Key Points
The outcome of relapsed/refractory TriNHL is better than that of relapsed/refractory de novo LBCL.
The toxicity profile of CAR T cells in relapsed/refractory TriNHL is acceptable.
Visual Abstract
Anti-CD19 chimeric antigen receptor (CAR) T cells have shown impressive results in the treatment of relapsed/refractory aggressive large B-cell lymphomas (LBCLs). However, the prognostic value of the LBCL histological subtype in the context of CAR T-cell therapy is unclear. Here, we report the prognostic value of transformed indolent non-Hodgkin lymphoma (TriNHL; N = 110) confirmed by an expert pathological review (LYMPHOPATH) vs de novo LBCL (N = 391) in the context of CAR T-cell therapy from 4 centers of the French DESCAR-T registry. After 1:1 propensity score matching (n = 170, 85 patients with TriNHL and 85 patients with de novo LBCL), the median follow-up was 19.4 months (95% confidence interval [CI], 12.0-25.1) for patients with TriNHL and 18.5 months (95% CI, 13.8-24.8) for patients with LBCL. The 1-year progression-free survival rate was significantly better (55.8%; 95% CI, 43.6-66.4) in the TriNHL group than in the de novo LBCL group (31.7%; 95% CI, 21.4-42.6; hazard ratio, 0.54; 95% CI, 0.36-0.82; P = .0034). The best overall response rate and complete response rate were 82.4% and 63.5%, respectively, whereas they were 63.5% and 50.6%, respectively, for the TriNHL group compared with the de novo LBCL group. The 1-year overall survival was also longer in the TriNHL group than in the de novo LBCL group (72.1%, [95% CI, 59.6-81.4] vs 50.7%, [95% CI, 38.2-62.0]; P = .031). Similar findings were found via an inverse probability weighting statistical approach. No difference was observed in terms of toxicity. In conclusion, our matched-comparison study revealed a greater efficacy of CAR T-cell therapy, with a toxicity profile for patients with TriNHL comparable with that for patients with LBCL.
Introduction
Anti-CD19 chimeric antigen receptor (CAR) T cells have shown impressive results in the treatment of relapsed/refractory (R/R) aggressive large B-cell lymphoma (LBCL) as second- or higher-line therapy, leading to approval of this treatment for patients with LBCL.1-10
However, LBCLs are a heterogeneous histological group that, according to the fifth edition of the World Health Organization classification, places transformed indolent non-Hodgkin lymphoma (TriNHL) as a peculiar category.11 The pivotal trials of second- or third-line CAR T-cell therapies included a diversity of LBCL histological subtypes without considering this diversity in the results.1,3,5,6,8-10 Similarly, many real-world evidence (RWE) studies have described significant proportions of patients with TriNHL who have received CAR T cells.12-19 Although the prognosis of patients with TriNHL is poor at diagnosis and at first-line treatment with R-CHOP (rituximab, cyclophosphamide, doxorubicin, Oncovin [vincristine], and prednisone) or R-CHOP–like regimens,20 there are still limited data on the effectiveness of CAR T-cell therapy for this specific histological subtype.
Initial retrospective results suggest that CAR T-cell therapy is effective in TriNHL and could halt the aggressive clinical course.21,22 Similarly, some studies have suggested better efficacy in the TriNHL subgroup than in the de novo LBCL subgroup.19,22-24
In this study, we assessed the outcomes and efficacy of the TriNHL histological subgroup in comparison with the de novo LBCL subgroup after CAR T-cell infusion, using a large real-world database.
We conducted an exhaustive individual patient data-based matched comparison considering patients with histological confirmation of either TriNHL or de novo LBCL in a large RWE patient population from the French DESCAR-T registry who were treated with axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisa-cel). We herein report, via adapted propensity score matching (PSM) analysis, the prognostic value of TriNHL vs LBCL in the context of CAR T-cell therapy.
Methods
Study design and patients
All patients with TriNHL or de novo LBCL from 4 French centers (Hôpital Saint-Louis, Assistance Publique–Hôpitaux de Paris, Paris; Centre Hospitaler Lyon Sud, Hospices Civils de Lyon, Pierre Benite; Groupe Hospitalo-Universitaire Chenevier Mondor, Assistance Publique–Hôpitaux de Paris, Créteil; and Hôpital Universitaire de Bordeaux, Bordeaux) who were treated, after at least 2 previous lines of treatment, with commercial CAR T-cell therapy from 1 July 2018 to 19 June 2023 and included in the DESCAR-T registry sponsored by the Lymphoma Academic Research Organization were considered. We defined TriNHL as transformed from follicular lymphoma (tFL) or marginal zone lymphoma (tMZL). Transformed Waldenström macroglobulinemia or Richter transformation were not included in this study. The diagnosis of indolence and transformation was confirmed by a concordant clinical history and a local expert pathological review from the LYMPHOPATH network.25 Transformation at the time of CAR T cells was confirmed on a biopsy performed within 4 months before CAR T-cell infusion and after the previous line of treatment. This timeframe accommodated the necessary duration for diagnosis, screening, and the establishment of CAR T-cell treatment by a multidisciplinary team and the vein-to-vein CAR T-cell process.26 Data were exported from the registry on 1 September 2023. The study complied with the French legal, regulatory, and patient information requirements of research not involving the human person and data reuse (RNIPH-MR004). DESCAR-T is registered under the ClinicalTrials.gov identifier: NCT04328298.27 The study was conducted under the responsibility of the Lymphoma Academic Research Organization.
Outcomes
The primary outcome was the progression-free survival (PFS) assessed by a local investigator. The secondary outcomes were the overall survival (OS), best overall response rate (ORR), complete response rate (CRR), duration of response (DOR), and safety. PFS was defined from the date of CAR T-cell infusion to the date of first documented relapse, progressive disease, date of last follow-up, or death from any cause, whichever came first. OS was defined from the date of CAR T-cell infusion to the date of death from any cause or the date of last follow-up. The response was assessed according to the Lugano 2014 criteria and was based on 18fluorodeoxyglucose positron emission tomography at the following approximate time points: at least before lymphodepletion and at 1, 3, 6, 9, and 12 months after CAR T-cell infusion.28 The best response rate was considered. DOR was defined from the date of first response (partial or complete) to the date of first documented relapse, date of last follow-up, or death from any cause, whichever occurred first. Hematological toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0). Hematological toxicities were reported in patients without initiation of a new treatment for progression or relapse after CAR T-cell infusion. Cytokine release syndrome (CRS) and neurotoxicity (immune effector cell–associated neurotoxicity syndrome [ICANS]) were graded according to the consensus criteria from the American Society for Transplantation and Cellular Therapy.29
Matching procedures
Both matching procedures, namely, PSM and weighting by matching weights (MWs),30 have been described previously in the context of CAR T-cell research.19
PSM was used to create a balanced covariate distribution between the TriNHL cohort and the de novo LBCL cohort. Propensity scores were estimated via a multivariate logistic regression model with the type of NHL (TriNHL vs de novo LBCL) as the dependent variable. An exhaustive list of covariates, previously described by Bachy et al19 and presented in supplemental Table 1, was used for PSM. For all matching parameters except continuous variables (no missing values could be used for continuous parameters in PSM), missing data were considered a distinct category for PSM. In the case of missing data for 1 parameter, the value was always <10%. PSM was performed considering a 1:1 match without replacement and with optimal matching applying a caliper width of the propensity score set at 0.1.
Weighting with the MW method was used as another approach to compare outcomes between patients with TriNHL and those with de novo LBCL and to further validate the PSM analysis. MW is based on the propensity score and results in a pseudopopulation that is balanced regarding the distribution of patient covariates in each group. The methodology underlying propensity score–based matched comparisons and differences with adjustment approaches have been reviewed elsewhere.31
Subgroup analyses
Two subgroup analyses were originally planned: the outcomes according to the subtype of TriNHL (tFL or tMZL) and the outcomes according to the CAR T-cell products (axi-cel and tisa-cel).
Statistical analysis
Survival distributions were compared through the log-rank test. Response rates and toxicities were compared via the χ2 test. A 2-sided P value <.05 was considered significant. No adjustment was performed for multiple testing. Survival curves were generated by using the Kaplan-Meier estimation method. Statistical analyses were performed with SAS software, version 9.4.
Results
Patient characteristics and outcomes
Between July 2018 and September 2023, 110 patients from 4 French centers (Hôpital Universitaire Saint-Louis; Centre Hospitaler Lyon Sud; Groupe Hospitalo-Universitaire Chenevier Mondor; and Hôpital Universitaire de Bordeaux) who had a clinical history and biopsy-proven TriNHL and received CAR T-cell infusion after at least 2 previous lines of therapy underwent infusion with commercial CAR T cells for axi-cel or tisa-cel treatment and were registered in the French DESCAR-T registry. The patient characteristics are presented in Table 1. A total of 107 patients were included in the final population for matching, and 3 were excluded (supplemental Figure 1A). During the same period, 391 patients included in the DESCAR-T registry from the same 4 centers presented with de novo LBCL and received CAR T-cell infusion after at least 2 lines of treatment and with compatible matching variables; these patients were included in the final population for matching (supplemental Figure 1A).
Patient characteristics before the matching procedures are presented in Table 1.
PSM
A propensity score is the conditional probability that a patient presented with TriNHL or de novo LBCL given a set of observed covariates. In this study, the aim of PSM was to balance covariates between the TriNHL and de novo LBCL groups to account for all possible measured confounding variables (supplemental Table 1). These variables have already been shown in the literature to have an impact on PFS and OS after CAR T-cell therapy and are potential confounders.12-14,17,19,32 After stringent PSM of 13 parameters (supplemental Table 1), the absolute values of the standardized mean differences (SMDs) were <0.1 for all except 4 of the matching covariates (C-reactive protein elevated, year of the multidisciplinary team meeting, lactate dehydrogenase elevated, and Ann Arbor stage), which were nevertheless ≤0.25 (supplemental Figure 1B). After 1:1 matching, 85 patients were included in the TriNHL group: 60 patients (72.3%) presented with tFL, 23 (27.7%) presented with tMZL, and 2 presented with TriNHL without any other specificity. Eighty-five patients were included in the de novo LBCL group: 71 patients (83.5%) presented diffuse LBCL with no other specificity, 5 (5.9%) presented primary mediastinal BCL, and 9 (10.6%) presented high-grade BCL. The patient characteristics after the matching procedures are listed in Table 1. The matching procedure allowed balancing, particularly of the Ann Arbor stage, the number of previous treatment lines, patient age at infusion, the sex ratio, and the product of CAR T cell. Regarding the previous treatment lines, we considered all of them, whether they were used for the indolent form or the aggressive form. After matching, the main previous treatment lines were similar in both groups (supplemental Table 2).
In the 1:1 matched population (n = 170, 85 patients with TriNHL and 85 patients with de novo LBCL), after a median follow-up of 18.5 months (95% confidence interval [CI], 13.8-24.8) for the de novo LBCL group and 19.4 months (95% CI, 12.0-25.1) for the TriNHL group, the PFS was significantly longer in the TriNHL group than in the de novo LBCL group (P = .0029), with a better median PFS (18.6 months [95% CI, 5.5-36.6] vs 3.1 months [95% CI, 2.8-5.6], respectively), and a better 1-year PFS in the TriNHL group (55.8%; 95% CI, 43.6-66.4) than in the de novo LBCL group (31.7%, [95% CI, 21.4-42.6]; hazard ratio [HR], 0.54 [95% CI, 0.36-0.82]; P = .0034; Figure 1A; Table 2).
PFS and best response rate after PSM. (A) PFS after the infusion of CAR T cells according to histology (P = .0029). (B) Best response rate according to histology. Time-to-event data were assessed via Kaplan-Meier curves, and the estimated rates at specific time points were computed with 95% CIs via the Greenwood formula. Response rates are expressed as percentages with 95% CIs according to the exact Pearson-Clopper method and were compared via a χ2 test. CR, complete response; PR, partial response.
PFS and best response rate after PSM. (A) PFS after the infusion of CAR T cells according to histology (P = .0029). (B) Best response rate according to histology. Time-to-event data were assessed via Kaplan-Meier curves, and the estimated rates at specific time points were computed with 95% CIs via the Greenwood formula. Response rates are expressed as percentages with 95% CIs according to the exact Pearson-Clopper method and were compared via a χ2 test. CR, complete response; PR, partial response.
Weighting via MWs
After MWs, the absolute values of the SMDs were <0.1 for all matching covariates (supplemental Table 1). MW was used to support the findings of the PSM analysis and to allow for proper comparisons between the 2 populations of patients with TriNHL and de novo LBCL. After a median follow-up of 18.5 months for TriNHL (95% CI, 12.1-24.9) and 18.5 months for de novo LBCL (95% CI, 14.2-23.8), the PFS was significantly better in the TriNHL group than in the de novo LBCL group (P = .0010), with a 1-year PFS of 55.9% (95% CI, 44.5-65.9) vs 31.7% (95% CI, 24.9-38.7) and a median PFS of 18.3 months for patients with TriNHL (95% CI, 6.4 to not available [NA]) vs 3.8 months for patients with de novo LBCL (95% CI, 3.0-5.6; HR, 0.58 [95% CI, 0.39-0.86]; P = .0064; supplemental Figure 2).
Secondary end points in the matched population
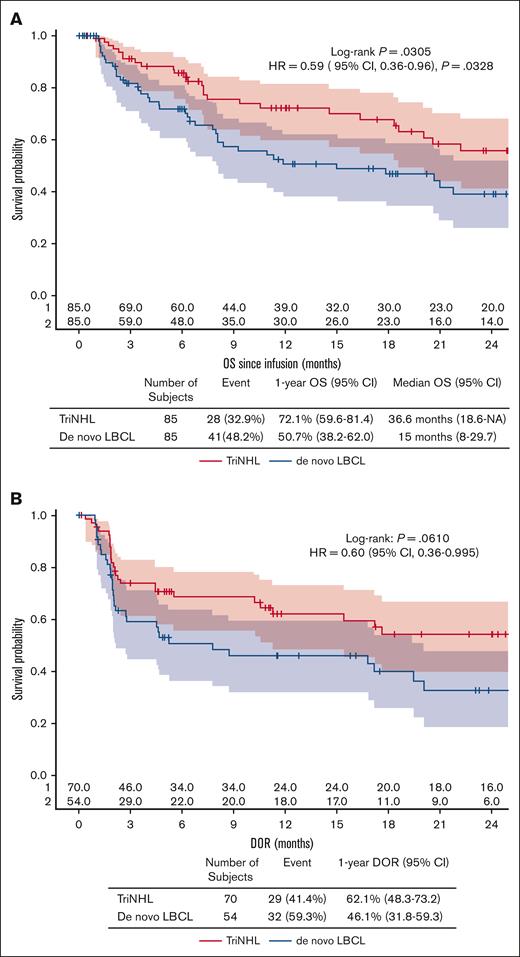
In the 1:1 matched population, the best ORR and CRR was 82.4% (95% CI, 72.6-89.8) and 63.5% (95% CI, 52.4-73.7), respectively, for patients with TriNHL, and 63.5% (95% CI, 52.4-73.7) and 50.6% (95% CI, 39.5-61.6), respectively, for patients with de novo LBCL (best ORR, P = .010; best CRR, P = .121; Figure 1B; Table 2). The OS was also significantly better in the TriNHL group than in the de novo LBCL group (P = .0305), with a median OS of 36.6 months (95% CI, 18.6 to NA) vs 15 months (95% CI, 8-29.7), and a better 1-year OS for the TriNHL group (72.1%; 95% CI, 59.6-81.4) than for the de novo LBCL group (50.7% [95% CI, 38.2-62.0]; HR, 0.59 [95% CI, 0.36-0.96]; P = .0328; Figure 2A; Table 2). The DOR was also longer for the TriNHL group than for the de novo LBCL group, with a median DOR of 26.7 months (95% CI, 11.3 to NA) vs 7.8 months (95% CI, 2.1-20.1) and a 1-year DOR of 62.1% (95% CI, 48.3-73.2) vs 46.1% (95% CI, 31.8-59.3), respectively (HR, 0.60; 95% CI, 0.363-0.995; Figure 2B; Table 2).
OS and DOR after PSM. (A) OS after the infusion of CAR T cells according to histology (P = .0305). (B) DOR according to histology (P = .0610). Response rates are expressed as percentages with 95% CIs according to the exact Pearson-Clopper method and were compared via a χ2 test. Time-to-event data were assessed via Kaplan-Meier curves, and the estimated rates at specific time points were computed with 95% CIs via the Greenwood formula.
OS and DOR after PSM. (A) OS after the infusion of CAR T cells according to histology (P = .0305). (B) DOR according to histology (P = .0610). Response rates are expressed as percentages with 95% CIs according to the exact Pearson-Clopper method and were compared via a χ2 test. Time-to-event data were assessed via Kaplan-Meier curves, and the estimated rates at specific time points were computed with 95% CIs via the Greenwood formula.
Histology at post–CAR T-cell relapse
Overall, 34 patients with TriNHL presented with progression or relapse after CAR T-cell therapy. The relapses after CAR T-cell failure were classified as biopsy-proven TriNHL in 70.6% (n = 24) of patients, as aggressive in terms of the clinical or imaging features but not proven by biopsy in 26.5% (n = 9) of patients; and as biopsy-proven indolent disease in 1 patient (MZL).
Safety in propensity score–matched populations
In the matched population (n = 170), there was no difference according to either CAR T-cell–specific toxicity or hematological toxicity (Table 3).
Sixty-seven (78.8%) and 71 (83.5%) patients in the TriNHL and de novo LBCL groups, respectively, experienced CRS of any grade (P = .4326), and 2 (2.4%) and 4 (4.7%) patients (P = .4058), respectively, experienced CRS of grade ≥3 (Table 3).
Twenty-five (29.4%) and 28 (32.9%) patients in the TriNHL and de novo LBCL groups, respectively, presented ICANS of any grade (P = .6194), and 5 (5.9%) and 4 (4.7%) patients (P = .7320), respectively, presented ICANS of grade ≥3. No grade 5 CRS or ICANS was observed (Table 3).
With respect to hematological toxicity, at 1 month after CAR T-cell therapy, cytopenia of any grade remained unresolved in 46 (57.5%) patients with TriNHL and 49 (62.8%) patients with de novo LBCL. Neutropenia of grade ≥3 was observed in 37 (43.5%) patients in the TriNHL group and 41 (48.2%) patients in the de novo LBCL group (P = .5381). Thrombocytopenia of grade ≥3 was detected in 25 (29.4%) and 31 (36.5%) patients, respectively (P = .3275). Anemia of grade ≥3 was detected in 16 (18.8%) and 24 (28.2%) patients, respectively (P = .1480; Table 3).
No difference was detected regarding unresolved cytopenia at 3 months (Table 3).
Subgroup analyses
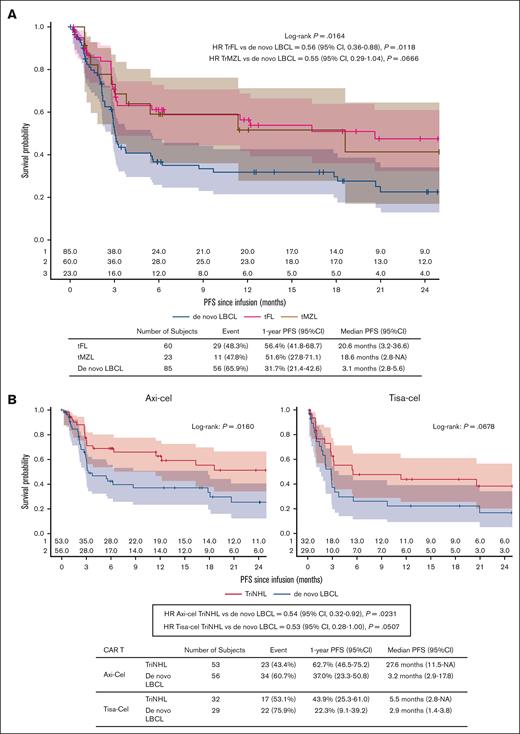
First, outcomes according to the subtype of TriNHL (tFL or tMZL) were assessed in the PSM population. The PFS was significantly longer in the tFL group (n = 60) than in the de novo LBCL group (n = 85), with 1-year PFS of 56.4% (95% CI, 41.8-68.7) vs 31.7% (95% CI, 21.4-42.6), respectively (HR, 0.56; 95% CI, 0.36-0.88; P = .0118; Figure 3A). A “positive” trend was also observed for PFS in the tMZL group (n = 23) compared with the de novo LBCL group (1-year PFS: 51.6% [95% CI, 27.8-71.1] vs 31.7% [95% CI, 21.4-42.6]; HR, 0.55 [95% CI, 0.29-1.04]; P = .0666; Figure 3A). No difference in 1-year PFS was observed between tMZL and tFL (1-year PFS: 56.4% [95% CI, 41.8-68.7] vs 51.6% [95% CI, 27.8-71.1], respectively; HR, 1.03 [95% CI, 0.51-2.06]; P = .9384).
PFS according to the indolent subgroup and CAR T-cell product after PSM. (A) PFS after infusion of CAR T cells according to the indolent subgroup (P = .0164). (B) PFS after the infusion of CAR T cells according to the CAR T-cell product: axi-cel (left graph) or tisa-cel (right graph). Time-to-event data were assessed via Kaplan-Meier curves, and the estimated rates at specific time points were computed with 95% CIs via the Greenwood formula. NA, not available.
PFS according to the indolent subgroup and CAR T-cell product after PSM. (A) PFS after infusion of CAR T cells according to the indolent subgroup (P = .0164). (B) PFS after the infusion of CAR T cells according to the CAR T-cell product: axi-cel (left graph) or tisa-cel (right graph). Time-to-event data were assessed via Kaplan-Meier curves, and the estimated rates at specific time points were computed with 95% CIs via the Greenwood formula. NA, not available.
Second, for patients receiving axi-cel, the PFS was still significantly longer in the TriNHL group (n = 53) than in the de novo LBCL group (n = 56; 1-year PFS: 62.7% [95% CI, 46.5-75.2] vs 37.0% [95% CI, 23.3-50.8], respectively; HR, 0.54 [95% CI, 0.32-0.92]; P = .0231; Figure 3B). A similar trend was observed for patients receiving tisa-cel (n = 32 and n = 29 for TriNHL tisa-cel and de novo LBCL tisa-cel, respectively; 1-year PFS: 43.9% [95% CI, 25.3-61.0] vs 22.3% [95% CI, 9.1-39.2], respectively; HR, 0.53 [95% CI, 0.28-1.00]; P = .0507; Figure 3B).
Impact of treatments received for indolent lymphoma and transformation at diagnosis
We performed an analysis to verify the impact of treatments received by patients for their indolent lymphomas preceding transformation. We compared the PFS and OS rates of patients in the TriNHL group who received no treatment for indolent lymphoma (n = 29) with those of patients with TriNHL who received at least 1 line of treatment before transformation (n = 56). There was no difference in either the median PFS (18.6 months [95% CI, 3.2 to NA] vs 16.4 months [95% CI, 3.0 to NA]; P = .94) or the median OS (not reached, [95% CI, 7.3 to NA] vs 27.3 months [95% CI, 18.3 to NA]; P = .47) between the patients with TriNHL who were previously untreated for their indolent lymphomas and patients who received such treatment (supplemental Figure 3). A small number of patients with TriNHL (n = 16) had previously been treated with bendamustine, which prevents us from analyzing its impact.
In the TriNHL group before matching, we also observed no difference in terms of OS and PFS between patients who experienced indolent transformation at diagnosis (n = 15) and those whose histological transformation occurred after diagnosis (n = 92; supplemental Figure 4). However, the number of patients with a transformation at diagnosis was low.
Discussion
Although CAR T cells have improved the survival of patients with LBCL and continue to be useful in modifying the disease management, optimization of their application is still necessary, especially with the development of new treatments such as bispecific therapies. One of the elements that is still poorly understood is the significant histopathological heterogeneity of LBCL. In fact, the inclusion of patients in therapeutic trials was not conducted with consideration of this diversity, and the comparative results that we have to date are essentially derived from secondary or multivariate analyses of real-life cases. Some studies suggest that CAR T-cell therapy is effective in patients with TriNHL, possibly with better efficacy than that in patients with de novo LBCL.19,21-24 The recent results published by Thiruvengadam et al conclude, through multivariate analysis, a better outcome for TriNHL.22 However, on the one hand, these are the results of secondary multivariate analyses by the studies of Bachy et al and Kuhnl et al19,24; on the other hand, the studies were not statistically designed for the comparison of the 2 groups (TriNHL vs de novo LBCL) and presented many potential cofounding factors between the 2 groups that must be considered for a robust comparison.22,23 Additionally, only 2 of these studies had histologically confirmed TriNHL before CAR T-cell infusion.21,24
To our knowledge, this study is the first to statistically and robustly compare the responses to CAR T-cell therapy on the basis of the histopathological subtype after at least 2 lines of treatment in real life. The PSM and MW methods address nearly most measurable confounding factors affecting treatment efficacy.
A total of 107 patients from 4 French centers in the DESCAR-T registry presented with TriNHL histologically proven just before CAR T-cell infusion. A total of 391 patients from the same centers presented, during the same period, de novo LBCL with compatible matching data. After 1:1 stringent matching, 85 patients were included in each group for our statistical study. After a median follow-up of >1.5 years, the median PFS (18.6 months vs 3.1 months), 1-year PFS (55.8% vs 31.7%), median OS (36.6 months vs 15 months), and 1-year OS (72.1% vs 50.7%) were better for the TriNHL group than for the de novo LBCL group. Our cohort of de novo LBCL was representative of the World Health Organization 2022 classification, including traditional histology: diffuse LBCL with no other specificity, primary mediastinal BCL, and high-grade BCL.11 Importantly, the median PFS and OS of the patients with de novo LBCL, who represents most of the LBCLs, were close to those described in the historic pivotal clinical trials ZUMA-1 (median OS, 25.8 months; median PFS, 5.9 months)2,33 and JULIET (median OS, 11.1 months; median PFS, 2.9 months).4 Additionally, various RWE studies show median PFS ranging from 3 to 8.6 months and median OS between 11.2 and 21.8 months, supporting the representativeness of our de novo LBCL cohort.14,17-19,24,32
As we have already discussed and implemented on our DESCAR-T registry, PSM and MWs are used to address confounding factors by indication in real-life studies.19 Although PSM was used to establish our results, MWs were used for sensitivity analysis to confirm these findings. In our study, both techniques similarly revealed that patients with TriNHL have better outcomes than patients with de novo LBCL administered CAR T-cell therapy after at least 2 previous lines of treatment, confirming preliminary results from multivariate or subgroup analyses in the literature.4,19,22 The SMDs were higher for PSM compared with MWs; however, the results were similar, indicating that they do not depend on the comparison method used.
With respect to other markers of response to CAR T-cell therapy, the efficacy was greater in the TriNHL group than in the de novo LBCL group. Indeed, the best ORR and CRR were 82.4% and 63.5% vs 63.5% and 50.6% for the TriNHL and de novo LBCL groups, respectively. Again, the best ORR and CRR of the de novo LBCL group were also, as PFS and OS, close to the response rates reported in pivotal clinical studies and real-life cohorts.1-4,19,24 These better responses to CAR T-cell therapy show the efficacy of this treatment for this histological subtype of LBCL and confirm certain preliminary results from subgroup or multivariate analyses of the literature.4,5,17,22
Our first subgroup analysis also revealed better PFS in patients with TriNHL than in those with de novo LBCL, PSM and MWs, regardless of the histological type of indolent lymphoma, whether FL or MZL. Significance was not reached for the tMZL group compared with the de novo LBCL group, probably because of the small sample size (n = 23) and the shorter median follow-up (11.5 months). Our second subgroup analysis demonstrated a better PFS of patients with TriNHL than of patients with de novo LBCL regardless of the CAR T-cell product used (axi-cel or tisa-cel).
With respect to the specific toxicities of CAR T cells, there was no difference in the percentages of patients experiencing ICANS and CRS. Similarly, there was no difference in the rate of grade ≥3 disease, but this rate was relatively low in our 2 groups compared with that in the literature. This lack of increase in the rate of specific toxicity of CAR T cells between TriNHL and de novo LBCL is consistent with what has been reported in previous studies.18,32,34 Moreover, no difference in the rates of hematological and infectious complications was found, which was in accordance with the few results in the literature.35
Our study has several limitations. First, most of the patient data were collected retrospectively; however, DESCAR-T is a monitored registry with high-quality control. Second, to ensure the robustness of the histopathological diagnosis, only patients from 4 DESCAR-T centers were included, which could have led to selection bias; however, to limit such bias, “center” was 1 of the matching factors.
Notably, all known confounding factors for efficacy after therapy with CAR T-cells were accounted for in the PSM and MWs approaches to ensure that the comparisons between the 2 groups were robust and balanced and that there were no missing data for continuous parameters; in the case of missing data, the value was always <10%, limiting the introduction of significant residual uncontrolled bias.
Considering these positive results, the use of CAR T cells should be presently discussed as part of the treatment strategy for TriNHL, which is recognized as a high-risk lymphoma in the context of immunochemotherapy. Our study also emphasizes the importance of biopsy in the follow-up of indolent lymphomas to rapidly detect transformed forms. Given the recent excellent results of CAR T cells in the second-line treatment of R/R LBCL compared with the reference treatment, it is crucial to study these results specifically in patients with TriNHL to deduce the best therapeutic sequence for these patients.6,9,36 Similarly, the results of CAR T-cell therapy in nontransformed indolent lymphoma invite the study of the impact of this intervention earlier in the management of TriNHL to identify the best treatment timing and therapeutic sequences.37,38
To date, the reason for the aforementioned treatment differences remains poorly understood. Differences in the tumor microenvironment (TME) may be partly responsible for the differences in response to CAR T cells. A recent study uncovered the changes in the TME during the transformation of FLs.39 The TME affects the effectiveness of CAR T-cell treatment in LBCLs.40 Therefore, comparing the TMEs of patients with TriNHL with those of patients with de novo LBCL could provide insights into their different outcomes on CAR T-cell therapy.
In conclusion, this study is, to our knowledge, the first to use robust statistical analysis using PSM and MWs, along with a rigorous pathological review, confirming the efficacy of CAR T cells in the treatment of TriNHL. Compared with de novo LBCL, TriNHL, which is historically associated with worse outcomes and an aggressive clinical course, has a better outcome in the context of CAR T-cell treatment for R/R LBCL.
Acknowledgments
The authors thank the patients and their families, all the investigators and their staff involved in data collection and analyses, and the Lymphoma Academic Research Organization for study organization and support.
Authorship
Contribution: P. Stephan, P. Sesques, J.C., R.D.B., E.B., and C.T. conceptualized and designed the study; P. Stephan, P. Sesques, and V.D. analyzed and interpreted the data; P. Stephan and P. Sesques wrote the manuscript; and all authors were responsible for the provision of study material or patients, collection and assembling of data, and final approval of the manuscript, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: P. Stephan reports research funding (to institution) from AbbVie and BeiGene, and a mobility grant from Institut Servier. R.D.B. served on the scientific advisory board of Novartis, Kite/Gilead, Janssen, and Bristol Myers Squibb (BMS); served as a conference speaker for Novartis, Kite/Gilead, Pfizer, AbbVie, and Incyte; and received travel accommodation from Kite/Gilead. L.R. reports travel grants from Kite and served on the advisory boards for Janssen and BMS. V.M. reports having consulted for EusaPharma and BeiGene. S.L.G. reports honoraria and travel support from, and membership of advisory boards of, Novartis, Kite/Gilead, and Janssen. R.H. has received honoraria from Kite/Gilead, Novartis, BMS/Celgene, Incyte, Janssen, Merck Sharp and Dohme, Takeda, Amgen, AbbVie, and Roche, and is a member on the board of directors or advisory committees of Kite/Gilead, Novartis, BMS/Celgene, Tessa Therapeutics, AbbVie, and Roche. F.-X.G. received honoraria from Janssen, Kite/Gilead, Novartis, BMS, and AstraZeneca. F.L. received honoraria from Takeda and AstraZeneca; served on the advisory board of Miltenyi, Kyowa Kirin, and BMS; received travel funds from Roche, Gilead, and AbbVie; and received research funds from BMS and Roche. E.B. reports membership on the board of directors or advisory committees of, consultancy with, and honoraria from AbbVie, Roche, and Takeda; reports honoraria from ADC Therapeutics; received research funding from Amgen; reports membership on the board of directors or advisory committees of, and honoraria from, BeiGene and Incyte; received honoraria and research funding from BMS; received honoraria from Janssen, Kite (a Gilead Company), Novartis, and Pfizer; reports consultancy with Janssen and Kite (a Gilead Company); and reports travel or accomodation from BMS, Kite (a Gilead Company), Novartis, and Pfizer. C.T. reports a speaker role with AbbVie, Amgen, Bayer, BMS/Celgene, Gilead Sciences Inc, Incyte Corporation, Janssen, Kite, Novartis, Roche, and Takeda; received travel and accommodation expenses from AbbVie, Amgen, Bayer, BMS/Celgene, Gilead Sciences Inc, Janssen, Kite, Novartis, Roche, and Takeda; received research grants from Janssen and Roche; and reports consultancy with/honoraria from AbbVie, Amgen, Bayer, BMS/Celgene, Gilead Sciences Inc, Novartis, Roche, Incyte Corporation, Janssen, Kite, and Takeda. P. Sesques reports honoraria from and advisory/consultancy role with Janssen, Roche, BMS, Chugai, Novartis, and Kite/Gilead. The remaining authors declare no competing financial interests.
Correspondence: Pierre Sesques, Hematology Department, Lyon Sud Hospital, 165 Chemin du Grand Revoyet, 69410 Pierre-Bénite, France; email: pierre.sesques@chu-lyon.fr.
References
Author notes
The data sets generated and/or analyzed during this study are not publicly available because of proprietary considerations. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. Data requests pertaining to the manuscript may be made to the corresponding author, Pierre Sesques (pierre.sesques@chu-lyon.fr). Requests will be processed within 12 weeks.
The full-text version of this article contains a data supplement.