In this issue of Blood Advances, Darwin et al1 review a wide-ranging body of preclinical and early clinical studies that highlight the impact of antibiotics on the dynamic interactions between the intestinal microbiome and cellular therapies. Broad-spectrum antibiotics improve infection outcomes for immunosuppressive treatments such as allogeneic hematopoietic stem cell transplantation (allo-HCT) and immune effector cell therapies. However, as the authors show, the possible influence of these agents on the intestinal microbiome and consequently, on other cell therapy clinical outcomes, cannot be overstated.
In this review, the authors examine the role of antibiotics, particularly those that affect anerobic flora, on shifting the overall composition of the intestinal microbiome. For many patients undergoing allo-HCT, there is baseline intestinal microbial dysbiosis hallmarked by low microbial diversity and emergence of a dominant taxa.2 The baseline dysbiosis is then further exacerbated by antibiotic use, mucosal injury, diet changes, and the other changes inherent to the transplant process.3 Studies have correlated the severity of transplant-associated dysbiosis with critical outcomes. The most well-defined relationship is between microbiome disruption and the development of gastrointestinal (GI) graft-versus-host disease (GVHD).4 As work begins to uncover the underlying mechanisms underpinning these relationships, an area of interest includes the intersection of the microbiome and cellular metabolism. A healthy gut microbiome promotes the generation of metabolites such as the short chain fatty acid (SCFA) butyrate, which is essential for maintenance of the mucosal barrier, and plays a key role in immune regulation. Decreased butyrate production by the intestinal microbiome results from dysbiosis associated with antibiotics and GI GVHD.5 Prospective studies looking to restore the metabolic function of the microbiome in allo-HCT patients through prebiotic, probiotic, and postbiotic approaches are ongoing. Prior studies also correlate disruption of the microbiome with outcomes of immune effector cell therapy such as chimeric antigen receptor (CAR) T-cell therapy and tumor infiltrating lymphocytes. This points to the ability of the microbiome to affect distant and indirect immune signaling and composition. Large clinical studies reporting the effects of antibiotics on clinical outcomes are currently limited to CAR T-cell therapy. Studies by Smith et al6 and Stein-Thoeringer et al6,7 investigated the effect of exposure to antibiotics in the weeks preceding administration of CD19 CAR T cells. Both studies demonstrated inferior progression-free survival and overall survival in patients exposed to antibiotics before cell infusion. The effects were more pronounced when exposure to high-risk antibiotics was considered. Smith et al6 defined high-risk antibiotics as piperacillin-tazobactam, imipenem/cilastatin, and meropenem. Stein-Thoeringer et al7 included cefepime and ceftazidime in addition to piperacillin-tazobactam and meropenem. Interestingly, both studies noted an increased incidence of immune effector cell–associated neurotoxicity syndrome but not cytokine release syndrome in the high-risk antibiotic cohorts. As the authors note, in a syngeneic HCT model, administration of gut flora decontamination disrupted the blood brain barrier and allowed for more fluid passage of immune cells into the cerebrospinal fluid.8
There are multiple proposed trials studying approaches to manipulate the microbiome to harness its influence on the immune system, ameliorate engineered T-cell therapy toxicity and potentiate the response. Preclinical data showed that beta-hydroxybutyrate (BHB), the principal metabolite in ketosis, can support CAR T-cell proliferation.9 A clinical trial investigating the effects of administering BHB on the intestinal microbiome of lymphoma patients undergoing CD19 CAR T cells is underway. Previous studies showed that exposure to antibiotics that affect gram positive, obligate, anerobic intestinal microbes was a risk factor for worse outcomes with CAR T-cell therapy and it decreased prevalence of species that produce SCFAs was an independent risk factor for worse outcomes. Preclinical data showed that SCFAs butyrate and pentanoate led to elevated production/release of CD25, tumor necrosis factor-α, and interferon-gamma, leading to enhanced activity of cytotoxic T cells. Our group has shown that butyrate production by the microbiome could be augmented by administration of defined quantities of a prebiotic in the allo-HCT population.10 A similar approach would be of interest in adaptive cell therapies. We also agree with the authors that much work is left to be done to determine the appropriate approach to antibiotic stewardship in this patient population that is affected by profound and long-term immunosuppression.
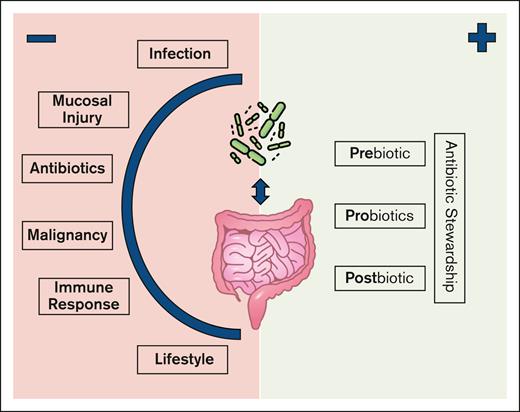
The microbiome is influenced by more than therapeutic antibiotics (see figure). Various other individual patient and disease factors play a role in shaping their composition. Furthermore, the web of individual signals, codependencies, spatial relationships, and downstream influences make isolating specific causal connections between the microbiome, the immune system, and patient outcomes challenging. Stein-Thoeringer et al7 identified weak associations between individual microbiome species and clinical outcomes. These associations became stronger when narrowed to patients that received high-risk antibiotics. Conversely, when Stein-Thoeringer et al7 applied these findings to a predictive model, the inclusion of patients that received the same high-risk antibiotics led to worse predictive accuracy in their validation cohort.
The intestinal microbiome in patients receiving cellular therapy is influenced by multiple patient and disease-specific factors. Possible interventions may target different phases to promote beneficial microbiome-host interactions.
The intestinal microbiome in patients receiving cellular therapy is influenced by multiple patient and disease-specific factors. Possible interventions may target different phases to promote beneficial microbiome-host interactions.
There is still much left to understand about the role of commensal microorganisms in both the genesis and treatment of cancer. For instance, significant work has focused on the GI flora’s impact on outcomes; however, the pulmonary system also has a distinct microbiome. The resident lung flora influences outcomes in pathologies such as sepsis and acute respiratory distress syndrome11 but we are only beginning to understand its impact on lung GVHD. Future work will inform how we can preserve a healthy microbiome and maximize its ability to make these dynamic therapies even more impactful.
Conflict-of-interest disclosure: The authors declare no competing financial interests.