Key Points
Outcomes in pediatric tAML have improved significantly. Prior radiation and adverse cytogenetics are the most relevant prognosticators.
Patients who received transplant with NEL with 2 instead of 4 preceding induction cycles achieved the best survival rates.
Visual Abstract
Therapy-related acute myeloid leukemia (AML; tAML) is one of the most feared therapy-emergent complications. This study aims to determine the clinical and pathological significance and define therapeutic implications and poor prognosticators in pediatric tAML. We analyzed a total of 119 pediatric patients (aged 2-20 years) who were centrally diagnosed with tAML within the Acute Myeloid Leukemia Berlin-Frankfurt-Münster (AML-BFM) study group between 1993 and 2019. Compared with de novo AML, tAML was associated with decreased white blood count and involvement of the central nervous system. Latency to tAML was inversely correlated with age at primary malignancy. Patients with tAML were more likely to have abnormal karyotypes, overrepresenting KMT2A rearrangements, the unfavorable cytogenetics –7/del(7q), as well as complex and monosomal karyotypes, whereas core-binding AML was underrepresented. The occurrence of stratification-relevant molecular genetics was comparable with de novo AML, whereas CEBPAdm was absent in tAML. Survival rates in tAML improved from 10% ± 6% in AML-BFM 1993/1998 to 50% ± 10% in the registries 2012/2017; however, this is still worse than de novo AML. Hematopoietic stem cell transplantation (HSCT) in no evidence of leukemia (NEL; <5% blasts) after 2 induction cycles greatly improved survival. Adverse cytogenetics, previous ionizing radiation (>35 Gy), and latency ≤1 year were identified as the strongest poor prognosticators. Over the past 26 years, outcome and survival significantly improved in pediatric tAML. Our results suggest that HSCT in NEL after 2 induction cycles is the most promising therapeutic approach for achieving improved survival. Radiation, adverse cytogenetics, and latency ≤1 year should be considered as poor prognosticators in pediatric tAML.
Introduction
During the past decades, improved prognosis in cancer has been achieved through optimized cytotoxic therapies. However, these modalities are still associated with toxicity-related complications. Therapy-related acute myeloid leukemia (AML; tAML) is one of the severest complications after chemotherapy (CTx), ionizing radiation therapy (RT), or immunosuppressive therapy of antecedent disease.1
According to the fifth edition of the 2022 World Health Organization classification, tAML is grouped together with therapy-related myelodysplastic syndrome and myeloproliferative neoplasms as therapy-related myeloid neoplasms.2 In recent years, the clinical relevance of tAML has grown, because more patients were diagnosed with cancer and became eligible for intensive poly-CTx, resulting in improved survival rates and higher risk of therapy-related malignancies.3,4 Although tAML has long been assumed to mainly arise from CTx-induced DNA damages, pathogenesis has now been attributed to a dynamic, clonal-driven process with multiple mechanisms, including the induction of pro-oncogenic mutation, genomic instability, and selection of resistant preleukemic clones, prompting evolution to tAML in a treatment-induced abnormal environment.1,5,6 Clinically, therapy-related toxicities substantially complicate therapeutic management, resulting in poor outcome and survival.7-11 In adults, tAML has mainly been associated with the accumulation of leukemia- and patient-related risk factors, including adverse cytogenetics, older age, and comorbidities.10,12,13 However, in pediatrics, little is known about the clinical and pathological significance of this difficult-to-treat entity.9,11,14,15 Thus, careful evaluation of prognosticators and pathobiology are required to mitigate unfavorable courses of diseases, optimize treatment and management, and ultimately improve outcome in pediatric tAML. This multicenter analysis evaluates the clinicopathological significance of tAML in a large pediatric cohort diagnosed over 26 years. Moreover, we report on prognostic achievements, therapeutic developments, and strategies that have been applied over the past 3 decades within the German Acute Myeloid Leukemia Berlin-Frankfurt-Münster (AML-BFM) study group. Finally, we conclude with tAML-specific therapeutic strategies that should form the basis for improved treatment guidelines by the AML-BFM group.
Methods
Within 5 different study periods of the AML-BFM study group in Germany between 1993 and 2019, a total of 119 patients were diagnosed with tAML according to the current World Health Organization criteria.2 All patients with tAML considered for analysis have a history for CTx, ionizing RT, or immunosuppressive therapy for a previous, unrelated disease. Sixty-one patients with tAML were compared with 944 patients with de novo AML, both groups were diagnosed from 2004 to 2019 (supplemental Table 1; supplemental Figure 1). Pediatric patients with de novo AML were enrolled within the AML-BFM study 2004 (04) and the registries 2012 and 2017 (R12/17), as reported previously.16-18 Patients with missing essential data (n = 8) and those who were not initially diagnosed with tAML within the AML-BFM study group or received pretreatment for tAML at a foreign center (n = 24) were excluded (supplemental Table 1; supplemental Figure 1).
AML was treated according to the respective treatment recommendations of the corresponding AML-BFM studies. Patients with tAML who were eligible for hematopoietic stem cell transplantation (HSCT) received 4 or 2 induction cycles according to the respective recommendations in AML-BFM 1993/1998 (93/98), 04, or R12/17, before HSCT. All AML-BFM studies and registries were approved by the ethic committees and conducted according to the Declaration of Helsinki and the principles of Good Clinical Practice. Written informed consent was obtained from each patient and their legal guardians.
Genetic analysis
AML samples were centrally characterized for morphology following the French-American-British (FAB) classification, as well as for immunophenotype and genetic profile, as part of the standard diagnostic procedures at the AML-BFM laboratories in Münster (for AML-BFM 93 and 98), Hannover (AML-BFM 04 and R12), and Essen, Germany. Classical cytogenetics were centrally analyzed in the AML-BFM reference laboratories in Giessen (AML-BFM 93 and 98) and Hannover, Germany (AML-BFM 04, R12, and R17).19,20 Karyotypes were described following the International System of Human Cytogenetic Nomenclature. A total of 54 AML-related genes were evaluated by next-generation sequencing (NGS) using the TruSight Myeloid Panel (Illumina, San Diego, CA) in 26 patients with tAML and 444 with de novo AML who were diagnosed since 2012. Only molecular genetic variants assessed as pathogenetically relevant were considered for mutational analysis.
Statistics
Definition of treatment response was based on the Cancer and Leukemia Group B criteria for complete remission (CR), CR with partial regeneration (CRp), and nonresponse (NR).21 No evidence of leukemia (NEL) was morphologically defined as <5% blasts in bone marrow or peripheral blood, without extramedullary involvement. Latency to tAML was defined as the time period from primary disease to the diagnosis of tAML. Death within the first 6 weeks of treatment was considered early death. Overall survival (OS) was defined as the time from diagnosis to death or last follow-up and event-free survival (EFS) as the time to relapse, secondary malignancy, death, or the date of last follow-up. For survival analyses of HSCT-associated subgroups, the time from HSCT (in tAML) to the corresponding event or censoring was evaluated. Survival rates were estimated by the Kaplan-Meier method, and the differences were calculated by 2-sided log-rank test. Patient characteristics between groups were compared using Pearson χ2 or Fisher exact testing for categorical variables and Mann-Whitney U or unpaired Welch t test for continuous variables. A P value <.05 was considered statistically significant. Hazard ratios (HRs) and 95% confidence intervals were calculated using the Cox proportional hazard model. For statistical analysis and illustrations, SPSS software version 28.0 (Chicago, IL), R software version 4.0.2, and GraphPad Prism 8.3 were applied. Python 3.9.13 environment with pandas 2.2.0 was used for data frame operations, lifelines 0.28.0 for survival analysis, and matplotlib 3.8.2 for graph generation.
Results
Characteristics of pediatric tAML
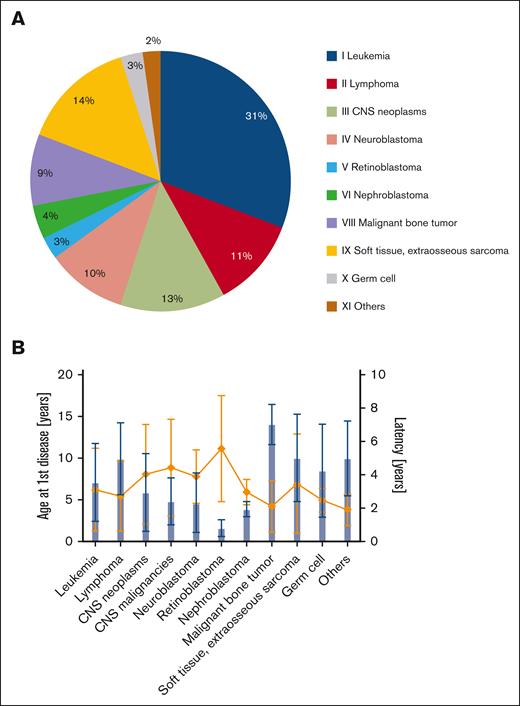
In the period between 1993 and 2019, tAML was diagnosed in 119 children with a history of cytotoxic therapy for an unrelated disease within the AML-BFM study group (supplemental Table 1). Over the study period, the annual frequency of tAML largely remained stable. Patients were aged between 2.2 and 20 years, with an even sex ratio of 1:1 (Table 1). Most of the patients (57%) were diagnosed and treated for solid tumors as primary cancer. Among these, soft tissue sarcomas were the most frequent ones (14%), followed by central nervous system (CNS) neoplasms (13%), including CNS malignancies (9%; Figure 1A). At the time of primary disease, 31% of patients were diagnosed with hematologic malignancies (leukemia) and 11% with lymphomas. Six children (5%) presented with nonmalignant diseases, including 5 with low-grade CNS tumors and 1 with hemophagocytic lymphohistiocytosis. On average, the age at primary disease was 7.8 years (range, 0.3-18), and the mean latency to tAML was 3.3 years (range, 0.2-13.4), with a median latency of 2.3 years (Figure 1B). Correlation analysis suggests a weak inverse relationship between latency to tAML and age at the time of primary disease (r = –0.2; P= .03; supplemental Figure 2). For the initial disease, most patients (89%) received intensive poly-CTx, whereas a minority of 11% had low-intensity or no CTx. Among patients who underwent cytotoxic pretreatment, 90% received alkylating agents as the treatment for their tAML-preceding malignancy, followed by mitotic inhibitors (84%), topoisomerase inhibitor (80%), and anthracyclines (77%; supplemental Table 2A). Twenty-nine patients with tAML (24%) received RT (≥35 Gy) as part of their treatment at the time of primary diagnosis, and 26 of them concomitantly received CTx.
Clinical characteristics in pediatric tAML, diagnosed between 1993 and 2019
| Variables . | tAML . | tAML . | De novo AML . |
|---|---|---|---|
| Total cohort, N | 119 | 61 | 944 |
| Period of sampling | 1993-2019 | 2004-2019 | |
| Gender, female, n (%) | 57 (48) | 29 (48) | 474 (50) |
| Age, median (range), y | 11.1 (2.2-20) | 11 (2-19) | 8.4 (0-19) |
| FAB classification, n (%) | |||
| M0 | 9 (8) | 1 (1.6) | 27 (3.2) |
| M1 | 11 (10) | 4 (6.5) | 115 (13) |
| M2 | 15 (13) | 7 (11) | 200 (24) |
| M4 | 29 (26) | 14 (22) | 127 (15) |
| M4eo | 2 (2) | 1 (1.6) | 68 (8.1) |
| M5 | 43 (38) | 28 (45) | 193 (23) |
| M6 | 3 (3) | 1 (1.6) | 13 (1.5) |
| M7 | 1 (1) | 0 (0) | 78 (9.3) |
| Other | 3 (3) | 4 (6.5) | 9 (1.0) |
| n.a. | 6 (5) | 2 (3.2) | 115 (12) |
| CNS involvement, n (%) | 32 (27) | 13 (21) | 106 (12) |
| WBC, ×109/L | |||
| Mean (range) | 36 (0.5-263) | 33 (1-211) | 47 (0.3-585) |
| n.a. | 5 | 1 | 125 |
| Platelet, ×109/L | |||
| Mean (range) | 79 (8-403) | 74 (8-305) | 86 (2-897) |
| n.a. | 9 | 0 | 123 |
| Bone marrow blasts, % | |||
| Mean (range) | 66 (4-99) | 65 (4-99) | 64 (3-99) |
| n.a. | 0 | 0 | 133 |
| Peripheral blood blasts, % | |||
| Mean (range) | 43 (0-99) | 37 (0-96) | 45 (0-99) |
| n.a. | 1 | 0 | 190 |
| Cytogenetics, n (%) | |||
| Normal | 16 (16) | 8 (14) | 185 (20) |
| Complex∗ | 24 (24) | 12 (21) | 149 (16) |
| CBF | 3 (3.0) | 2 (3.2) | 192 (21) |
| t(8;21)(q22;q22), RUNX1::RUNX1T1 | 1 (1.0) | 1 (1.7) | 115 (12) |
| inv(16)/t(16;16)(p13;q22), CBFB::MYH11 | 2 (2.0) | 1 (1.7) | 77 (8.5) |
| KMT2Ar, 11q23 | 47 (47) | 31 (55) | 200 (22) |
| t(9;11)(p21;q23), KMT2A::MLLT3 | 27 (27) | 16 (29) | 90 (9.9) |
| t(11;19)(q23;p13.3), KMT2A/MLLT1 | 3 (3.0) | 3 (5.3) | 16 (1.7) |
| t(4;11)(q21;q23), KMT2A/AFF1 | 3 (3.0) | 2 (3.6) | 3 (0.3) |
| –5/del(5q) | 5 (5.1) | 3 (5.3) | 16 (1.7) |
| –7/del(7q) | 12 (12) | 6 (11) | 32 (3.5) |
| Trisomy +8 | 7 (7.1) | 6 (11) | 109 (12) |
| Monosomy | 21 (21) | 11 (20) | 66 (7) |
| Missing | 20 (17) | 5 (7.9) | 39 (4.1) |
| Molecular genetics, n (%) | |||
| CEBPAdm | 0 (0) | 0 (0) | 26 (6) |
| NPM1 | 1 (4) | 1 (4) | 33 (7) |
| FLT3-ITD# | 2 (8) | 2 (8) | 56 (13) |
| WT1 | 2 (8) | 2 (8) | 40 (9) |
| NRAS | 3 (12) | 3 (12) | 60 (13) |
| KRAS | 2 (8) | 2 (8) | 21 (5) |
| Missing | 35 (57) | 35 (57) | 445 (53) |
| Variables . | tAML . | tAML . | De novo AML . |
|---|---|---|---|
| Total cohort, N | 119 | 61 | 944 |
| Period of sampling | 1993-2019 | 2004-2019 | |
| Gender, female, n (%) | 57 (48) | 29 (48) | 474 (50) |
| Age, median (range), y | 11.1 (2.2-20) | 11 (2-19) | 8.4 (0-19) |
| FAB classification, n (%) | |||
| M0 | 9 (8) | 1 (1.6) | 27 (3.2) |
| M1 | 11 (10) | 4 (6.5) | 115 (13) |
| M2 | 15 (13) | 7 (11) | 200 (24) |
| M4 | 29 (26) | 14 (22) | 127 (15) |
| M4eo | 2 (2) | 1 (1.6) | 68 (8.1) |
| M5 | 43 (38) | 28 (45) | 193 (23) |
| M6 | 3 (3) | 1 (1.6) | 13 (1.5) |
| M7 | 1 (1) | 0 (0) | 78 (9.3) |
| Other | 3 (3) | 4 (6.5) | 9 (1.0) |
| n.a. | 6 (5) | 2 (3.2) | 115 (12) |
| CNS involvement, n (%) | 32 (27) | 13 (21) | 106 (12) |
| WBC, ×109/L | |||
| Mean (range) | 36 (0.5-263) | 33 (1-211) | 47 (0.3-585) |
| n.a. | 5 | 1 | 125 |
| Platelet, ×109/L | |||
| Mean (range) | 79 (8-403) | 74 (8-305) | 86 (2-897) |
| n.a. | 9 | 0 | 123 |
| Bone marrow blasts, % | |||
| Mean (range) | 66 (4-99) | 65 (4-99) | 64 (3-99) |
| n.a. | 0 | 0 | 133 |
| Peripheral blood blasts, % | |||
| Mean (range) | 43 (0-99) | 37 (0-96) | 45 (0-99) |
| n.a. | 1 | 0 | 190 |
| Cytogenetics, n (%) | |||
| Normal | 16 (16) | 8 (14) | 185 (20) |
| Complex∗ | 24 (24) | 12 (21) | 149 (16) |
| CBF | 3 (3.0) | 2 (3.2) | 192 (21) |
| t(8;21)(q22;q22), RUNX1::RUNX1T1 | 1 (1.0) | 1 (1.7) | 115 (12) |
| inv(16)/t(16;16)(p13;q22), CBFB::MYH11 | 2 (2.0) | 1 (1.7) | 77 (8.5) |
| KMT2Ar, 11q23 | 47 (47) | 31 (55) | 200 (22) |
| t(9;11)(p21;q23), KMT2A::MLLT3 | 27 (27) | 16 (29) | 90 (9.9) |
| t(11;19)(q23;p13.3), KMT2A/MLLT1 | 3 (3.0) | 3 (5.3) | 16 (1.7) |
| t(4;11)(q21;q23), KMT2A/AFF1 | 3 (3.0) | 2 (3.6) | 3 (0.3) |
| –5/del(5q) | 5 (5.1) | 3 (5.3) | 16 (1.7) |
| –7/del(7q) | 12 (12) | 6 (11) | 32 (3.5) |
| Trisomy +8 | 7 (7.1) | 6 (11) | 109 (12) |
| Monosomy | 21 (21) | 11 (20) | 66 (7) |
| Missing | 20 (17) | 5 (7.9) | 39 (4.1) |
| Molecular genetics, n (%) | |||
| CEBPAdm | 0 (0) | 0 (0) | 26 (6) |
| NPM1 | 1 (4) | 1 (4) | 33 (7) |
| FLT3-ITD# | 2 (8) | 2 (8) | 56 (13) |
| WT1 | 2 (8) | 2 (8) | 40 (9) |
| NRAS | 3 (12) | 3 (12) | 60 (13) |
| KRAS | 2 (8) | 2 (8) | 21 (5) |
| Missing | 35 (57) | 35 (57) | 445 (53) |
Patients with tAML compared with de novo AML diagnosed between 2004 and 2019.
n.a., not available; ITD, internal tandem duplication.
Complex karyotype defined as ≥3 chromosomal aberrations, including at least 1 structural aberration.
Distribution of primary disease–related characteristics in pediatric tAML. (A) Distribution of primary diseases preceding tAML in 119 pediatric patients, classified according to the International Classification of Childhood Cancer-3 (ICCC-3). (B) Age at primary disease (blue) and latency period to tAML (orange) in years by different antecedent diseases. ICCC-3 XI others includes 2 patients previously diagnosed with squamous cell carcinoma and hemophagocytic lymphohistiocytosis.
Distribution of primary disease–related characteristics in pediatric tAML. (A) Distribution of primary diseases preceding tAML in 119 pediatric patients, classified according to the International Classification of Childhood Cancer-3 (ICCC-3). (B) Age at primary disease (blue) and latency period to tAML (orange) in years by different antecedent diseases. ICCC-3 XI others includes 2 patients previously diagnosed with squamous cell carcinoma and hemophagocytic lymphohistiocytosis.
tAML compared with de novo AML
From 2004 to 2019, tAML was compared with patients with de novo AML enrolled in AML-BFM 04 and AML-BFM R12/17, representing the German pediatric AML cohort (Table 1). Among all patients with AML, tAML occurred in 61 of 1005 pediatric patients (6.1%) and was significantly correlated with older age (11 vs 8.4 years; P= .005). Gender was evenly distributed between both cohorts (female, 48% vs 50%). tAML was more frequently assigned as monocytic phenotype (AML-FAB M5) than de novo AML (45% vs 23%; P < .001), whereas AML-FAB M2 was underrepresented (11% vs 24%; P= .03), and megakaryoblast morphology was absent in the tAML cohort (0% vs 9.3%; P= .01). CNS was more frequently involved in tAML (21% vs 12%; P= .04). However, in patients having a history of antecedent CNS diseases, the number of CNS-positive tAML (3/32 [9%]) was not increased compared with CNS-negative tAML (12/87 [14%]; χ2 [1, N = 119] = 0.54; P= .5). The proportions of leukemic blasts in bone marrow were comparable between both cohorts (65% vs 64%; P= .7), whereas the proportion of blasts in peripheral blood was slightly reduced in tAML compared with de novo AML (37% vs 45%; P= .09). At the time of diagnosis, pediatric patients with tAML presented with lower platelets (74×109/L vs 86×109/L; P= .1) and white blood cell (WBC) count (33×109/L vs 47×109/L; P= .04) than de novo AML. WBC count and platelets were particularly reduced in patients with tAML who were previously exposed to spinal and lumbosacral RT (16×109/L vs 47×109/L; P= .004 and 69×109/L vs 86×109/L; P= .04, respectively). The high-risk cytogenetic aberrations –7/del(7q) and monosomic karyotype were significantly overrepresented in patients with tAML (11% vs 4% [P= .02] and 20% vs 7% [P= .006]). A complex karyotype was detected in 21% of tAML compared with 16% in the de novo AML group (P = .4). The favorable core-binding factor (CBF) fusions RUNX1::RUNX1T1 and CBFB::MYH11 were underrepresented in tAML (1.7% vs 12% [P= .01] and 1.7% vs 8.5% [P= .05], respectively). Compared with de novo AML, KMT2A rearrangement (KMT2Ar) was overrepresented in tAML, making it the most common balanced translocation (55% vs 22%; P < .001). This was particularly evident for the fusion KMT2A::MLLT3 (29 vs 9.9%; P < .001). KMT2Ar-tAML tended to associate with exposure to topoisomerase inhibitor in primary malignancy (41% vs 29%; P= .08), whereas there was no other strong association between further primary disease– and tAML-related characteristics (supplemental Table 2B). High-throughput sequencing by NGS revealed even distribution of the stratification-relevant molecular genetics FLT3-internal tandem duplication, NPM1, and WT1 in tAML and de novo AML (8% vs 13% [P= .8]; 4% vs 7% [P= .7]; and 8% vs 9% [P= .9], respectively), whereas CEBPAdm was completely absent in tAML (0% vs 6%; P= .9; supplemental Table 3).
Evolving outcome and survival in tAML
Over the past 26 years, the 10-year OS and EFS rates for tAML increased significantly from AML-BFM 93 (10% ± 5.5% and 6.7% ± 4.6%) to R12/17 (50% ± 9.8% and 45.5% ± 9.9%, respectively; stratified log-rank P < .001; Figure 2). Similarly, CR and NR rates improved from 34% to 69% (P= .004) and 59% to 19% (P < .001), respectively (supplemental Table 4). However, over the last 15 years, survival rates in tAML were still inferior to those in de novo AML (OS, 37% ± 6.2% vs 77.9% ± 1.4%; EFS, 36.1% ± 6.2% vs 60.7% ± 1.6%; P < .001), with largely comparable OS and EFS rates in tAML (Figure 3). In tAML, response to therapy was inferior compared with de novo AML, with markedly decreased rates of NEL (69% vs 89%; P < .001) and higher rates of NR to induction therapy (31% vs 10%; P < .001; supplemental Table 4B). In cause-specific multivariable regression models adjusted for HSCT, high-risk AML, complex karyotype, KMT2Ar, and age, tAML carried the highest relative risk for poor OS (HR, 4.8; P < .001) and EFS (HR, 2.6; P < .001; supplemental Table 5).
Probability of OS and EFS in pediatric tAML stratified by AML-BFM study periods over the past 26 years. The median follow-up was 14.4 years for (A) OS (95% confidence interval [CI], 11.3-17.6) and 14.4 years for (B) EFS (95% CI, 11.3-17.3).
Probability of OS and EFS in pediatric tAML stratified by AML-BFM study periods over the past 26 years. The median follow-up was 14.4 years for (A) OS (95% confidence interval [CI], 11.3-17.6) and 14.4 years for (B) EFS (95% CI, 11.3-17.3).
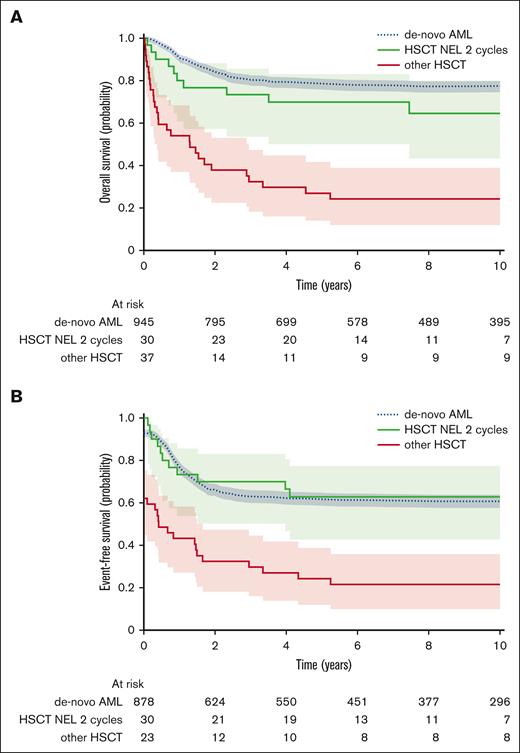
Probability of OS and EFS stratified by treatment regimes in pediatric tAML compared with de novo AML diagnosed from 2004. HSCT NEL was morphologically defined <5% blasts and no extramedullary blasts.
Probability of OS and EFS stratified by treatment regimes in pediatric tAML compared with de novo AML diagnosed from 2004. HSCT NEL was morphologically defined <5% blasts and no extramedullary blasts.
Therapeutic implication in pediatric tAML
Treatment recommendations for tAML have changed from 4 induction cycles in AML-BFM 93 and 98 to 2 induction cycles in R12/17. This change in treatment strategy was associated with improvements in 10-year survival rates of 55.1% ± 9% vs 25.7% ± 8% OS (stratified log-rank P = .03) and 53.6% ± 8% vs 22.3% ± 8% EFS (stratified log-rank P = .02; supplemental Table 6). The improved outcome was accompanied by higher transplantation rates in patients with tAML recognized within the AML-BFM R12/17 arm than the AML-BFM 93/98 arm (92% vs 34%; P < .001). Most patients in both groups underwent HSCT in NEL (80% and 88%). Although the proportions of HSCT in CR (46%) and CRp (42%) were almost equal in AML-BFM R12/17, in 93/98, more patients received HSCT in CR (65%; P= .2) and fewer in CRp (15%; P= .05). Although the 10-year survival rates were only slightly superior in patients with tAML who underwent transplant in CR (OS, 53.2% ± 8%; EFS, 51.4% ± 8%) compared with those undergoing HSCT in CRp (OS, 47.4% ± 12%; EFS, 42.9% ± 12%; stratified log-rank P= .9 and .7, respectively), HSCT in NEL compared with HSCT with evidence of leukemia (>5% blasts) significantly improved the 10-year OS to 51.6% ± 7% vs 4.8% ± 3% (stratified log-rank P < .001) and the 10-year EFS to 48.5% ± 7% vs 4.8% ± 3% (stratified log-rank P < .001; supplemental Table 6). Importantly, HSCT in NEL after 2 induction cycles achieved even superior survival rates of 64.5% ± 9% 10-year OS and 62.8% ± 9% EFS, which almost matched the OS of 77.4% ± 1% and EFS of 60.7% ± 2% achieved in de novo AML (stratified log-rank P= .1 and .8, respectively) for the same period (Figure 3). In contrast, patients who did not undergo HSCT had devastating outcomes, with 10-year OS and EFS rates of 5.9% ± 3%, and all patients undergoing HSCT in NR died (10-year OS, 0% ± 0%).
tAML-related prognosticators
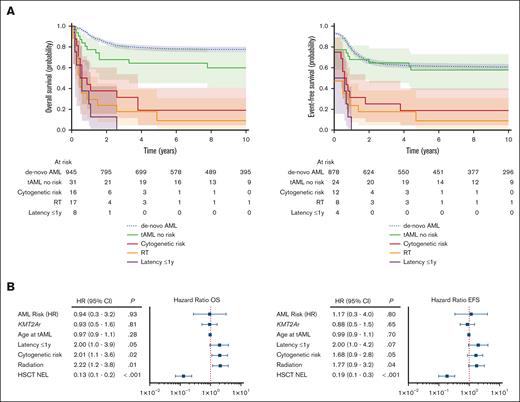
To detect tAML-related prognostic markers, survival in disease- and patient-related subgroups were differentially assessed (Figure 4). Ten-year OS and EFS rates were significantly worse in tAML harboring adverse cytogenetics –7/del(7q) and complex and monosomy karyotypes (supplemental Table 7). Prior exposure to RT was strongly linked with adverse cytogenetics in tAML (46% vs 17%; P= .002), compared with tAML without a history of RT. In addition, previously RT-treated patients with tAML had worse 10-year OS (5.2% ± 4.6%) and 10-year EFS (5.2% ± 4.6%) than those having no previous RT (OS, 33.6% ± 5.2%; EFS, 31.7% ± 5%; stratified log-rank P= .003 and .004, respectively; Figure 4A). We found the strongest prognostic power for a latency period to tAML of 1 year. Latency to tAML ≤1 year and preceding CNS malignancies showed decreased 10-year OS (0% ± 0% vs 29.4% ± 4.5% and 0% ± 0% vs 27.8% ± 4.7%) and EFS rates (0% ± 0% vs 27.87% ± 4.4% and 0% ± 0% vs 25.8% ± 4.6%; stratified log-rank for OS, P < .001 and P= .001; for EFS, P= .003 and .002, respectively), whereas other primary disease–related characteristics did not relevantly affect survival in tAML. In proportional hazard models adjusted for relevant confounders, prior RT showed the strongest poor prognostic power, followed by adverse cytogenetics and latency ≤1 year for OS (HR, 2.1 [P= .01]; HR, 2.0 [P= .02]; HR, 2 [P= .05]) and EFS (HR, 1.8 [P= .04]; HR, 1.7 [P= .05]; HR, 2.0 [P= .07], respectively; Figure 4B). In contrast to de novo AML, high-risk allocation as performed for de novo AML according to the corresponding AML-BFM protocols showed no prognostic significance in the tAML cohort (OS [HR, 0.9; P= .8] and EFS [HR, 1.3; P= .7]; supplemental Table 5).
tAML-related prognostic factors. (A) Probability of OS and EFS stratified by risk-related factors in pediatric tAML compared with de novo AML diagnosed from 2004. The median follow-up was 10.9 years for OS (95% CI, 10.2-11.5) and 10.5 years for EFS (95% CI, 9.9-11.2). (B) Forest plots of multivariate proportional hazards models for OS and EFS in pediatric tAML. Latency ≤1 year to tAML; cytogenetic risk encompassed –7/del(7q), monosomy, and complex karyotype; HSCT NEL is morphologically defined as <5% blasts and no extramedullary blasts. AML-HR, high-risk assignment according to the corresponding AML-BFM protocol; KMT2Ar, lysine methyltransferase 2A rearrangement.
tAML-related prognostic factors. (A) Probability of OS and EFS stratified by risk-related factors in pediatric tAML compared with de novo AML diagnosed from 2004. The median follow-up was 10.9 years for OS (95% CI, 10.2-11.5) and 10.5 years for EFS (95% CI, 9.9-11.2). (B) Forest plots of multivariate proportional hazards models for OS and EFS in pediatric tAML. Latency ≤1 year to tAML; cytogenetic risk encompassed –7/del(7q), monosomy, and complex karyotype; HSCT NEL is morphologically defined as <5% blasts and no extramedullary blasts. AML-HR, high-risk assignment according to the corresponding AML-BFM protocol; KMT2Ar, lysine methyltransferase 2A rearrangement.
Discussion
This large-scale analysis comprehensively assesses the evolving significance of pediatric tAML within the German AML-BFM study group over the past 26 years. We show improving outcomes, highlight therapeutic implications, and identify prognostic markers, defining a heretofore undescribed risk population in tAML. Between 2004 and 2019, the estimated frequency of AML was 6%, which is comparable with previous studies in adult tAML.7,8,22 Population-based data suggest a stable proportion of tAML among AML over the observation period. Similar to previous reports,3,4,7,8,15,22 our results reveal significantly worse treatment response and survival rates in tAML than de novo AML. However, here, we demonstrate a dramatic improvement in outcome in pediatric tAML over the past 3 decades. Prognostic differences became particularly clear when comparing patients with tAML treated according to the AML-BFM 93/98 recommendations with those treated within AML-BFM R12/17. Besides well-known improvements in the quality of supportive care and refined transplant modalities, major differences between both treatment groups were the reduction from 4 to 2 induction cycles before HSCT and a higher proportion of HSCT in NEL/CRp in AML-BFM R12/17. In view of the only slight differences in prognosis between HSCT in CR and CRp, we conclude that there is no benefit of waiting for full hematologic recovery after achieving NEL (<5% blasts) before proceeding to HSCT. Results of survival analysis indicate that HSCT in NEL substantially contributes to improved survival in tAML, and the clinical importance becomes particularly evident facing fatal prognosis in patients who underwent transplant in NR or did not undergo HSCT. The improved outcomes after 2 induction cycles suggest that 2 courses of CTx, rather than 4, are not only sufficient but likely the best approach before HSCT. This approach also reduces therapy-related toxicities, which is particularly beneficial for patients with tAML who have already received first-line treatment. Reducing CTx intensity is both feasible and strategically advantageous for better patient outcomes. Ultimately, the prognosis of patients with tAML who underwent transplant in NEL after 2 induction cycles almost equaled that of de novo AML. Therefore, our results indicate that HSCT in NEL after 2 induction cycles is the most promising therapeutic approach to improve survival in pediatric tAML. To further refine treatment recommendations for pediatric tAML, incorporating MRD data, particularly flow cytometry–based measurable residual disease (MRD), might be valuable. Given the historical and comprehensive nature of the data set and that MRD was not established as a standard procedure at the time most patients were diagnosed, further research incorporating MRD data would be needed to enhance treatment recommendations for pediatric tAML.
Although EFS has widely been acknowledged as a reliable and accurate end point that is less affected by HSCT or salvage therapy,23 our data reveal that OS and EFS were virtually indistinguishable in tAML, suggesting that patients with tAML are unlikely to be salvaged in case of further events, thus supporting the need for refined therapeutic regimes as first-line treatment in tAML.
Considering unfavorable outcomes, previous RT, associated adverse cytogenetics, including complex karyotype, monosomy, and –7/del(7q), and latency ≤1 year were identified as the most powerful prognosticators independently predicting poor survival in pediatric tAML. Because the probability of survival in patients with tAML lacking these risk markers almost reached the level of patients with de novo AML, it seems likely that the identified risk factors relevantly contribute to poor prognosis in pediatric tAML. In contrast to the identified poor prognosticators, high-risk assignment stratification as it is performed for de novo AML showed no prognostic relevance in tAML, which may point to different underlying risk-related factors driving unfavorable outcomes in tAML and de novo AML and may further support the notion that tAML and de novo AML present 2 distinct entities.
In line with previous reports,4,8,22 our data reveal a relevant overrepresentation of adverse cytogenetics in pediatric tAML, enforcing the pathogenetic impact in tAML. Although CBF-translocated tAML has been reported in adult patients, linked with older age,8,24,25 the recurrent aberration were virtually absent in pediatric tAML, which may underpin the assumption of age-related differences as well as an unfavorable cytogenetic profile in tAML. Complementary sequencing analysis provides additional insights into the molecular genetic signature of pediatric tAML. Besides an even distribution of most of AML and risk-related aberrations, the absence of CEBPAdm in the pediatric tAML samples analyzed fits the described unfavorable genetic profile in tAML. Together with the observed rarity of CBF AML, our findings seem to contradict the concept of oncogenic mutation promoting the evolution to tAML. The herein described unfavorable genetic profile in tAML may indicate a distinct pathological impact that confers poor prognosis in pediatric tAML. This hypothesis is based on limited molecular genetic data, and therefore, further, more comprehensive genetic analyses are required. Given the limited availability of NGS data, particularly regarding germ line mutations or other genetic alterations associated with familial cancer predisposition syndromes, such as TP53 and GATA2, we are unable to draw detailed conclusions on this aspect in our pediatric tAML cohort.
Given the association between adverse cytogenetics and prior exposure to RT, it can be assumed that RT may promote the development of high-risk tAML by inducing high-risk–associated genetic mutations (ie, complex karyotypes). This reinforces the notion of RT-induced genomic instability as an underlying mechanism in pediatric tAML, which has previously been proposed for CTx-induced tAML.5,13,24,26 On the contrary, independent prognostic power of RT and adverse cytogenetics support separate underlying mechanisms, which could independently contribute to pathogenesis in tAML, thus arguing against a simple post hoc relationship between RT and adverse cytogenetics as a single underlying mechanism. Other putative pathomechanisms in the development of tAML include an abnormal environment characterized by stromal and hematopoietic damage due to previous toxic treatment.27,28 Consistent with previous studies,8 we found tAML with antecedent RT to be accompanied by reduced platelets and WBC count, particularly in RT involving pelvis and lumbosacral spine. These results enforces the hypothesis that prior RT, especially involving hematopoietic-relevant regions, may lead to the depletion of healthy hematopoiesis, thereby promoting the development of tAML.27-29 Among chemotherapeutic agents, the described correlation between prior topoisomerase treatment and KMT2Ar-altered tAML lends strength to the known assumption of topoisomerase inhibitor–mediated induction of oncogenic fusions as another mechanism driving the pathogenesis in pediatric tAML.1,5,30 Considering these findings, which highlight different pathogenetic concepts and the diversity of preceding cytotoxic agents for different initial diseases, it seems likely that multiple, interacting, and not mutually exclusive pathogenetic factors promote leukemogenesis in pediatric tAML.
Overall, this comprehensive analysis provides data from a large multicenter study. We demonstrate improved survival rates over the past 26 years in pediatric tAML. Given the association of optimized outcome with HSCT in NEL and the change in treatment regimen from 4 to 2 cycles of induction, we infer novel therapeutic implications suggesting HSCT in NEL after 2 cycles of induction. We were able to define unfavorable cytogenetics and previous RT as the strongest independent poor prognosticators, shedding light on putative underlying pathomechanisms that may contribute to a better understanding of the development of tAML. Altogether, we provide practice-changing treatment recommendations for pediatric tAML. The results shown here form the necessary foundation for future tAML-specific studies within the AML-BFM study group to implement improved guidelines into clinical practice to refine future treatment and ultimately improve prognosis in pediatric tAML.
Acknowledgments
The authors acknowledge Lukas Böckmann for valuable assistance in data management. The authors thank J.E. Müller for competent data management. The authors are grateful to patients and their families for participating. The authors thank all colleagues, data managers, and technicians of the cooperating hospitals for their efforts and valuable contributions.
The AML-BFM study group received grant (110244) funding by the Deutsche Krebshilfe e.V. This work was supported by the Open Access Publication Fund of the University of Duisburg-Essen. N.N. and S.S. were supported by the Clinician Scientist Program of the University Medicine Essen, funded by the Deutsche Forschungsgemeinschaft.
Authorship
Contribution: D.R., K.W., and S.S. devised the study, verified and interpreted the data, and wrote the manuscript; D.R., U.C., N.v.N., M.S., E.A., U.D., K.W., and S.S. collected and compiled the data; D.R., U.C., N.v.N., and K.W. provided administrative support; D.N. supported and reviewed data analysis; D.R., U.C., and K.W. were responsible for trial conduct; E.A., N.N., M.S., D.N., U.D., N.v.N., D.R., K.W., and S.S. read and commented on the manuscript; M.S. and N.v.N. were responsible for trial genetics and analysis of sequencing data and read and commented on the manuscript; D.R., K.W., and S.S. were responsible for submitting and revising the manuscript; and all authors had access to all data of the study and agreed with the final decision to submit for publication.
Conflict-of-interest disclosure: D.R. reports consultancy, research funding, and speakers' bureau fees from Medac; serving on a speakers’ bureau for EUSA and Merck Sharp & Dohme; membership on an entity's board of directors or advisory committees and speakers' bureau with Cerus; research funding from and serving on a speakers’ bureau with bluebird bio; consultancy and membership on an entity's board of directors or advisory committees with Immedica; honoraria from Novartis; and research funding from Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
A complete list of the members of the AML-BFM Study Group appears in the supplemental Appendix.
Correspondence: Dirk Reinhardt, Society of Pediatric Oncology and Hematology (German Society of Pediatric Oncology and Hematology gGmbH), Holsterhauser Platz 2, 45147 Essen, Germany; email: dirk.reinhardt@gpoh-trials.org.
References
Author notes
The data reported in this article have been deposited in the Xclinical Marvin database of the AML-BFM Study Group (accession numbers for the RNA-fusion and next-generation sequencing data are SUB13582559 and SUB12982048, respectively).
Original data are available on request from corresponding author, Dirk Reinhardt (dirk.reinhardt@gpoh-trials.org) or author, Katarina Waack (waack.katharina@gpoh-trials.org).
The full-text version of this article contains a data supplement.

![Probability of OS and EFS in pediatric tAML stratified by AML-BFM study periods over the past 26 years. The median follow-up was 14.4 years for (A) OS (95% confidence interval [CI], 11.3-17.6) and 14.4 years for (B) EFS (95% CI, 11.3-17.3).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/11/10.1182_bloodadvances.2024014728/2/m_blooda_adv-2024-014728-gr2.jpeg?Expires=1764960930&Signature=elJu1e7BAZ3cTA-p1i46Tdcf16mhtcwFKnaHJueIsDYoLlMS0m6eGIMy-0UWAQEiZmtFoK~Z-L1EZ0QnWb-UoPN51sht-b0-XzRu9sTVLC99S3KERdo06PKbO9zipQdpAz7JBAcH4IoNb0zpglM8gsplwOzYKXfcJoAWs7fAU3lWLXZ~2WD6lnteysjsP5owrfeOcL77b7R8g2vNL~XZQ66e~KYwod3H6UIfaxxxNysz~UH16O6SOSxOZj1wxeHJmng5zAcF6M0tmsqhjEWXKy7aSM1j0yhYogluxv8sgNDmCp~Umia66x1esQr0SmzfaA1jwUvUuvF2rJwSMCRBIg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)