coulter CytoFLEX S analyzer and replated in fresh medium supplemented with cytokines.
Apoptosis assay
To quantify the percentage of apoptosis induction in Clec12a knockout cells, cells were stained with Annexin V–allophycocyanin (APC) according to the manufacturer’s protocol (BD Pharmingen; catalog no. 550474). Cells were acquired using a BD LSRII flow cytometer (BD Biosciences, Heidelberg, Germany), and the percentage of Annexin V–positive cells was quantified.
Cell cycle analysis
Cells were labeled in vitro using 10 μM of bromodeoxyuridine for 12 hours. Cells were stained according to the manufacturer’s protocol (BD Pharmingen catalog no. 557892). Cell cycle analysis was performed using a BD LSRII flow cytometer (BD Biosciences, Heidelberg, Germany).
Clonogenic progenitor assay
Colony-forming cell assays from transduced mouse BM cells were performed using methylcellulose (MethoCult M3234; STEMCELL Technologies, Inc, Vancouver, BC, Canada) supplemented with 10 ng/mL murine IL-3, 10 ng/mL human IL-6, 50 ng/mL stem cell factor, and 3 U/mL erythropoietin (PeproTech, Hamburg, Germany). One thousand Clec12a wild-type or Clec12a knockout NUP98::NSD1+NRASG12D-immortalized mouse BM cells were plated in duplicate with 3 independent clones for each round of plating. Colonies were observed and counted under the Olympus CKX31 (Olympus, Tokyo, Japan) microscope after 7 days of plating.
Morphologic analysis
Cytospins were prepared from cells and stained with Wright-Giemsa stain. Morphology was analyzed with a Zeiss Axioscope A1 using the Axiocamera 5s microscope and Zeiss immersol medium, and captured images with 1000× magnification were processed with the Zen 2.6 lite (blue) software (Zeiss, Jena, Germany).
Antibodies for flow cytometry
For immunophenotyping of murine cells, used antibodies were Gr-1-APC Cy7 (clone-RB6-8C5) from BD Biosciences and CD11b-APC (M 1/70) from eBioscience. Clec12a expression was quantified using the Clec12A-APC (REA594; Miltenyi Biotec) antibody. Flow cytometry data were analyzed using the FlowJo software (version 10.0.7, TreeStar, Ashland, OR).
RNA extraction, complementary DNA synthesis, and quantitative reverse transcriptase PCR
Total RNA was isolated using the RNeasy kit (Qiagen), according to the manufacturer’s protocol. RNA was used to prepare complementary DNA using the reverse transcription kit (Thermo Fisher Scientific), according to the manufacturer’s instructions.
Quantitative reverse transcriptase polymerase chain reaction (PCR) was performed using SYBR green (Qiagen) on a StepOne Plus cycler (Thermo Fisher Scientific) as previously described.32 Relative expression of the target gene to the housekeeping gene Abl1 was quantified using the 2–ΔΔCT method. The primer sequences are listed in supplemental Table 2.
Gene expression profiling and analysis
Single-cell sorting of NUP98::NSD1+NRASG12D/Clec12a wild-type and NUP98::NSD1+NRASG12D/Clec12a knockout cells was done in triplicate. RNA was extracted as mentioned previously and sequenced at the Helmholtz Center for Infection Research (Braunschweig) using paired end-read 50-bp chemistry on a NovaSeq 6000 instrument (Illumina, Berlin, Germany). Sequencing reads were aligned to the mouse genome GRCm38 using GENCODE version m2233 transcriptome annotations. The comparison was executed with the main alignment program, STAR34 and Bowtie2.35 Mapping to the transcriptome and gene expression analysis was performed using TopHat and Cufflinks36 with the use of GENCODE annotation of genes and alternative transcript variants. Fragments per kilobase per million and transcripts per million values were calculated to quantify the expression of genes and transcripts with the help of program High-throughput sequencing (HTSeq)37 and DESeq2analysis.38 The Broad Institute Gene Set Enrichment Analysis (GSEA) software (http://www.broad.mit.edu/gsea/msigdb/) and the David online tool (https://davidbioinformatics.nih.gov/) were used for analysis of differentially regulated pathways. The expression of Clec12a in the NUP98::NSD1 doxycycline-regulated system was assessed using the GSE134784 data set and as described by Schmoellerl et al.31 Raw single-cell RNA sequencing data were obtained from Gene Expression Omnibus entry GSE116256 (van Galen et al).39 Transcript counts of each cell were normalized to 10 000, and CLEC12A counts are presented in the ”Results.”
Statistical analysis
Pairwise comparisons were carried out by Student t test for continuous variables. A P value of <.05 was considered statistically significant. The log-rank test was used to compare survival differences between the 2 groups, and Kaplan-Meier curves were used to present it. All statistical analyses and preparation of graphs were executed with GraphPad Prism version 7 software (GraphPad Software, La Jolla, CA), and graphs were prepared using Adobe Illustrator CS5.1 (Adobe Systems GmbH, Munich, Germany).
Results
CLEC12A expression is regulated by NUP98::NSD1
To study the expression of CLEC12A in genetic subgroups of AML, we used the gene expression cohorts of Leucegene and Balgobind et al (GSE17855).3,40 The Leucegene cohort showed that patients with AML had higher expression levels of CLEC12A compared with healthy controls (Figure 1A). Moreover, it showed higher expression of CLEC12A in patients with NUP98::NSD1+ AML compared with other subtypes of AML. Apart from NUP98::NSD1, high expression of CLEC12A was also observed in t(8,21) AML in the Leucegene cohort. In both cohorts, the relative expression of CLEC12A was higher in patients with NUP98::NSD1+ AML compared with cytogenetically normal patients with AML (Figure 1A-B). In addition, single-cell RNA sequencing data from van Galen et al reveal that CLEC12A is expressed in normal HSCs at low levels compared with malignant HSCs, across different AML subtypes (supplemental Figure 1; supplemental Table 4).39
![Clec12a expression is regulated by NUP98::NSD1. (A) CLEC12A mRNA expression among different cytogenetic AML subgroups, derived from the Leucegene cohort (NUP98::NSD1, n = 7; CN-AML, n = 168; N/K-RASmut, n = 87; N/K-RASwt, n = 350; complex KT, n = 139; CBFB::MYH11, n = 29; CEBPAmut, n = 16; MLL:MLLT3, n = 42; PML::RARA, n = 15; RUNX1::RUNX1T1, n = 21; normal CD34+, n = 17). Values are in log (FPKM). (B) CLEC12A mRNA expression among different AML KTs and mutations, derived from the Balgobind et al40 cohort (GSE17855; NUP98::NSD1, n = 11; CN-AML, n = 39; MLL::MLT3, n = 47; N/K-RASmut, n = 41; N/K-RASwt, n = 196; CEBPAmut, n = 16; cKITmut, n = 18; FLT3-ITD, n = 48; CBFB::MYH11, n = 27; PML::RARA, n = 19; RUX1::RUNX1T1, n = 28; other, n = 45; unknown, n = 25; NPM1mut, n = 17). Values are in normalized microarray intensity (RMA). (C) Reverse transcriptase PCR revealing Clec12a mRNA expression in murine BM cells transduced with either NRASG12D, NUP98::NSD1, or NUP98::NSD1+NRASG12D (n = 3; mean ± standard error of the mean [SEM]). (D) Flow cytometric representation of Clec12a protein expression in murine hematopoietic BM cells transduced with NUP98::NSD1+NRASG12D. Cells were gated on GFP+BFP+. (E) Normalized Clec12a expression for NUP98 CTL and NUP98::NSD1 murine-expressing cells. Clec12a is highly expressed in NUP98::NSD1-expressing cells (off Dox) compared with NUP98 CTL cells and is strongly downregulated if NUP98-NSD1 expression is shut off (day 3_on_Dox and day 5_on_Dox). Data are presented as boxplots in which the center line represents the median, the boundaries of the box indicate the first (25th percentile) and third (75th percentile) quartiles, and the whiskers extend to 1.5× interquartile range from the hinges. Data points beyond this range are revealed as individual dots and represent outliers. (F) Publicly available NUP98::NSD1 chromatin immunoprecipitation-sequencing data reveal binding of NUP98::NSD1 to regions with proximal or distal enhancer-like signatures according to the ENCODE candidate cis-Regulatory Elements atlas (GSE112928). CTL, control; Dox, doxycycline; FPKM, fragments per kilobase per million; H3K4me3, histone 3, lysine 4 trimethylation; KT, karyotype; mRNA, messenger RNA; RMA, robust multi-array analysis.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/15/10.1182_bloodadvances.2024015739/2/m_blooda_adv-2024-015739-gr1f.jpeg?Expires=1761665144&Signature=AePhp3By1I0YOR6UH44dsJWRl81PTH1Fj0cgsFUQ8mJB8zo32A6rCyivwWNNdCujauZ02i-LfhH00IFJ0sLaRUxE6FZIF6MpwUIcYWR5Y-eaqCd5JkixUjzVEy3NIZsCi4MJdgagyEBTtknk7hfWDVLSzsoyJIQhL~XXbq-MQbAdwv2IBHVWSauBOQOWmnV4EIqu1V6yfj4l6k2YVm6UXva7Nho82GZYCO5kjIKrzXxUpxnKH7xFgMCghXXiudXFYuQUmbscqNeDv68WuefDWY3Jt5lFgnZ87LAGlV-DYOYukmFdHB3nDzKKDLjLsYjz90YNjI2eG22HN4XHz7dZUg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Clec12a expression is regulated by NUP98::NSD1. (A) CLEC12A mRNA expression among different cytogenetic AML subgroups, derived from the Leucegene cohort (NUP98::NSD1, n = 7; CN-AML, n = 168; N/K-RASmut, n = 87; N/K-RASwt, n = 350; complex KT, n = 139; CBFB::MYH11, n = 29; CEBPAmut, n = 16; MLL:MLLT3, n = 42; PML::RARA, n = 15; RUNX1::RUNX1T1, n = 21; normal CD34+, n = 17). Values are in log (FPKM). (B) CLEC12A mRNA expression among different AML KTs and mutations, derived from the Balgobind et al40 cohort (GSE17855; NUP98::NSD1, n = 11; CN-AML, n = 39; MLL::MLT3, n = 47; N/K-RASmut, n = 41; N/K-RASwt, n = 196; CEBPAmut, n = 16; cKITmut, n = 18; FLT3-ITD, n = 48; CBFB::MYH11, n = 27; PML::RARA, n = 19; RUX1::RUNX1T1, n = 28; other, n = 45; unknown, n = 25; NPM1mut, n = 17). Values are in normalized microarray intensity (RMA). (C) Reverse transcriptase PCR revealing Clec12a mRNA expression in murine BM cells transduced with either NRASG12D, NUP98::NSD1, or NUP98::NSD1+NRASG12D (n = 3; mean ± standard error of the mean [SEM]). (D) Flow cytometric representation of Clec12a protein expression in murine hematopoietic BM cells transduced with NUP98::NSD1+NRASG12D. Cells were gated on GFP+BFP+. (E) Normalized Clec12a expression for NUP98 CTL and NUP98::NSD1 murine-expressing cells. Clec12a is highly expressed in NUP98::NSD1-expressing cells (off Dox) compared with NUP98 CTL cells and is strongly downregulated if NUP98-NSD1 expression is shut off (day 3_on_Dox and day 5_on_Dox). Data are presented as boxplots in which the center line represents the median, the boundaries of the box indicate the first (25th percentile) and third (75th percentile) quartiles, and the whiskers extend to 1.5× interquartile range from the hinges. Data points beyond this range are revealed as individual dots and represent outliers. (F) Publicly available NUP98::NSD1 chromatin immunoprecipitation-sequencing data reveal binding of NUP98::NSD1 to regions with proximal or distal enhancer-like signatures according to the ENCODE candidate cis-Regulatory Elements atlas (GSE112928). CTL, control; Dox, doxycycline; FPKM, fragments per kilobase per million; H3K4me3, histone 3, lysine 4 trimethylation; KT, karyotype; mRNA, messenger RNA; RMA, robust multi-array analysis.
![Clec12a expression is regulated by NUP98::NSD1. (A) CLEC12A mRNA expression among different cytogenetic AML subgroups, derived from the Leucegene cohort (NUP98::NSD1, n = 7; CN-AML, n = 168; N/K-RASmut, n = 87; N/K-RASwt, n = 350; complex KT, n = 139; CBFB::MYH11, n = 29; CEBPAmut, n = 16; MLL:MLLT3, n = 42; PML::RARA, n = 15; RUNX1::RUNX1T1, n = 21; normal CD34+, n = 17). Values are in log (FPKM). (B) CLEC12A mRNA expression among different AML KTs and mutations, derived from the Balgobind et al40 cohort (GSE17855; NUP98::NSD1, n = 11; CN-AML, n = 39; MLL::MLT3, n = 47; N/K-RASmut, n = 41; N/K-RASwt, n = 196; CEBPAmut, n = 16; cKITmut, n = 18; FLT3-ITD, n = 48; CBFB::MYH11, n = 27; PML::RARA, n = 19; RUX1::RUNX1T1, n = 28; other, n = 45; unknown, n = 25; NPM1mut, n = 17). Values are in normalized microarray intensity (RMA). (C) Reverse transcriptase PCR revealing Clec12a mRNA expression in murine BM cells transduced with either NRASG12D, NUP98::NSD1, or NUP98::NSD1+NRASG12D (n = 3; mean ± standard error of the mean [SEM]). (D) Flow cytometric representation of Clec12a protein expression in murine hematopoietic BM cells transduced with NUP98::NSD1+NRASG12D. Cells were gated on GFP+BFP+. (E) Normalized Clec12a expression for NUP98 CTL and NUP98::NSD1 murine-expressing cells. Clec12a is highly expressed in NUP98::NSD1-expressing cells (off Dox) compared with NUP98 CTL cells and is strongly downregulated if NUP98-NSD1 expression is shut off (day 3_on_Dox and day 5_on_Dox). Data are presented as boxplots in which the center line represents the median, the boundaries of the box indicate the first (25th percentile) and third (75th percentile) quartiles, and the whiskers extend to 1.5× interquartile range from the hinges. Data points beyond this range are revealed as individual dots and represent outliers. (F) Publicly available NUP98::NSD1 chromatin immunoprecipitation-sequencing data reveal binding of NUP98::NSD1 to regions with proximal or distal enhancer-like signatures according to the ENCODE candidate cis-Regulatory Elements atlas (GSE112928). CTL, control; Dox, doxycycline; FPKM, fragments per kilobase per million; H3K4me3, histone 3, lysine 4 trimethylation; KT, karyotype; mRNA, messenger RNA; RMA, robust multi-array analysis.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/15/10.1182_bloodadvances.2024015739/2/m_blooda_adv-2024-015739-gr1f.jpeg?Expires=1761665144&Signature=AePhp3By1I0YOR6UH44dsJWRl81PTH1Fj0cgsFUQ8mJB8zo32A6rCyivwWNNdCujauZ02i-LfhH00IFJ0sLaRUxE6FZIF6MpwUIcYWR5Y-eaqCd5JkixUjzVEy3NIZsCi4MJdgagyEBTtknk7hfWDVLSzsoyJIQhL~XXbq-MQbAdwv2IBHVWSauBOQOWmnV4EIqu1V6yfj4l6k2YVm6UXva7Nho82GZYCO5kjIKrzXxUpxnKH7xFgMCghXXiudXFYuQUmbscqNeDv68WuefDWY3Jt5lFgnZ87LAGlV-DYOYukmFdHB3nDzKKDLjLsYjz90YNjI2eG22HN4XHz7dZUg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Clec12a expression is regulated by NUP98::NSD1. (A) CLEC12A mRNA expression among different cytogenetic AML subgroups, derived from the Leucegene cohort (NUP98::NSD1, n = 7; CN-AML, n = 168; N/K-RASmut, n = 87; N/K-RASwt, n = 350; complex KT, n = 139; CBFB::MYH11, n = 29; CEBPAmut, n = 16; MLL:MLLT3, n = 42; PML::RARA, n = 15; RUNX1::RUNX1T1, n = 21; normal CD34+, n = 17). Values are in log (FPKM). (B) CLEC12A mRNA expression among different AML KTs and mutations, derived from the Balgobind et al40 cohort (GSE17855; NUP98::NSD1, n = 11; CN-AML, n = 39; MLL::MLT3, n = 47; N/K-RASmut, n = 41; N/K-RASwt, n = 196; CEBPAmut, n = 16; cKITmut, n = 18; FLT3-ITD, n = 48; CBFB::MYH11, n = 27; PML::RARA, n = 19; RUX1::RUNX1T1, n = 28; other, n = 45; unknown, n = 25; NPM1mut, n = 17). Values are in normalized microarray intensity (RMA). (C) Reverse transcriptase PCR revealing Clec12a mRNA expression in murine BM cells transduced with either NRASG12D, NUP98::NSD1, or NUP98::NSD1+NRASG12D (n = 3; mean ± standard error of the mean [SEM]). (D) Flow cytometric representation of Clec12a protein expression in murine hematopoietic BM cells transduced with NUP98::NSD1+NRASG12D. Cells were gated on GFP+BFP+. (E) Normalized Clec12a expression for NUP98 CTL and NUP98::NSD1 murine-expressing cells. Clec12a is highly expressed in NUP98::NSD1-expressing cells (off Dox) compared with NUP98 CTL cells and is strongly downregulated if NUP98-NSD1 expression is shut off (day 3_on_Dox and day 5_on_Dox). Data are presented as boxplots in which the center line represents the median, the boundaries of the box indicate the first (25th percentile) and third (75th percentile) quartiles, and the whiskers extend to 1.5× interquartile range from the hinges. Data points beyond this range are revealed as individual dots and represent outliers. (F) Publicly available NUP98::NSD1 chromatin immunoprecipitation-sequencing data reveal binding of NUP98::NSD1 to regions with proximal or distal enhancer-like signatures according to the ENCODE candidate cis-Regulatory Elements atlas (GSE112928). CTL, control; Dox, doxycycline; FPKM, fragments per kilobase per million; H3K4me3, histone 3, lysine 4 trimethylation; KT, karyotype; mRNA, messenger RNA; RMA, robust multi-array analysis.
To determine whether NUP98::NSD1 induces CLEC12A expression, we cloned NUP98::NSD1 and the control NRASG12D into MSCV-IRES-GFP and MSCV-IRES-BFP vectors, respectively,14 and immortalized murine BM cells using NUP98::NSD1, NRASG12D, or both vectors. Expression of NUP98::NSD1 and NUP98::NSD1+NRASG12D led to a 132-fold and 226-fold overexpression of Clec12a compared with NRASG12D-immortalized cells, respectively (Figure 1C). This massive upregulation was confirmed on protein level using flow cytometry (Figure 1D) and in a doxycycline-regulated expression system for NUP98::NSD1 (Figure 1E; supplemental Figure 2). We analyzed chromatin immunoprecipitation followed by deep sequencing data from Franks et al41 to determine whether NUP98::NSD1 binds to Clec12a regulatory regions. We found enriched NUP98::NSD1 binding across the entire Clec12a gene that was not observed in cells expressing wild-type NUP98 (Figure 1F). These binding regions overlap putative cis-regulatory elements as defined by the ENCODE registry of candidate cis-regulatory elements, which are DNase hypersensitive sites that also reveal enrichment of H3K4me3, H3K27ac, or CTCF binding.42 These data suggest that NUP98:NSD1 might bind to cis-regulatory elements involved in the regulation of Clec12a expression.
Knockout of Clec12a in NUP98::NSD1+NRASG12D-immortalized cells
We next studied the effects of Clec12a inactivation in NUP98::NSD1-induced leukemia. Our earlier study demonstrated that NUP98::NSD1+NRASG12D-immortalized cells developed aggressive leukemia with short leukemia latency in mice,14 so we used this model for our in vivo studies. To deplete the expression of Clec12a from NUP98::NSD1+NRASG12D-immortalized murine BM cells, we designed 3 sgRNAs against Clec12a using the CCTop tool. We selected 3 sgRNAs, Clec12a sgT6, Clec12a sgT44, and Clec12a sgT47, which bind to exon 1, exon 2, and exon 4 of Clec12a, respectively (supplemental Figure 3A). We cloned these sgRNAs into the Cas9-expressing lentiviral vector L40C-CRISPR.EFS.dTomato, and the dTomato reporter was used to select sgRNA-transduced cells (Figure 2A). A sgRNA against the lacZ gene was used as a transduction control. CRISPR/Cas9-mediated Clec12a deletion was validated on genomic, transcript, and protein levels. Bulk-sorted cells were used for transcript and flow cytometry analysis. Quantitative PCR analysis showed an ∼50% reduction of the messenger RNA level in Clec12a sgT6-, Clec12a sgT44-, and Clec12a sgT47-transduced cells compared with lacZ-transduced cells (Figure 2B). Flow cytometry analysis revealed a complete loss of Clec12a surface expression in all Clec12a sgT6-, Clec12a sgT44-, and Clec12a sgT47-transduced cells (Figure 2C).
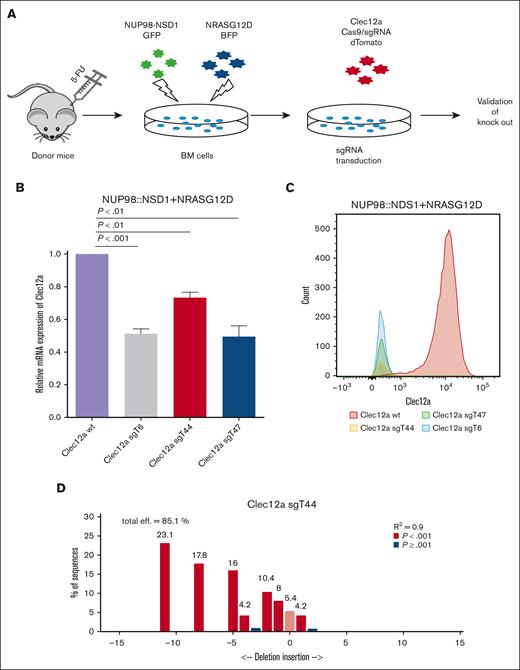
Knockout of Clec12a in NUP98::NSD1+NRASG12D-immortalized cells. (A) Experimental outline showing the generation of a Clec12a deletion in the NUP98::NSD1+NRASG12D model. (B) Gene expression of Clec12a in NUP98::NSD1+NRASG12D-immortalized cells transduced with 3 different sgRNAs against Clec12a and Clec12a WT. Clec12a WT cells were transduced with CTL lacZ vector. (C) Clec12a protein expression in Clec12a WT and knockout NUP98::NSD1+NRASG12D cells analyzed by flow cytometry. Cells were gated on GFP+BFP+dTomato+. Clec12a WT cells were transduced with CTL lacZ vector. (D) Sanger sequencing shows the genomic deletion of Clec12a in NUP98::NSD1+NRASG12D-immortalized cells using sgRNA Clec12a sgT44. 5-FU, 5-fluoruracil; BFP, blue fluorescent protein. GFP, green fluorescent protein; WT, wild-type.

Knockout of Clec12a in NUP98::NSD1+NRASG12D-immortalized cells. (A) Experimental outline showing the generation of a Clec12a deletion in the NUP98::NSD1+NRASG12D model. (B) Gene expression of Clec12a in NUP98::NSD1+NRASG12D-immortalized cells transduced with 3 different sgRNAs against Clec12a and Clec12a WT. Clec12a WT cells were transduced with CTL lacZ vector. (C) Clec12a protein expression in Clec12a WT and knockout NUP98::NSD1+NRASG12D cells analyzed by flow cytometry. Cells were gated on GFP+BFP+dTomato+. Clec12a WT cells were transduced with CTL lacZ vector. (D) Sanger sequencing shows the genomic deletion of Clec12a in NUP98::NSD1+NRASG12D-immortalized cells using sgRNA Clec12a sgT44. 5-FU, 5-fluoruracil; BFP, blue fluorescent protein. GFP, green fluorescent protein; WT, wild-type.
Sanger sequencing verified the deletion of Clec12a on the genomic level for all 3 Clec12a sgRNAs (Figure 2D; supplemental Figure 3B-C). Untransduced mouse BM cells demonstrated low expression of Clec12a similarly to Clec12A knockout cells compared with control Clec12A wild-type cells (supplemental Figure 4). In summary, we confirmed knockout of Clec12a in NUP98::NSD1+NRASG12D-immortalized cells.
Inactivation of Clec12a in NUP98::NSD1+NRASG12D cells reveals reduction in proliferation kinetics in vitro
We next compared the oncogenic potential of Clec12a knockout and wild-type NUP98::NSD1+NRASG12D cells in vitro. Clec12a knockout NUP98::NSD1+NRASG12D cells proliferated slower than wild-type cells but had similar growth kinetics after a few days (Figure 3A). However, there was a loss of dTomato+ Clec12a sgRNA-transduced cells over time, suggesting that untransduced cells had a competitive advantage and overgrew the transduced cells (Figure 3B). We found a small but significant decrease in colony-forming units in Clec12a knockout NUP98::NSD1+NRASG12D cells (Figure 3C). The proportion of cells in G0/G1 cell cycle phases were increased in Clec12a knockout cells (gated on dTomato+ cells), and there was a concomitant reduction of cells in the S phase (Figure 3D). Furthermore, cells in G2/M were slightly but significantly increased. The proportion of apoptotic cells was increased in Clec12a knockout cells (Figure 3E). Immunophenotypic analysis revealed a small proportional increase in the mature myeloid (CD11b+Gr1+) population but no difference in CD11b+Gr1– expression (supplemental Figure 5A-B). Morphologic analysis using cytospin preparations did not reveal substantial differences between wild-type and Clec12a knockout cells (Figure 3F), similar to the overall morphology of colony-forming cells. In summary, the loss of Clec12a is associated with reduced growth of NUP98::NSD1+NRASG12D-immortalized cells, which results from changes in cell cycle and apoptosis.

Clec12a KO NUP98::NSD1+NRASG12D cells demonstrate less proliferation kinetics in vitro. (A) Proliferation of Clec12a WT and KO NUP98::NSD1+NRASG12D cells in 12 days (mean ± SEM). (B) Growth of dTomato+ sgRNA-transduced and -untransduced cells over time in the proliferation assay (mean ± SEM). (C) Cumulative CFC yield is revealed for an initial plating of 1000 Clec12a WT and KO NRASG12D+NUP98::NSD1 transduced cells (mean ± SEM). (D) Frequency of cell cycle phases in Clec12a WT and KO NUP98::NSD1+NRASG12D cells (mean ± SEM). Cells were gated on dTomato+ cells, which correspond to Clec12a KO cells. (E) Proportion of apoptosis in Clec12a WT and KO NUP98::NSD1+NRASG12D cells (mean ± SEM). (F) Representative Wright-Giemsa–stained cytospin preparations of Clec12a WT and KO NUP98::NSD1+NRASG12D-transduced mouse BM cells (1000×). CFC, colony-forming cell; KO, knockout.

Clec12a KO NUP98::NSD1+NRASG12D cells demonstrate less proliferation kinetics in vitro. (A) Proliferation of Clec12a WT and KO NUP98::NSD1+NRASG12D cells in 12 days (mean ± SEM). (B) Growth of dTomato+ sgRNA-transduced and -untransduced cells over time in the proliferation assay (mean ± SEM). (C) Cumulative CFC yield is revealed for an initial plating of 1000 Clec12a WT and KO NRASG12D+NUP98::NSD1 transduced cells (mean ± SEM). (D) Frequency of cell cycle phases in Clec12a WT and KO NUP98::NSD1+NRASG12D cells (mean ± SEM). Cells were gated on dTomato+ cells, which correspond to Clec12a KO cells. (E) Proportion of apoptosis in Clec12a WT and KO NUP98::NSD1+NRASG12D cells (mean ± SEM). (F) Representative Wright-Giemsa–stained cytospin preparations of Clec12a WT and KO NUP98::NSD1+NRASG12D-transduced mouse BM cells (1000×). CFC, colony-forming cell; KO, knockout.
Differential gene expression between Clec12a wild-type and knockout NUP98::NSD1+NRASG12D cells
We performed gene expression profiling to identify the pathways that are differentially regulated by Clec12a in NUP98::NSD1-mediated leukemogenesis. We found a differential gene expression profile for NUP98::NSD1+NRASG12D/Clec12a knockout cells compared with NUP98::NSD1+NRASG12D/Clec12a wild-type cells (Figure 4A). Kyoto Encyclopedia of Genes and Genomes pathway analysis revealed that NUP98::NSD1+NRASG12D/Clec12a knockout cells had strongly downregulated cell cycle and DNA replication pathways, whereas a broad range of metabolic pathways was upregulated, including purine, arginine, and proline metabolism (Figure 4B-D). In addition, NUP98::NSD1+NRASG12D/Clec12a knockout cells demonstrated an enrichment in the apoptotic pathway (supplemental Figure 6A). Meis1, which plays a vital role in the maintenance of AML cells,43 was found to be downregulated in NUP98::NSD1+NRASG12D/Clec12a knockout cells (supplemental Table 1). We also observed an increase in reactive oxygen pathways in NUP98::NSD1+NRASG12D/Clec12a knockout cells, which might lead to depletion of Clec12a sgRNA-expressing dTomato+ cells (supplemental Figure 6B). In summary, Clec12a is required for NUP98::NSD1 leukemia to enhance cell cycle, inhibit apoptosis, and control a broad range of metabolic pathways.
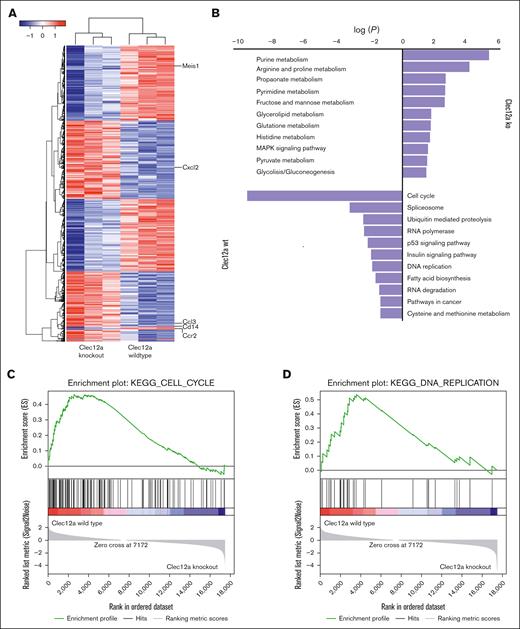
Differential gene expression between Clec12a WT and KO NUP98::NSD1+NRASG12D cells. (A) Heat map showing differential gene expression between Clec12a KO and WT NUP98::NSD1+NRASG12D cells. (B) KEGG pathway analysis showing enrichment of different pathways between Clec12a KO and WT NUP98::NSD1+NRASG12D cells. (C) Enrichment plot for the gene set KEGG_CELL_CYCLE of GSEA comparing Clec12a KO and WT NUP98::NSD1+NRASG12D cells. (D) Enrichment plot for the gene set KEGG_DNA_REPLICATION of GSEA comparing Clec12a KO and WT NUP98::NSD1+NRASG12D cells. GSEA, Gene Set Enrichment Analysis; KEGG, Kyoto Encyclopedia of Genes and Genomes.

Differential gene expression between Clec12a WT and KO NUP98::NSD1+NRASG12D cells. (A) Heat map showing differential gene expression between Clec12a KO and WT NUP98::NSD1+NRASG12D cells. (B) KEGG pathway analysis showing enrichment of different pathways between Clec12a KO and WT NUP98::NSD1+NRASG12D cells. (C) Enrichment plot for the gene set KEGG_CELL_CYCLE of GSEA comparing Clec12a KO and WT NUP98::NSD1+NRASG12D cells. (D) Enrichment plot for the gene set KEGG_DNA_REPLICATION of GSEA comparing Clec12a KO and WT NUP98::NSD1+NRASG12D cells. GSEA, Gene Set Enrichment Analysis; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Clec12a is required for NUP98::NSD1-induced leukemogenesis in vivo
To understand the leukemogenic potential of Clec12a knockout NUP98::NSD1+NRASG12D-immortalized cells, we transplanted an equal number of wild-type and Clec12a knockout cells into mice. After 4 weeks of transplantation, we checked the engraftment of cells in the PB. Because the sgRNA-carrying vectors express dTomato, we checked the expression of dTomato+ cells as a marker for engraftment of sgRNA+ cells. PB analysis at 4 weeks revealed a lower engraftment and a lower WBC count in Clec12a knockout compared with wild-type mice (supplemental Figure 7A-B), with similar levels of hemoglobin and platelet counts (supplemental Figure 7C-D). The cumulative analysis of knockout mice from all Clec12a knockout clones revealed a significant increase in leukemia latency in Clec12a-deficient mice (Figure 5A). Individual analysis of all Clec12a sgRNA groups also revealed a significant survival advantage in all 3 sgRNAs (supplemental Figure 8A). At the time of euthanasia, WBC counts were higher, and hemoglobin and platelet counts were lower in the Clec12a knockout mice compared with wild-type AML (Figure 5B-D). Moreover, mice transplanted with NUP98::NSD1+NRASG12D/Clec12a knockout cells had a significantly lower spleen weight at the time of euthanasia (Figure 5E). Although 100% of the engrafted cells in BM, spleen, and PB of control mice were dTomato+ (thus transduced), a much lower number of dTomato+ cells were detected in Clec12a knockout mice (mean, 16%) indicating that the death of mice in Clec12a knockout groups was likely caused by the outgrowth of untransduced cells (Figure 5F). These results together demonstrate that NUP98::NSD1-induced leukemia requires the expression of Clec12a.
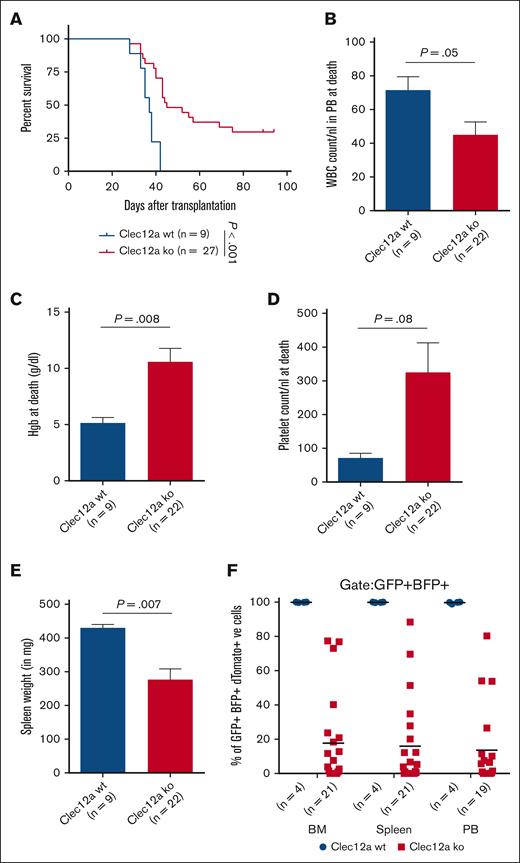
Clec12a is required for NUP98::NSD1-induced leukemogenesis in vivo. (A) Survival of mice that received transplants of NUP98::NSD1+NRASG12D cells transduced with lacZ (n = 9) or Clec12a sgRNAs (n = 27; sgT6, n = 10; sgT44, n = 9; sgT47, n = 8). (B) WBC count in PB at the time of death in mice that received transplants of Clec12a WT and KO NUP98::NSD1+NRASG12D cells (mean ± SEM). (C) Hemoglobin levels in PB at the time of death in mice that received transplants of Clec12a WT and KO NUP98::NSD1+NRASG12D cells (mean ± SEM). (D) Platelet count in PB at the time of death in mice that received transplants of Clec12a WT and KO NUP98::NSD1+NRASG12D cells (mean ± SEM). (E) Average spleen weight at the time of death in mice that received transplants of Clec12a WT and KO NUP98::NSD1+NRASG12D cells (mean ± SEM). (F) Engraftment of dTomato+ cells in the BM, spleen, and PB of Clec12a WT and Clec12a KO NUP98NSD1+NRASG12D cells at the time of death. Some mice died before PB analysis could be performed.

Clec12a is required for NUP98::NSD1-induced leukemogenesis in vivo. (A) Survival of mice that received transplants of NUP98::NSD1+NRASG12D cells transduced with lacZ (n = 9) or Clec12a sgRNAs (n = 27; sgT6, n = 10; sgT44, n = 9; sgT47, n = 8). (B) WBC count in PB at the time of death in mice that received transplants of Clec12a WT and KO NUP98::NSD1+NRASG12D cells (mean ± SEM). (C) Hemoglobin levels in PB at the time of death in mice that received transplants of Clec12a WT and KO NUP98::NSD1+NRASG12D cells (mean ± SEM). (D) Platelet count in PB at the time of death in mice that received transplants of Clec12a WT and KO NUP98::NSD1+NRASG12D cells (mean ± SEM). (E) Average spleen weight at the time of death in mice that received transplants of Clec12a WT and KO NUP98::NSD1+NRASG12D cells (mean ± SEM). (F) Engraftment of dTomato+ cells in the BM, spleen, and PB of Clec12a WT and Clec12a KO NUP98NSD1+NRASG12D cells at the time of death. Some mice died before PB analysis could be performed.
Discussion
Here, we identify Clec12a as a target gene of the NUP98::NSD1 oncogene that is required to promote cell cycle, inhibit apoptosis, and control metabolism, and, thereby, mediates the leukemogenic potential of NUP98::NSD1. Knockout of Clec12a using 3 different sgRNAs in NUP98::NSD1+NRASG12D-induced leukemia efficiently prevented leukemia development in vivo. These results suggest that inhibition of Clec12a may have a therapeutic potential in NUP98::NSD1+ AML.
We observed a proliferative disadvantage of NUP98::NSD1+NRASG12D/Clec12a knockout cells in vitro and in vivo together with a reduction in colony-forming potential. Low expression of Meis1 might associate with a reduction in the colony-forming ability of Clec12a knockout cells as it is associated with leukemic self-renewal.44 We observed a strong depletion of Clec12a sgRNA+ cells in vivo but a comparatively weaker reduction in oncogenic effects in vitro, indicating that a Clec12a-mediated interaction of AML cells with the microenvironment may be an important function.
Ligands for CLEC12A are poorly defined so far. Neumann et al demonstrated that uric acid crystals or unknown ligands on dead cells can act as Clec12a ligands and the immunoreceptor tyrosine-based inhibition motif of Clec12a in the cytoplasmic domain attenuates inflammation through negative regulation of Syk signaling.45 They did not find any significant changes in the number of monocytes, lymphocytes, neutrophils, and macrophages between Clec12a−/− and wild-type mice. However, Clec12a knockout mice had an increase in monosodium urate-induced reactive oxygen species (ROS) in BM cells and purified neutrophils from Clec12a−/− mice. Similarly, our gene set enrichment analysis revealed an increase in ROS in Clec12a knockout cells compared with wild-type NUP98::NSD1+NRASG12D cells. Zhang et al reported that loss of the chemokine receptor CXCR4 resulted in ROS elevation, DNA damage, and apoptosis in HSCs.46 Furthermore, a recent study by Ramakrishnan et al revealed that disruption of CXCR4 increased oxidative stress in KMT2A::MLLT3 leukemia and inhibits its progression.47 Therefore, loss of Clec12a might reduce the leukemic burden of NUP98::NSD1 leukemia by increasing oxidative stress, DNA damage, and apoptosis.
Another study described a role of CLEC12A for binding dendritic cells to endothelial cells and their transmigration and that CLEC12A antibody treatment inhibited the infiltration of dendritic cells to the central nervous system.48 These findings associate CLEC12A expression with transmigration, possibly to the hematopoietic niche. Migration of leukemic cells from BM to vessels and extramedullary tissues is associated with poor prognosis in leukemia.49,50 CLEC12A may be involved in this process and should be evaluated further.
The surface expression of CLEC12A allowed researchers to use different approaches to target LSCs in the last several years. The absence of its expression on T cells and higher expression in leukemic stem cells make it a potential target for chimeric antigen receptor T-cell therapy.18,21 Monoclonal antibodies against CLEC12A were found to have a cytotoxic effect through both antibody-dependent cell–mediated cytotoxicity and complement-dependent cytotoxicity.51 In addition, a bispecific T-cell redirecting antibody (MCLA-117) against CLEC12A was developed, which activates T cells and redirects them to eliminate CLEC12A+ AML cells.52 Anti-CLEC12A antibody-drug conjugates were found to have antitumor effects in an AML xenograft model.53,54 Furthermore, different chimeric antigen receptors were designed to target CLEC12A+ AML cells.55,56 These studies together indicate that successive therapies can be developed to treat CLEC12A+ NUP98::NSD1 AML.
In summary, our results highlight that Clec12a is a crucial contributor to NUP98::NSD1 and NRASG12D-mediated leukemogenesis and should be explored as a druggable target for the treatment of NUP98::NSD1+ AML.
Acknowledgments
The authors are thankful to Renate Schottmann, Kerstin Görlich, Martin Wichmann, and Nadine Kattre for their technical support. The authors also thank Robert Geffers from the Helmholtz Center for Infection Research (Braunschweig) for RNA sequencing and Matthias Ballmaier and the staff of the Cell Sorting Core Facility of Hannover Medical School. The authors acknowledge the help from staff of the Central Animal Facility supporting animal maintenance.
This study was supported by the Rudolf-Bartling Stiftung, an European Research Council grant under the European Union’s Horizon 2020 research and innovation program (number 638035); grant from Deutsche José Carreras Leukämie-Stiftung (16 R/2021 [M.H.]); and grants from Deutsche Krebshilfe (70114189, 70114478, and 70115044 [M.H.]).
Authorship
Contribution: S.M. and M.H. designed the research; S.M., F.C.C., R.G., C.K.L., B.O., H.S., and F.G. performed the experiments; S.M., F.C.C., R.G., C.K.L., H.S., R.H., T.E., and D.B.L. analyzed the data; S.M., F.C.C., and M.H. wrote the manuscript; and all authors read and agreed to the final version of the manuscript.
Conflict-of-interest disclosure: M.H. declares receiving honoraria from AbbVie, Bristol Myers Squibb, Janssen, Jazz Pharmaceuticals, Pfizer, Qiagen, Servier, and Sobi; providing paid consultancy for AvenCell Therapeutics, AbbVie, Astellas, Glycostem, Janssen, LabDelbert, Miltenyi, Novartis, Pfizer, PinotBio, and Servier; and receiving research funding to his institution from AbbVie, Servier, Astellas, BerGenBio, Glycostem, Jazz Pharmaceuticals, Karyopharm, Loxo Oncology, Novartis, and PinotBio. The remaining authors declare no competing financial interests.
Correspondence: Michael Heuser, Department of Hematology, Hemostasis, Oncology, and Stem Cell Transplantation, Hannover Medical School, Carl-Neuberg-Str 1, 30625 Hannover, Germany; email: heuser.michael@mh-hannover.de.
References
Author notes
S.M. and F.C.C. contributed equally to this study.
RNA sequencing data are available on the Gene Expression Omnibus (accession number GSE282047).
The full-text version of this article contains a data supplement.


