Key Points
There is little information regarding how DOACs have been incorporated for the management of children with, or at risk of, thrombotic events.
We identified barriers and inequalities in the use of DOACs, highlighting areas that can be improved to facilitate their use globally.
Visual Abstract
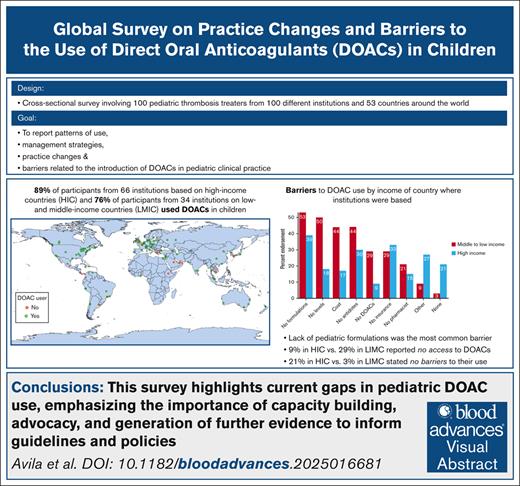
We aim to describe the patterns of use, practice changes, and emerging challenges after the approval of direct oral anticoagulants (DOACs) for thrombosis management and prevention in children. This cross-sectional survey-based study involved pediatric thrombosis treaters from different institutions around the world. The survey was distributed between January and August 2024. DOAC use and barriers were compared according to the country’s income (high-income countries [HICs] vs low- and middle-income countries [LMICs], per World Bank classification). In total, 100 of 103 respondents completed the survey, representing 100 different institutions from 96 cities in 53 countries (31% European, 25% North American, 24% Asian, 12% South American, 5% Oceanian, and 3% African). Eighty-five percent of respondents used DOACs (HICs, 89% vs LMICs, 76%; P = .09). Although DOACs were more commonly used than low molecular weight heparin (LMWH) or vitamin K antagonists (VKA) in outpatients, 91% respondents indicated that <50% of their patients were started on DOACs directly, and 62% reported that <50% of children on long-term LMWH/VKA had been switched to DOACs. Lack of pediatric formulations was the most common barrier (39% in HICs, 52% in LMICs); 9% of respondents in HIC vs 29% in LIMC reported no access to DOACs (P = .009), whereas 21% of respondents in HICs vs 3% in LIMCs stated no barriers to their use (P = .02), indicating inequality. Additional barriers included cost and clinician acceptability and limited knowledge, experience, and expertise. The results highlight current gaps in pediatric DOAC use, emphasizing the importance of capacity building, advocacy, and generation of further evidence to inform guidelines and policies.
Introduction
Thrombotic events are increasingly being recognized in the pediatric population. According to recent reports from the United States, the frequency of pediatric venous thrombosis increased 300%, escalating from 34 per 10 000 hospital admissions in 2001 to 106 per 10 000 hospital admissions in 2019.1,2 Although most of the data related to thrombotic events in children originate in high-income countries (HICs),3 pediatric thrombosis is a phenomenon recognized worldwide.3-10 Anticoagulants are the main strategy for the treatment or prevention of thrombotic events in adults and children, and include heparinoids, vitamin K antagonists (VKAs), and direct oral anticoagulants (DOACs).
Regulatory agencies, including the US Food and Drug Administration (FDA), European Medicines Agency (EMA), and Health Canada/Santé Canada, started approving DOAC use in adults in the late 2000s and early 2010s, after the publication of pivotal trials.11-15 At the time, dabigatran16 and rivaroxaban,17 and later apixaban18 and edoxaban,19 entered the early phases of pediatric clinical trials. This resulted in a never-before-seen achievement in pediatric thrombosis research: 6 randomized clinical trials investigating the efficacy and safety of DOACs for the treatment and prevention of thromboembolism in >1800 children were published within 5 years.20-25 As a result, the FDA and EMA approved dabigatran and rivaroxaban for the treatment of acute venous thromboembolism, and the FDA approved oral dabigatran pellets for secondary venous thromboembolism prophylaxis and primary prophylaxis in children with congenital heart disease undergoing Fontan palliative surgery. More recently, the EMA approved apixaban for the treatment and prevention of venous thromboembolism in children aged ≥28 days.
The introduction of DOACs profoundly changed the landscape of anticoagulation management in adults and children, also bringing challenges that were first recognized in the care of adult patients. Some of the challenges adult treaters faced were expected, such as a relative higher cost in contrast to VKAs26,27 and uncertainty in the translation of safety and efficacy of these agents to the populations that were underrepresented or excluded from clinical trials.28-30 Less-anticipated problems included the frequency of underdosing and overdosing because of perceived risk,31,32 the marginal improvement of adherence as compared with VKAs,33 the appropriateness of DOAC level monitoring, and the difficulty interpreting and managing drug level results.34
In addition to the aforementioned, other factors increase the complexity of the care of children with or at risk of thrombosis. Thrombotic events are less frequent in children than in adults, which limits the timely collection of robust data to guide clinical practice. Furthermore, the developmental and cognitive changes in pediatric patients also adds to this complexity, given their impact on the coagulation system, drug metabolism, and DOAC administration.
Despite becoming a global clinical reality, the changes and challenges in the management of children with thrombotic events have not been fully explored. Therefore, the goal of this study was to report the pattern of use, management strategies, practice changes, and barriers related to the introduction of DOACs in clinical practice across different institutions around the world from the perspective of clinicians involved in the care of children with thrombotic events.
Methods
In this cross-sectional study, we used an online survey to inquire about the institutional experience and practice of pediatric thrombosis treaters. Only 1 respondent per health care institution was invited to complete the survey. Survey respondents were identified through existing pediatric literature, the directory of the Scientific and Standardization Subcommittee on Pediatric and Neonatal Thrombosis and Haemostasis of the International Society on Thrombosis and Haemostasis, and snowball sampling, in which targeted respondents were asked to nominate potential pediatric thrombosis treaters from other institutions within their country or continent. The latter sampling technique was used to increase the participation of underrepresented regions outside North America and Europe. The survey was distributed using the Research Electronic Data Capture platform. Research ethics board approval was not required. Participants were informed that participation in the survey implied consent.
The survey asked about the total number of pediatric admissions and of venous thrombotic events in children per year at their institution, and whether DOACs were prescribed for pediatric patients at their institution (yes/no). For those using DOACs, the survey asked about the frequency of use of each agent (most to least used, not used, not available), percentage of outpatients receiving DOACs vs VKAs vs low molecular weight heparin (LMWH), percentage of children started on DOACs directly (including lead-in phase), and percentage of children on long-term VKAs/LMWH switched to DOACs. Additional questions included prescription of DOACs according to thrombosis affecting different vascular territories and across different pediatric subpopulations, with answers in a 3-point Likert item (always/very often, sometimes, never); respondents also had the option of indicating whether they did not see a particular type of thrombotic event or patient population. The survey also asked about protocol availability at their institution, management strategy in emergency surgery or major bleeding complications, whether they monitored DOAC levels (eg, laboratory testing), and whether they had made changes to their clinical services because of the implementation of DOACs. Lastly, participants were asked about barriers to DOAC use and to provide overall comments.
Throughout the study, “income level” refers to that of the country in which each institution was based, and was classified as high-, upper-middle-, lower-middle-, and low-income following the World Bank classification, fiscal year 2024.35 The institutions were divided into 2 groups according to their country’s income level: HICs and low- and middle-income countries (LMICs).
Responses were summarized using descriptive statistics, with medians (first quartile [Q1] to third quartile [Q3]) or percentages, as appropriate. Income level, hospital case volume based on pediatric admissions per year, and pediatric venous thrombotic events per year were compared between DOAC users and non-users using the Fisher exact test. Outpatient anticoagulant use in HICs vs LIMCs were compared using 2-way repeated measures mixed analysis of variance. A sensitivity analysis was conducted including only centers that followed up children with mechanical valves or antiphospholipid syndrome to explore the effect on outpatient anticoagulant use, because VKAs are expected to be used more frequently in these 2 populations. The main effects of the between and within factors (ie, income classification and anticoagulant type, respectively) were explored using Bonferroni adjusted pairwise comparisons. The percentage of patients started or switched to DOACs and barriers to the use of DOACs were also compared between HIC and LMIC using the Fisher exact test or the Pearson χ2 test, as appropriate. Analysis of DOAC use in specific vascular territories and patient populations excluded respondents who indicated that they did not provide care for these cases or patients. Analyses were carried out using R version 4.4.0.
Results
Characteristics of the institutions
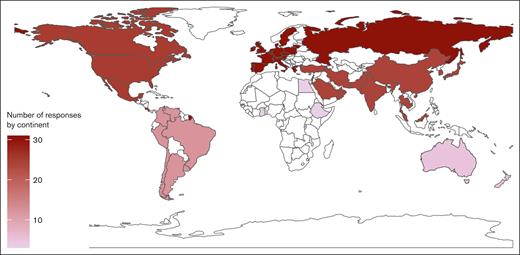
Of 103 invited participants, 100 completed the survey (97% response) between January and August 2024. The respondents represented 100 unique institutions from 96 cities in 53 countries and 6 continents (supplemental Appendix 1). In total, 31 institutions were based in Europe (31%), 25 in North America (25%), 24 in Asia (24%), 12 South America (12%), 5 in Oceania (5%), and 3 in Africa (3%). Figure 1 shows a map illustrating the countries represented in the survey and the number of respondents per continent.
Sixty-six institutions (66/100 [66%]) were from HICs and 34 (34%) from LMICs. The latter comprised 25 that were from upper-middle-income countries, 8 from lower-middle-income countries, and 1 from a low-income country. Of note, Venezuela was classified according to the latest available data reported in fiscal year 2021.36
In total, 94 of 100 (94%) of respondents were pediatric hematologists or pediatric hematologist-oncologists, 3 were pediatric cardiologists, 2 were general pediatricians, and 1 participant was an adult hematologist.
Most respondents (85/100 [85%]) used DOACs at their institutions. The visual abstract shows the geographical distribution of institutions (cities) according to DOACs use.
General DOAC use
We found no evidence of significant difference in DOAC use when comparing HICs and LMICs, or when comparing institutions according to the number of pediatric admissions per year (Table 1). Although all treaters from institutions with >200 pediatric venous thrombotic events per year used DOACs, the frequency of use in institutions with fewer thrombotic events per year varied (Table 1).
Characteristics of institutions in which DOACs were used vs not used
| Characteristic . | DOAC users . | P value∗ . | |
|---|---|---|---|
| No, n = 15 n (%) . | Yes, n = 85 n (%) . | ||
| World Bank income classification | |||
| HIC | 7 (11) | 59 (89) | .09 |
| LMIC | 8 (24) | 26 (76) | |
| Pediatric admissions per y | |||
| <5 000 | 3 (19) | 13 (81) | .78 |
| 5 000 to <10 000 | 3 (11) | 24 (89) | |
| 10 000-20 000 | 7 (17) | 34 (83) | |
| Pediatric venous thrombotic events per y | |||
| <50 | 7 (19) | 30 (81) | .004 |
| 50 to <100 | 2 (5) | 35 (95) | |
| 100 to <200 | 5 (50) | 5 (50) | |
| >200 | 0 (0) | 13 (100) | |
| Characteristic . | DOAC users . | P value∗ . | |
|---|---|---|---|
| No, n = 15 n (%) . | Yes, n = 85 n (%) . | ||
| World Bank income classification | |||
| HIC | 7 (11) | 59 (89) | .09 |
| LMIC | 8 (24) | 26 (76) | |
| Pediatric admissions per y | |||
| <5 000 | 3 (19) | 13 (81) | .78 |
| 5 000 to <10 000 | 3 (11) | 24 (89) | |
| 10 000-20 000 | 7 (17) | 34 (83) | |
| Pediatric venous thrombotic events per y | |||
| <50 | 7 (19) | 30 (81) | .004 |
| 50 to <100 | 2 (5) | 35 (95) | |
| 100 to <200 | 5 (50) | 5 (50) | |
| >200 | 0 (0) | 13 (100) | |
Fisher exact test.
Figure 2 shows the use/availability of each DOAC per respondent. Overall, rivaroxaban was the most used and most available agent, being available to 95% of respondents. Apixaban was the second most used (31%), and edoxaban was the most infrequently used or not available DOAC (93% of the respondents).
Lollipop plot of the proportion (y-axis) of use/availability for each DOAC among all respondents (N = 100).
Lollipop plot of the proportion (y-axis) of use/availability for each DOAC among all respondents (N = 100).
Outpatient DOAC use
When analyzing the relative proportion of outpatients on DOACs, LMWH, or VKAs for treatment or prophylaxis among DOAC users (Table 2), DOACs were used most frequently (median of 40%; Q1-Q3, 20%-62%), followed by LMWH (median, 30%; Q1-Q3, 15%-50%) and VKAs (median, 10%; Q1-Q3, 37%-25%). Two-way mixed repeated measures analysis of variance showed significant interaction between income classification level (HIC and LMIC) and type of outpatient anticoagulant use, which indicates that these 2 variables operate together to determine the extent to which each agent is used in an economy (Table 2; supplemental Appendix 2). Pairwise investigation of the main effects for income classification and for type of anticoagulant is shown in supplemental Appendix 3. Sensitivity analysis excluding institutions that did not follow up patients with mechanical valves or antiphospholipid syndrome showed only marginal changes in the results (supplemental Appendix 4).
Distribution of outpatient anticoagulant use and DOAC use according to income of country in which institutions were based
| Characteristic . | HICs (n = 59) . | LMICs (n = 26) . | P value∗ . |
|---|---|---|---|
| Outpatient anticoagulant use (median proportion, Q1-Q3) | |||
| DOACs | 45 (25-64) | 18 (5-53) | .02 for the interaction (see supplemental Appendix 2) |
| LMWH | 30 (15-47) | 47 (15-70) | |
| VKAs | 10 (10-25) | 15 (6-30) | |
| Percentage started on DOACs, n (%) | |||
| 0-10 | 34 (58) | 19 (73) | .47 |
| 11 to <25 | 9 (15) | 2 (8) | |
| 25 to <50 | 11 (19) | 2 (8) | |
| 50 to <75 | 2 (3) | 2 (8) | |
| ≥75 | 3 (5) | 1 (3) | |
| Percentage on long-term VKA/LMWH switched to DOACs, n (%)† | |||
| 0-10 | 14 (24) | 11 (44) | .20 |
| 11 to <25 | 13 (22) | 2 (8) | |
| 25 to <50 | 8 (13) | 5 (20) | |
| 50 to <75 | 14 (24) | 3 (12) | |
| ≥75 | 10 (17) | 4 (16) |
| Characteristic . | HICs (n = 59) . | LMICs (n = 26) . | P value∗ . |
|---|---|---|---|
| Outpatient anticoagulant use (median proportion, Q1-Q3) | |||
| DOACs | 45 (25-64) | 18 (5-53) | .02 for the interaction (see supplemental Appendix 2) |
| LMWH | 30 (15-47) | 47 (15-70) | |
| VKAs | 10 (10-25) | 15 (6-30) | |
| Percentage started on DOACs, n (%) | |||
| 0-10 | 34 (58) | 19 (73) | .47 |
| 11 to <25 | 9 (15) | 2 (8) | |
| 25 to <50 | 11 (19) | 2 (8) | |
| 50 to <75 | 2 (3) | 2 (8) | |
| ≥75 | 3 (5) | 1 (3) | |
| Percentage on long-term VKA/LMWH switched to DOACs, n (%)† | |||
| 0-10 | 14 (24) | 11 (44) | .20 |
| 11 to <25 | 13 (22) | 2 (8) | |
| 25 to <50 | 8 (13) | 5 (20) | |
| 50 to <75 | 14 (24) | 3 (12) | |
| ≥75 | 10 (17) | 4 (16) |
Fisher exact test.
“I don’t know” for n = 1.
Patients started on DOACs and switched to DOACs
Only 8 of 85 (9%) DOAC users indicated that more than half their patients were started on DOACs directly (including lead-in anticoagulation; Table 2), and only one-third (31/85 [36%]) switched more than half their patients on long-term VKAs or LMWH to DOACs. There was no statistically significant difference in the percentage started or switched to DOACs according to income classification (Table 2).
DOAC use according to affected vascular territory and patient population
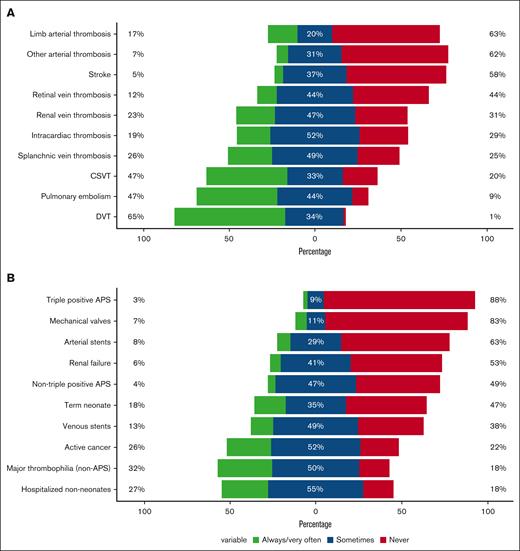
Figure 3 shows DOAC use according to thrombosis location for respondents providing care for affected patients (ie, excluding those who indicated that they did not provide care for patients affected by thrombosis in a given vascular territory or location). Overall, users commonly prescribed DOACs for the management of deep vein thrombosis and pulmonary embolism, and only 1% to 9% would “never” prescribe DOACs for these locations (Figure 3). In contrast, two-thirds of respondents (58%-63%) indicated that they would “never” prescribe DOACs for the management of arterial thrombosis, including arterial ischemic strokes.
DOAC prescription in specific scenarios. DOAC use according to thrombosis location (A) and patient population (B) among prescribers (n = 85). APS, antiphospholipid syndrome; CSVT, cerebral sinus venous thrombosis; DVT, to deep vein thrombosis.
DOAC prescription in specific scenarios. DOAC use according to thrombosis location (A) and patient population (B) among prescribers (n = 85). APS, antiphospholipid syndrome; CSVT, cerebral sinus venous thrombosis; DVT, to deep vein thrombosis.
When asked about DOAC prescription for specific patient populations, 78% to 82% of DOAC users stated that they “sometimes” to “always” prescribed DOACs to hospitalized children (nonneonates), patients with major thrombophilia (excluding antiphospholipid syndrome), and children with active cancer (Figure 3). Although 83% to 88% of respondents would “never” prescribe DOACs to children with mechanical valves or with triple-positive antiphospholipid syndrome, some respondents (3%-7%) indicated they “always/very often” used DOACs in these 2 contexts. Additional ineligible populations proposed by the respondents included patients with significant/severe liver disease or synthetic liver dysfunction; lack of established gastrointestinal tract (eg, because of malabsorption/total parenteral nutrition dependency/jejunal or nasogastric tube); renal dysfunction; vascular anomalies; catastrophic antiphospholipid syndrome; thrombotic storm; pregnancy; Ehlers-Danlos syndrome; recent neurosurgical procedure or intracranial bleeding; ventricular assist devices; younger patients because of problems with formulations; patients with a body weight of <2.6 kg or >100 to 140 kg; patients prescribed drugs that can lead to interactions; and patients with nonadherence.
DOAC protocols, bleeding, and perioperative management
Of 85 DOAC users, 33 (39%) indicated that their institution had a standardized protocol to guide DOAC reversal for bleeding events. As shown in Table 3, stopping DOACs was the most common strategy for the management of emergency surgery and major bleeding events. One-third to half of the respondents administered 4-factor prothrombin complex concentrate, (fresh) frozen plasma, and DOAC antidotes. Strategies indicated under the category “other” included tranexamic acid, local measures for bleeding events, and dialysis (if appropriate). One respondent indicated that perioperative management (from holding to reversing) depended on the type of surgery, and that they used DOAC level monitoring to guide the management of bleeding events.
Management strategies for emergency surgery and major bleeding among DOAC users (N = 85)
| Scenario . | Strategy . | n (%) . |
|---|---|---|
| Management strategy for children on DOACs undergoing emergency surgery | Stop DOAC | 81 (95) |
| Four-factor prothrombin complex concentrate | 39 (46) | |
| (Fresh) frozen plasma | 26 (31) | |
| DOAC antidote | 19 (22) | |
| Recombinant factor VIIa | 14 (16) | |
| Activated prothrombin complex concentrate | 14 (16) | |
| Three-factor prothrombin complex concentrate | 11 (13) | |
| Other | 7 (8) | |
| Management strategy for DOAC reversal in children with major bleeding | Stop DOAC | 77 (91) |
| Four-factor prothrombin complex concentrate | 45 (53) | |
| (Fresh) frozen plasma | 37 (44) | |
| DOAC antidote | 27 (32) | |
| Recombinant factor VIIa | 29 (34) | |
| Activated prothrombin complex concentrate | 17 (20) | |
| Three-factor prothrombin complex concentrate | 12 (14) | |
| Other | 11 (13) |
| Scenario . | Strategy . | n (%) . |
|---|---|---|
| Management strategy for children on DOACs undergoing emergency surgery | Stop DOAC | 81 (95) |
| Four-factor prothrombin complex concentrate | 39 (46) | |
| (Fresh) frozen plasma | 26 (31) | |
| DOAC antidote | 19 (22) | |
| Recombinant factor VIIa | 14 (16) | |
| Activated prothrombin complex concentrate | 14 (16) | |
| Three-factor prothrombin complex concentrate | 11 (13) | |
| Other | 7 (8) | |
| Management strategy for DOAC reversal in children with major bleeding | Stop DOAC | 77 (91) |
| Four-factor prothrombin complex concentrate | 45 (53) | |
| (Fresh) frozen plasma | 37 (44) | |
| DOAC antidote | 27 (32) | |
| Recombinant factor VIIa | 29 (34) | |
| Activated prothrombin complex concentrate | 17 (20) | |
| Three-factor prothrombin complex concentrate | 12 (14) | |
| Other | 11 (13) |
DOAC level monitoring
DOAC users most frequently had no access to drug-specific laboratory monitoring (30/85 [35%]). One-third monitored DOAC levels in some patients only (28/85 [33%]). At opposite ends, 15 users indicated they did not monitor levels (15/85 [18%]), whereas 12 monitored levels in most (9/85 [11%]) or all (3/85 [4%]) patients. The context for pursuing laboratory monitoring included concerns regarding gastrointestinal absorption (intestinal failure, short-gut syndrome, inflammatory bowel disease, and protein losing enteropathy), excretion (renal dysfunction), drug interactions, and patient adherence. Additional scenarios for level monitoring were emergency situations (eg, bleeding/need for reversal), prematurity or patients who were small for gestational age, patients with a body weight of <30 kg, patients who are overweight or with obesity, athletes, patients with recurrent thrombosis, situations in which it is critical to achieve acceptable levels, and nonstandard administration (such as tablet crushing/mixing with water).
Institutional changes after DOAC implementation
In terms of institutional adjustments among DOAC users, 42 of 85 (49%) created protocols, 27 of 85 (32%) made no changes, 18 of 85 (21%) introduced laboratory monitoring of levels, 17 of 85 (20%) included pharmacy support for DOAC management, 8 of 85 (9%) started an anticoagulation clinic, and 4 (5%) hired personnel. Other changes involved developing handouts for families, initiating an inpatient thrombosis service, training personnel, and implementing strategies to decrease costs for families (such as adding DOACs to the hospital formulary).
Barriers to DOAC use
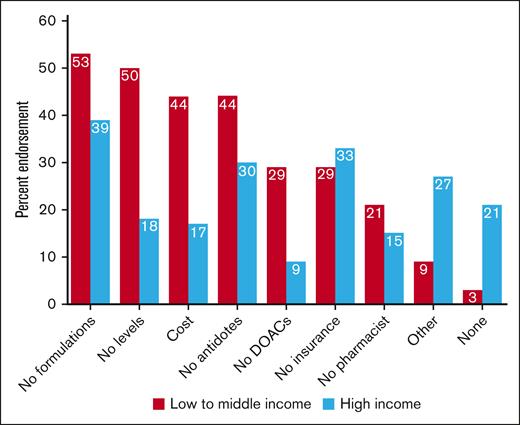
The most common barriers to using DOACs in children were the lack of pediatric formulations (44/100 [44%]), lack of specific antidotes (35/100 [35%]), lack of drug insurance coverage (32/100 [32%]), lack of access to laboratory monitoring (29/100 [29%]), drug cost (26/100 [26%]), other barriers (21/100 [21%]), no access to a pharmacist (17/100 [17%]), and no availability of DOACs (16/100 [16%]); 15 of 100 (15%) of respondents indicated that they encountered no barriers to DOAC at their institution. Additional barriers were lack/limited pediatric data for less conventional indications (such as childhood cancer); lengthy process to obtain approval/drug coverage; absence of national licensing; prescribing restrictions; acceptability by other specialists (cardiologists, intensivists); and limited experience, expertise, and knowledge (including, for example, periprocedural management).
When comparing barriers according to income classification (Figure 4), lack of pediatric formulations, no availability of laboratory monitoring, cost, and lack of access to specific antidotes were the most common barriers in LMICs, whereas lack of formulations, lack of coverage/insurance, lack of specific antidotes, and other barriers were more frequent in HICs. Endorsement to the following barriers was significantly different in HIC vs LMIC: DOACs not available (9% vs 29%, respectively; P = .009), lack of access to DOAC level monitoring (18% vs 50%, respectively; P < .001), cost (17% vs 44%, respectively; P = .003), and other barriers (27% vs 9%, respectively; P = .03). There was also a significant difference in the lack of barriers to DOAC use when comparing HICs (21% no barriers) vs LMICs (3% no barriers); P = .02.
Barriers to DOAC use by income of country in which institutions were based for all respondents (N = 100).
Barriers to DOAC use by income of country in which institutions were based for all respondents (N = 100).
General comments of the participants on the introduction of DOACs to pediatric clinical care largely highlighted the challenges (lack of pediatric formulations and associated variability in dosing, limited resources, constraints of local policies/regulations, costs/coverage, limited experience of providers, lack of levels or antidotes, and resistance to change or strong preferences by other teams because of legacy set up for anticoagulation management within the institution), but also mentioned advantages, including ease of use and lower treatment costs in some settings (“They have facilitated management,” “It has revolutionized the treatment of venous thromboembolism,” “DOACs have been revolutionary because of low cost in the low-middle-income setting. There is a huge difference in the cost of LMWHs versus DOACs”), and opportunities (“I am using the introduction of DOACs as a reason to create a thrombosis service and to standardise protocols throughout my hospital.”).
Discussion
This survey investigated the practices, challenges, and barriers related to the introduction of DOACs in the clinical setting from the perspective of practitioners involved in the care of children with or at risk of thrombotic events around the globe.
The sampling strategy used in the survey allowed us to collect diverse institutional perspectives as shown by the distribution of respondents per continent (24% from Asia, 56% from Europe and North America). This diversity is in line with the International Society on Thrombosis and Haemostasis membership distribution in 2020,37 which was 37% European, 31% North American, 11% Asian, 9% for Latin American, 6% for Oceanian, 3% for West Asian and Middle Eastern, and 3% African.
As expected, because of the multiple advantages of DOACs as compared with VKAs and LMWH,38 most of the respondents indicated that they used these agents at their institutions. There was no difference in DOAC use according to country income or volume of pediatric admissions per year. However, all the institutions with the highest volume of pediatric venous thrombotic events per year (>200) used DOACs, suggesting that providing care to a larger number of affected patients could catalyze the implementation of new management strategies, likely because of the availability of more resources and faster experience accrual.
DOACs use was only slightly higher in HIC vs LMIC institutions. It is possible that the global participation of multiple HIC and LMIC sites in the dabigatran and rivaroxaban pediatric trials facilitated the adoption of these agents globally.
Rivaroxaban and edoxaban were the most and least commonly used DOACs, respectively. These findings are in line with a recent report in adult patients analyzing the trends in DOAC consumption according to pharmaceutical sales data in 65 countries. The study showed rivaroxaban contributed to the largest proportion of DOAC consumption in 70% of the countries, whereas edoxaban accounted for <20%.26 Some reasons to explain the higher use of rivaroxaban in pediatrics include its early approval; availability as an oral solution; and existing clinical trial data for specific populations, such as patients with cancer39 and cardiac conditions.22
The lower use of VKAs in the outpatient setting reported in this study likely reflects its overall lower use in pediatrics. In the United States, LMWH was the most common anticoagulant used in hospitalized children with venous thrombotic events between 2001 and 2007, accounting for ∼30% to 50% of admissions with this diagnosis, in contrast to a 10% VKA use in the same context.1 A follow-up study showed that LMWH was still the most frequently used agent in 2019, accounting for ∼60% of the pediatric admissions with venous thrombosis, whereas the percentage of VKA use had declined to 4% and the use of DOACs had increased to 3% by the same year.2
In most centers, less than half the patients were started on DOACs or had been switched to DOACs. In fact, two-thirds of respondents indicated that they started ≤10% of their patients directly on DOACs (including lead-in phase) and that they had switched <50% of their patients on long-term anticoagulation to DOACs.
Most respondents would not use these anticoagulants for children with mechanical valves or triple-positive antiphospholipid syndrome. Two large randomized clinical trials investigated the efficacy of dabigatran40 and apixaban41 vs VKAs for the prevention of thromboembolic events in adult patients with mechanical valves. Both trials showed excess thromboembolic events in the DOAC arm, leading to their premature termination. Similarly, randomized trials in adults with antiphospholipid syndrome showed excess arterial events, including stroke, among those who received DOACs vs VKAs.42 These results, which led to the recommendation to avoid DOACs particularly in patients with triple-positive antiphospholipid syndrome or with antiphospholipid syndrome and a history of arterial events,43-49 explain the low use of DOACs in children with triple-antiphospholipid syndrome positivity reported in this survey.
Although one of the main advantages of DOACs has historically been the lack of or limited need for level monitoring, as their use expanded in adult clinical practice, it became clear that monitoring could be useful in 2 main scenarios: emergencies (bleeding events, emergency surgery) and suspected suboptimal dosing.50 The latter is relevant to pediatrics, because children who can be dosed using adult formulations without concerns regarding drug absorption, excretion, interactions, or weight extremes are not necessarily the norm. In fact, most thrombotic events affect younger patients,1,51 for whom pediatric formulations are not readily available in both HICs and LMICs. This makes it difficult to prescribe the exact dosing per weight reported in pediatric trials. The problem that arises with level monitoring, however, is that the interpretation of DOAC levels is unclear and known to be complex in adult patients in view of the significant interpatient and intrapatient variability.52,53
Lack of pediatric formulations was the main barrier, regardless of country income classification. Importantly, the equal frequency of respondents who had no barriers and those who had no access to DOACs (15% for both) indicates health inequalities in access to medication.
The accumulation of data from trials and real-world experience in adult patients led to the incorporation of DOACs to the Essential Medicines List in 2019,54 but they have yet to be included in the Essential Medicines List for Children (in which VKAs was included in the first pediatric edition in 2007; LMWH was included in 2019). Because the addition of medicines to the Essential Medicines List can trigger actions to improve equitable access and facilitate advocacy,55-57 pediatric studies that can support all the criteria for inclusion of DOACs (relevance, efficacy and safety, and cost-effectiveness) are necessary.
Country income affects the cost-effectiveness of DOACs. Systematic reviews of studies in adults with atrial fibrillation showed that whereas DOACs may be more cost-effective than VKAs in HICs, VKAs may be more cost-effective in LMICs.58,59 These cost-effectiveness results may also apply to LMWH, which is used more frequently than VKAs in children. For example, DOACS were found to be more cost-effective than LMWH for thromboembolism prevention after total hip or knee replacement in adults in HICs only.60 Overall, uptake of DOACs in pediatric clinical practice is influenced by formulation and indication approved by local regulatory agencies, further suggesting the importance of pediatric studies in these areas.
There are limitations to this study. Although we inquired about practices and challenges at the institutional level, it is possible that there might be variations in practice in centers with >1 thrombosis treater that were not be captured (eg, perception of barriers, or comfort using DOACs in different territories). Two strategies were in place to overcome this problem: the survey questions were designed to inquire about the health care institution, and the most experienced treaters were selected to answer the survey. In addition, although we aimed to represent the views of health care institutions around the world, the number of centers not identified, and therefore not invited to participate, remains unknown. However, every effort was made to identify treaters outside North America and Europe, as shown by the wide distribution of responding countries.
In conclusion, this survey shows the summary of the collective knowledge and experience accumulated since the introduction of DOACs in pediatric clinical practice, and highlights current challenges. The results emphasize the critical need for clinicians, researchers, and scientific organizations working together to build capacity, advocate for drug access and appropriate formulations, and generate the evidence needed to inform guidelines and policy.
The goal of these actions is to provide effective, safe, patient-centered quality health care that is timely, equitable, integrated, and efficient,61 to ultimately improve the outcomes of children at risk or affected by thrombotic events.
Authorship
Contribution: M.L.A. designed the study, supported study conduction, analyzed and interpreted the data, and wrote the manuscript; E.D.R. and J.V. critically reviewed the manuscript; and L.R.B. designed and conducted the study, interpreted the data, and critically reviewed the manuscript.
Conflict-of-interest disclosure: L.R.B. received research funding from Pfizer for participation in trial NCT02464969, entitled Apixaban for the Acute Treatment of Venous Thromboembolism in Children.
Correspondence: Leonardo R. Brandão, Division of Paediatric Haematology/Oncology, The Hospital for Sick Children, Tenth Floor, Room 10-14-038, 175 Elizabeth St, Toronto, ON M5G 2G3, Canada; email: leonardo.brandao@sickkids.ca.
References
Author notes
Original data are available on request from the corresponding author, Leonardo R. Brandão (leonardo.brandao@sickkids.ca).
The full-text version of this article contains a data supplement.