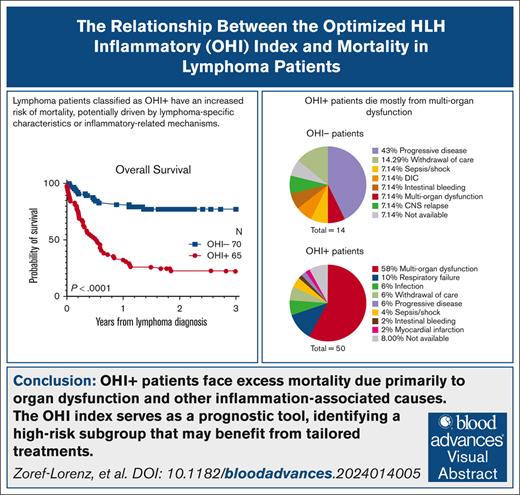
Key Points
The excess mortality predicted by the OHI index in patients with lymphoma is predominantly caused by multiorgan dysfunction.
Incorporation of etoposide into lymphoma-directed therapy for those deemed OHI+ at diagnosis is associated with improved OS.
Visual Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a hyperinflammatory syndrome that complicates hematologic malignancies. The Optimized HLH Inflammatory (OHI) index, based solely on the combined elevation of soluble CD25 (sCD25; >3900 U/mL) and ferritin (1000 ng/mL) levels, predicts mortality more effectively than the conventional HLH criteria. This study aimed to determine whether mortality in OHI-positive (OHI+) patients is primarily caused by lymphoma or inflammation-related causes. In a multicenter, retrospective study of patients with newly diagnosed lymphoma with sCD25 and ferritin measurements available, patients were classified as OHI+ or OHI negative (OHI−). The 1-year and 3-year overall survival (OS), event-free survival, and causes of death were analyzed, along with predicted vs observed mortality based on other lymphoma-relevant prognostic indexes. Among 135 patients with lymphoma (70 OHI− and 65 OHI+), OHI+ patients had significantly lower OS at 1 year (33% vs 81%; P < .0001) with a median survival of 190 days. OHI+ patients had a 13-fold increased mortality risk (odds ratio, 13.3; 95% confidence interval, 6.0-28.5) despite similar predicted OS based on conventional indexes (72% vs 65%; P = .46). Mortality causes differed significantly. A total of 58% of OHI+ patients died from multiorgan failure, whereas only 6% died from progressive lymphoma. In contrast, 43% of the OHI− patients died from lymphoma progression. The incorporation of etoposide into lymphoma-directed treatment was associated with improved OS in OHI+ T–cell lymphomas (P = .007). These findings underscore the clinical significance of the OHI index as a prognostic tool in lymphoma, to elucidate the mechanisms of mortality, and to identify a high-risk subgroup for which tailored treatments may lead to improved outcomes.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening inflammatory syndrome that may complicate hematologic malignancies (HMs).1,2 Malignancy-associated HLH has a poor prognosis with only a 10% to 20% long-term survival rate.1,3-6 HLH was reported in ∼1% of adults diagnosed with an HM (HM-HLH), but this may increase to 20% in some B- and T–cell lymphomas.7,8 T-cell lymphomas are the most common HMs in which HLH occurs, accounting for 35% of all cases, whereas B-cell lymphomas account for 32%.8,9 Its rapidity of onset and progression makes HM-HLH a hematologic urgency.2,10,11
HLH has long been clinically defined using the HLH-2004 diagnostic criteria developed to identify familial HLH, typically in infants.12 However, HM-HLH occurs mostly without evidence of familial inheritence.1 In this context, many of the HLH-2004 criteria may directly reflect the underlying malignancy rather than an abnormal inflammatory state and thus lead to both under- and overdiagnosis of HLH.13,14 We have developed a novel diagnostic and prognostic tool termed the Optimized HLH Inflammatory (OHI) index,15 which combines malignancy-optimized elevations of the 2 inflammatory biomarkers included in the HLH-2004 criteria,12 namely soluble CD25 (sCD25; >3900 U/mL) and ferritin (>1000 ng/mL), that successfully identifies patients with HM with HLH-spectrum hyperinflammation and excess mortality. However, it remains unclear whether the poor prognosis of patients with a positive OHI index is attributable to a more aggressive malignancy or an uncontrolled inflammatory process.
In this study, we aimed to determine whether mortality in patients with OHI-positive (OHI+) and OHI-negative (OHI−) lymphoma differed from that predicted by standard prognostic indexes and whether this mortality was uniquely associated with conditions related to abnormal inflammation or immune dysfunction.
Methods
Study design
All centers obtained approval from the institutional review boards and ethics committee for the study. We studied causes of death in a subgroup of patients with lymphoma derived from our previously published, retrospective, multicenter study of patients with various HMs.15 This cohort comprised patients with HMs for whom serum sCD25 and ferritin levels had been measured, either during investigation for HLH or as part of routine surveillance, and who had detailed data regarding their malignancy diagnosis, treatment, and causes of death between January 2012 and March 2020. The OHI status of patients was determined using the published thresholds for sCD25 (>3900 U/mL) and ferritin (>1000 ng/mL).15
Data collection
We collected patient data related to prognosis, causes of mortality, treatment, and lymphoma responses. The prognostic scores used were as follows: the International Prognostic Index (IPI) for T-cell and aggressive B-cell non-Hodgkin lymphoma (B-NHL),16 the International Prognostic Score for HL,17 and the Follicular Lymphoma International Prognostic Index for follicular lymphoma.18 Data on the date of diagnosis, histologic subtype (classified according to World Health Organization 2022), age at lymphoma diagnosis, complete blood counts with differential, serum albumin levels, lactate dehydrogenase levels, Ann Arbor stage, extranodal site involvement, Eastern Cooperative Oncology Group (ECOG) performance status, cell of origin (COO; germinal center B cell [GCB] or non-GCB), MYC, B-cell lymphoma (BCL)-2, and BCL-6 status assessed by fluorescence in situ hybridization, and bone marrow involvement were collected. Ann Arbor staging was chosen over Lugano staging to ensure consistency with the IPI calculation. We recorded survival at 1 year, 3 years, and at the time of the last follow-up. Data on the prespecified proximate and underlying causes of death were also collected (supplemental Table 1).
Inclusion criteria
Patients from our original cohort1 with any lymphoma subtype for whom both sCD25 and ferritin retrospective measurements were available at the initial lymphoma diagnosis were eligible for inclusion. These markers were measured either as part of routine surveillance policy (MD Anderson and Toyama centers) or because of HLH suspicion at all centers.
Exclusion criteria
Patients who received >1 cycle of chemotherapy before sCD25 and ferritin assessment were excluded from the analysis. This exclusion criterion was chosen to ensure that the OHI status primarily reflected the underlying lymphoma rather than being confounded by unrelated factors, such as infections that might arise as a consequence of treatment.
OS and EFS analysis
Overall survival (OS) and event-free survival (EFS) were calculated at 1 year and 3 years from the lymphoma diagnosis. Patients were censored at death, the last follow-up, and at 1 year or 3 years if follow-up was longer than the predefined time point. An event was defined as death, refractory, or relapsed disease.
Prognostic index comparison
To assess the prognostic relevance of the OHI status, we calculated lymphoma-relevant prognostic indexes at the time of the new-onset malignancies, collected the 3-year OS rate predicted for each patient,17-19 and compared the predicted OS between OHI+ and OHI− patients.
Details of treatment administered
We collected data regarding the first- and second-line treatments provided for HLH and the lines of therapy administered for lymphoma. Lymphoma treatment response after each line of therapy and at last follow-up or death were determined using the Lugano criteria.20
Causes of death
The proximate cause of death refers to the event or condition that directly led to an individual’s death. It is the primary cause in a sequence of events that ultimately led to death, without which the fatal outcome would not have occurred. The proximate cause was distinguished from underlying causes, which may have set the stage for the fatal event but are not the immediate reason for death. The proximate and underlying causes of mortality were collected based on predetermined etiologies in accordance with the Forensic Pathology, Autopsy, and Neuropathology Committees of the College of American Pathologists, in conjunction with the National Association of Medical Examiners, which are based on previous publications.21,22 Proximate or direct causes were heart failure, coronary artery disease, thrombotic stroke, hemorrhagic stroke, bleeding, unspecified, respiratory failure, sepsis/shock, infection (bacterial, viral, or fungal), acute kidney failure, multiorgan dysfunction, trauma, suicide, homicide, and progressive lymphoma. Underlying causes of death were heart failure, coronary artery disease, thrombotic stroke, hemorrhagic stroke, chemotherapy-induced thrombocytopenia, chemotherapy-induced neutropenia, immune compromise not otherwise defined, infection (bacterial, viral, or fungal), acute kidney failure, chronic kidney failure, hepatic failure, withdrawal of care, progressive lymphoma, and other cancers (supplemental Table 1).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 10.1.2. Descriptive statistics were used to summarize the demographic and clinical characteristics of the study population. Categorical variables are presented as frequencies and percentages, whereas continuous variables are described using means with standard deviations or medians with interquartile ranges depending on the data distribution.
Categorical variables were compared between OHI+ and OHI− groups using χ2 or Fisher’s exact tests when appropriate. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to quantify the predicted mortality risk vs the observed mortality risk.
The OS and EFS analyses were conducted using the Kaplan-Meier method, and differences between survival curves were assessed using log-rank tests, which calculated the P values, hazard ratios (HRs), and 95% CI.
All statistical tests were 2-tailed, and a P value < .05 was considered statistically significant.
Results
Our previous published cohort included 225 patients with various HMs.15 From these, 167 patients with lymphoma were included in this study.
In total, 30 patients with aggressive lymphoma who received >1 cycle of chemotherapy before sCD25 and ferritin assessment were excluded from the analysis. Two more patients were excluded because of missing data pertaining to the diagnosis of lymphoma. Thus, the final cohort comprised 135 patients (Table 1). Of these, 80 patients (59%) had B-NHL; among them, 43 patients had diffuse large B-cell lymphoma (DLBCL; 32%), 14 patients had intravascular large BCL (10%), and 8 patients had follicular lymphoma (6%). Furthermore, 41 patients (30%) had mature T-cell lymphoma and 16 patients (12%) had HL. Of those included in the cohort, 65 patients were classified as OHI+ and 70 as OHI−.
There were clear associations between the HLH-2004 criteria and OHI status, highlighting that OHI+ patients exhibited a distinct hyperinflammatory phenotype (supplemental Table 2) even when they did not fully meet the HLH-2004 criteria (supplemental Table 3). Notably, 86% of OHI+ patients fulfilled the HLH-2004 criteria, emphasizing the overlap between these conditions (supplemental Table 2).
Patients with OHI+ lymphoma exhibited decreased OS and EFS
The survival outcomes were inferior in OHI+ patients with a 1-year OS rate of 33% (95% CI, 22-44) as opposed to 81% (95% CI, 70-89) among OHI− patients, and the median OS was 190 days in the OHI+ group and not reached in the OHI− group (Table 2). These disparities were consistent among patients who were OHI+ and HLH-2004– with a 1-year OS rate of 44%. In contrast, patients who were OHI−/HLH-2004+ had a markedly higher 1-year OS of 82% (supplemental Table 3), demonstrating that the OHI index captures a severe hyperinflammatory state with an exceptionally poor prognosis independent of classical HLH classification. Thus, OHI+ patients had a significantly reduced OS (HR, 5.2; 95% CI, 3.0-8.8; P < .0001; supplemental Figure 1A; Table 2) and EFS (HR, 2.9; 95% CI, 1.8-4.6; P < .0001; supplemental Figure 1B; Table 2) compared to OHI− patients at 1 year. These inferior outcomes remained consistent across specific lymphoma subtypes, and the OS differences were more pronounced than the EFS differences.
In DLBCL, the most common lymphoma subtype, OHI+ patients had significantly worse 1-year OS (HR, 8.7; 95% CI, 3.0-25; P = .0006; supplemental Figure 2A; Table 2) and EFS (HR, 2.7; 95% CI, 1.1-6.4; P = .02; supplemental Figure 2B; Table 2). Similarly, in T-cell lymphoma, the 1-year OS was significantly lower in OHI+ patients (HR, 3.6; 95% CI, 1.6-8.3; P = .0007; supplemental Figure 2C; Table 2), and the EFS was inferior (HR, 2.6; 95% CI, 1.2-5.3; P = .01; supplemental Figure 2D; Table 2) when compared with OHI− patients. These findings suggest worse systemic outcomes and potentially impaired tumor control in OHI+ patients.
Patients with OHI+ lymphoma have inferior outcomes than those predicted by disease-relevant traditional indexes
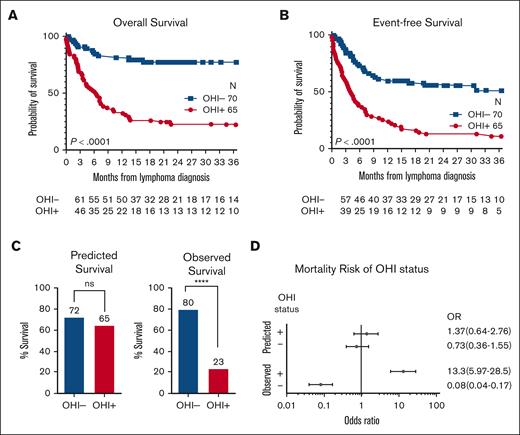
The survival outcomes discrepancies were more pronounced at 3 years with a 3-year OS rate of 23% (95% CI, 13-35) in the OHI+ group and 80% (95% CI, 68-88) in OHI− patient group (Table 2). OHI+ patients had a significantly reduced OS (HR, 5.4; 95% CI, 3.3-8.8; P < .0001; Figure 1A) and EFS (HR, 3.3; 95% CI, 1.1-5.1; P < .0001; Figure 1B) at 3 years.
Patients with OHI+ lymphoma have decreased 3-year OS and EFS. Kaplan-Meier curves comparing the 3-year OS (A) and EFS (B) between OHI− (70 patients) and OHI+ patients (65 patients). Statistical significance was determined using the log-rank (Mantel-Cox) test (∗∗∗∗P < .0001). An event was defined as death, refractoriness, progression, or relapse of lymphoma. (C) Predicted survival of OHI− and OHI+ patients based on the lymphoma-relevant prognostic indexes (left) and the observed survival (right). Statistics were calculated using the χ2 test. ∗∗∗∗P < .0001. (D) Forest plot of the OR and 95% CI of the predicted OHI+/− and the observed survival of patients with OHI+/− lymphoma. The number of patients at risk at each time point is shown beneath the survival curves. ns, nonsignificant.
Patients with OHI+ lymphoma have decreased 3-year OS and EFS. Kaplan-Meier curves comparing the 3-year OS (A) and EFS (B) between OHI− (70 patients) and OHI+ patients (65 patients). Statistical significance was determined using the log-rank (Mantel-Cox) test (∗∗∗∗P < .0001). An event was defined as death, refractoriness, progression, or relapse of lymphoma. (C) Predicted survival of OHI− and OHI+ patients based on the lymphoma-relevant prognostic indexes (left) and the observed survival (right). Statistics were calculated using the χ2 test. ∗∗∗∗P < .0001. (D) Forest plot of the OR and 95% CI of the predicted OHI+/− and the observed survival of patients with OHI+/− lymphoma. The number of patients at risk at each time point is shown beneath the survival curves. ns, nonsignificant.
The lymphoma-relevant IPI predicted 3-year OS rates of 72% and 65% for OHI− and OHI+ patients, respectively (P = .46; Figure 1C), suggesting no significant difference. However, the observed OS was markedly worse in OHI+ patients (P < .0001). This translated into a notably elevated 3-year mortality risk of >13-fold among OHI+ vs OHI− patients (OR, 13.3; 95% CI, 6.0-28.5; Figure 1D), whereas the IPI predicted that the mortality risk was not significantly different (OR, 1.37; 95% CI, 0.64-2.76; Figure 1D). These data indicate that traditionally recognized lymphoma-relevant prognostic factors do not explain the increased mortality risk among OHI+ patients.
Distinct malignancy characteristics among patients with OHI+ lymphoma
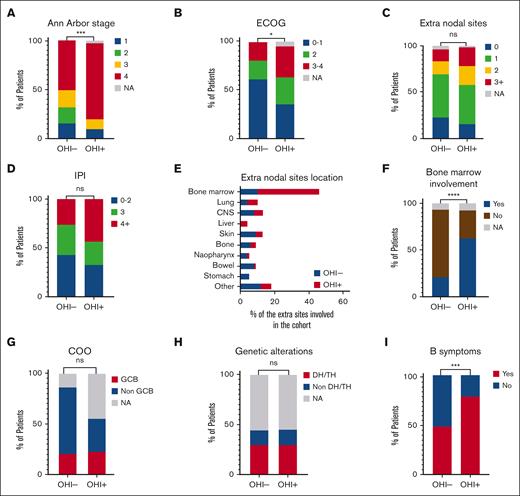
We analyzed the malignancy characteristics of all patients in the cohort. Certain disease features differed between the OHI+ and OHI− patients. OHI+ patients had a more advanced stage disease than OHI− patients (P = .002; Figure 2A). Thus, 77% of the OHI+ patients had stage IV lymphoma as opposed to 50% of the OHI− patients. Furthermore, a statistically significant difference was observed in patients’ performance status (P = .01; Figure 2B). A higher percentage of OHI+ patients had an ECOG score of 3 to 4 (32% in OHI+ vs 17% in OHI−). Conversely, fewer OHI+ patients had ECOG scores of 0 to 1 (35% vs 61% in OHI−), indicating a generally lower performance status in the OHI+ cohort. In contrast, the number of extranodal sites involved (Figure 2C) did not differ significantly between the groups (P = .87). Thus, overall, the lymphoma-relevant IPI did not differ between OHI+ and OHI− patients (P = .11; Figure 2D). However, the extranodal sites involved varied between the groups (Figure 2E). OHI− patients had bone marrow (15.1%), skin (14.6%), central nervous system (12.1%), and bowel (12.1%) involvement, whereas OHI+ patients primarily had bone marrow (54.5%) and lung (9.1%) involvement. Bone marrow involvement was significantly more common in OHI+ than OHI− patients (P < .0001; Figure 2F).
Some disease features differ between OHI+ and OHI− patients. Bar plots comparing OHI+ (sCD25 >3900 U/mL and ferritin >1000 ng/mL) and OHI− patients in terms of Ann Arbor stage (A); ECOG scores (B); extranodal sites (C); the lymphoma-relevant prognostic indexes (D); extranodal sites location as defined by positron emission tomography-computed tomography scan or bone marrow biopsy (E); bone marrow involvement (F); COO (G); genetic alterations (H); and B symptoms (I). Disease features were compared using the χ2 test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. CNS, central nervous system; DH/TH, double hit/triple hit lymphoma; NA, not available; ns, nonsignificant.
Some disease features differ between OHI+ and OHI− patients. Bar plots comparing OHI+ (sCD25 >3900 U/mL and ferritin >1000 ng/mL) and OHI− patients in terms of Ann Arbor stage (A); ECOG scores (B); extranodal sites (C); the lymphoma-relevant prognostic indexes (D); extranodal sites location as defined by positron emission tomography-computed tomography scan or bone marrow biopsy (E); bone marrow involvement (F); COO (G); genetic alterations (H); and B symptoms (I). Disease features were compared using the χ2 test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. CNS, central nervous system; DH/TH, double hit/triple hit lymphoma; NA, not available; ns, nonsignificant.
To evaluate whether OHI positivity was associated with specific biological features, we focused on the subgroup of patients with DLBCL, analyzing the COO and molecular abnormalities. The distribution of COO subtypes did not differ between the OHI− and OHI+ groups (P = .43; Figure 2G). In the OHI− group, 65% were classified as non-GCB subtype as opposed to 33% in the OHI+ group, whereas the GCB subtype accounted for 22% in both groups. Molecular abnormalities among patients with DLBCL also showed no significant differences between the groups (P = .99; Figure 2H).
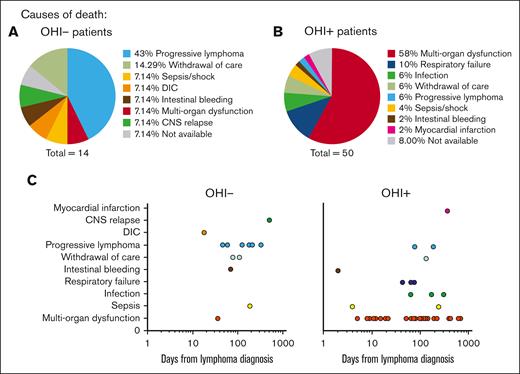
Excess mortality among OHI+ patients is predominantly attributed to early-onset MOF. Pie charts illustrating the proximate causes of mortality in OHI− (A) and OHI+ (B) patients (sCD25 >3900 U/mL and ferritin >1000 ng/mL). (C) Time to death from lymphoma diagnosis stratified by the proximate causes of mortality. CNS, central nervous system; DIC, disseminated intravascular coagulation.
Excess mortality among OHI+ patients is predominantly attributed to early-onset MOF. Pie charts illustrating the proximate causes of mortality in OHI− (A) and OHI+ (B) patients (sCD25 >3900 U/mL and ferritin >1000 ng/mL). (C) Time to death from lymphoma diagnosis stratified by the proximate causes of mortality. CNS, central nervous system; DIC, disseminated intravascular coagulation.
Notably, a significantly higher proportion of OHI+ patients presented with B symptoms than OHI− patients (78% vs 49%; P = .0004; Figure 2I). Furthermore, 86% of the OHI+ patients also met the HLH-2004 criteria as opposed to 15% of the patients in the OHI− group (P < .0001; Table 1), indicating a strong association between OHI+ and an inflammatory phenotype.
Thus, OHI+ status was not associated with IPI, double-/triple-hit lymphoma, or COO, indicating that OHI identified a novel, high-risk subgroup of patients with lymphoma who were not captured by the traditional prognostic indexes.
OHI+ patients died mainly because of multiorgan failure (MOF)
During the 3-year follow-up period, a total of 64 patients in the cohort died (14 in the OHI− group and 50 in the OHI+ group). Among the OHI− patients who died, the most common proximate cause of death was lymphoma progression, accounting for 6 cases (43%), as illustrated in Figure 3A. Progressive lymphoma was also the primary underlying cause of death in 70% of OHI− patients (supplemental Figure 3A).
In contrast, the most frequent proximate cause of death among OHI+ patients was MOF, responsible for 29 cases (58%; P = .02), as illustrated in Figure 3B. Other proximate causes included respiratory failure (10%), infections (6%), withdrawal of care (6%), progressive lymphoma (6%), and sepsis (4%). In addition, the OHI+ group demonstrated a broader range of underlying death causes as shown in supplemental Figure 3B. Progressive lymphoma accounted for 40% of deaths, followed by infections (23%) and MOF (6%). Additional factors, detailed in Figure 4B, underscore the complex and multifactorial nature of mortality in OHI+ patients. This distribution highlights the complex interplay among the factors that lead to mortality in OHI+ patients in contrast with what is seen in OHI− patients with mortality mostly attributed to progressive lymphoma.
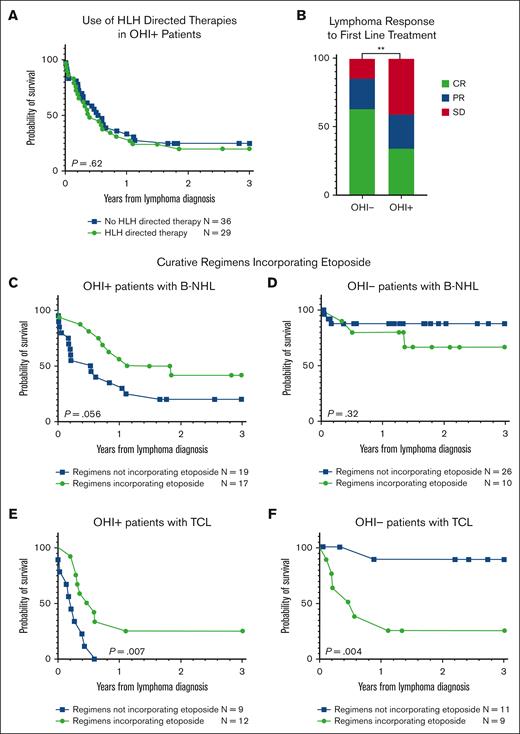
Incorporation of etoposide into the therapeutic regimens is associated with improved survival in patients with OHI+ lymphoma. Kaplan-Meier curves of OHI+ patients (sCD25 >3900 U/mL and ferritin >1000 ng/mL) in the cohort stratified by use of HLH-directed treatments (A). (B) Bar plots of the percentage of lymphoma responses as defined by the Lugano criteria after the first line of therapy. Statistical analyses were conducted using the Fisher exact test; Kaplan-Meier curves of patients with lymphoma in the cohort stratified by inclusion of etoposide in the malignancy-directed therapeutic regimen, in OHI+ (C) and OHI− (D) B-NHL, and OHI+ (E) and OHI− (F) T-cell lymphoma. The number at risk is presented for each group. Statistical analysis was performed using the log-rank (Mantel-Cox) test, and the P values are presented on the plots. CR, complete response; PR, partial response; SD, stable disease; TCL, T-cell lymphoma.
Incorporation of etoposide into the therapeutic regimens is associated with improved survival in patients with OHI+ lymphoma. Kaplan-Meier curves of OHI+ patients (sCD25 >3900 U/mL and ferritin >1000 ng/mL) in the cohort stratified by use of HLH-directed treatments (A). (B) Bar plots of the percentage of lymphoma responses as defined by the Lugano criteria after the first line of therapy. Statistical analyses were conducted using the Fisher exact test; Kaplan-Meier curves of patients with lymphoma in the cohort stratified by inclusion of etoposide in the malignancy-directed therapeutic regimen, in OHI+ (C) and OHI− (D) B-NHL, and OHI+ (E) and OHI− (F) T-cell lymphoma. The number at risk is presented for each group. Statistical analysis was performed using the log-rank (Mantel-Cox) test, and the P values are presented on the plots. CR, complete response; PR, partial response; SD, stable disease; TCL, T-cell lymphoma.
Incorporating etoposide in the malignancy-directed treatment regimen has a survival benefit in OHI+ patients with T-cell lymphoma
The diagnosis of HLH triggered a change in treatment in 29 of the OHI+ patients (45%). These included either administration of HLH-directed treatments or a change in the lymphoma treatment protocol. The HLH-directed treatments are summarized in supplemental Figure 4A and include dexamethasone alone in 29% of the patients (n = 8), dexamethasone in combination with etoposide in 25% of patients (n = 7) or in combination with of etoposide and tocilizumab in 14% of patients (n = 4), and a pulse of methylprednisolone in 11% of patients (n = 3). Other treatments were used less frequently (supplemental Figure 4A). Notably, HLH-directed therapy, irrespective of agent/s used, did not improve the OS when compared with patients for whom no treatment changes were implemented (P = .62), as shown in Figure 4A.
Regarding the lymphoma-directed treatments (supplemental Figure 4B-C), 89% of the OHI+ and 90% of the OHI− patients received malignancy-directed treatments. The most common treatment was the CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone) in both groups with 45.3% of the OHI+ patients (supplemental Figure 4B) and 45.7% of the OHI− patients (supplemental Figure 4C) receiving this treatment. In 9.4% of OHI+ patients, etoposide was added to the CHOP backbone, administered either as part of the dose-adjusted EPOCH (DA-EPOCH) regimen or the CHOEP regimen–both of which include etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin–each used in 4.7% of patients. Analysis of the lymphoma response to first-line treatment revealed superior results in the OHI+ group than in the OHI− group (P = .006; Figure 4B): Among those with response data available, 34% of the OHI+ group achieved a complete response to first-line treatment, 25% achieved a partial response, and 41% had stable or progressive disease, as illustrated in Figure 4B. By comparison, a complete response rate of 63% was achieved in the OHI− group, a partial response rate of 22%, and 15% had stable or progressive disease.
Notably, the incorporation of etoposide into malignancy-directed therapy was associated with improved 3-year OS among OHI+ patients. This trend approached statistical significance in B-NHL (HR, 0.5; 95% CI, 0.2-1.0; P = .056; Figure 4C), however, it showed no survival benefit in OHI− patients (HR, 2.2; 95% CI, 0.4-12; P = .32; Figure 4D).
Moreover, etoposide incorporation was significantly associated with improved 3-year OS in patients with OHI+ T-cell lymphoma (HR, 0.3; 95% CI, 0.1-0.9; P = .007; Figure 4E), whereas in patients with OHI− T-cell lymphoma, it was linked to significantly poorer survival (HR, 9.8; 95% CI, 2.0-47; P = .004; Figure 4F). Of note, these analyses included first-line treatment and all subsequent lines of treatment during the observation period.
Discussion
In this study, we demonstrated that patients with OHI+ lymphoma have higher mortality rates and lower EFS, which was not predicted by their disease-relevant IPI score. Importantly, OHI+ patients succumbed more frequently to MOF, respiratory failure, and infections rather than to the lymphoma itself. Despite OHI+ patients often presenting with more advanced Ann Arbor stage and lower performance status, their lymphoma-relevant IPI did not significantly differ from that of OHI− patients. In addition, although treatments that directly targeted HLH did not improve patient outcomes, incorporating etoposide into the anti-lymphoma chemotherapy regimen was associated with superior OS. This study confirms the unique prognostic value of the OHI index in lymphoma and underscores the distinct clinical trajectory of OHI+ patients, emphasizing the need for tailored therapeutic strategies.
The key finding of this study is that the most common causes of death among OHI+ patients were MOF, single organ failure, or infection, whereas the proximate cause of death was lymphoma in 6%. In contrast, OHI− patients mortality was primarily attributed to the progression of lymphoma. This clear difference suggests that the OHI identifies clinically significant inflammation, which may lead to premature death. This inflammation is likely related to the new-onset malignancy in these patients but is clearly maladaptive because it seems to drive morbidity and early mortality.
These findings suggest that the OHI index may help to identify a subset of patients with lymphoma who require alternative therapeutic strategies to manage dysregulated inflammation. Although incorporating etoposide into any line of malignancy-directed treatment was associated with inferior 3-year OS in OHI− T-cell lymphoma, it was strongly linked to improved 3-year OS among patients with OHI+ T-cell lymphoma and showed a near-statistically significant trend toward a survival benefit in patients with OHI+ B-NHL.
Thus, adding etoposide, an essential component of the HLH-2004 protocol,12 was associated with improved OS. This result aligns with a previous single-arm, open-label, phase 2 trial in which the DA-EPOCH regimen as first-line treatment for NHL-associated HLH demonstrated a 5-year OS rate of 73.1% ± 8.7%. However, it contrasts with the poor 3.4% ± 3.4% 12-month OS in T-cell lymphoma.23
Moreover, novel therapies,24-27 as well as incorporating etoposide into chemotherapy regimens such as the DEP regimen (liposomal doxorubicin, etoposide, and high-dose methylprednisolone),28,29 should be rigorously investigated in these patients. The Adult HLH Working Group of the Histiocyte Society is conducting a multicenter international study to gather real-world evidence and evaluate the efficacy of different treatment strategies in lymphoma-associated hyperinflammation (ClinicalTrials.gov identifier: NCT05898477). These treatment strategies may be investigated using the simplified OHI index in prospective, placebo-controlled trials in the future.
OHI+ patients exhibited a significantly higher mortality rate than expected based on traditional prognostic tools, such as lymphoma-relevant IPI, and was not associated with high-risk COO classification and molecular abnormalities, such as double-/triple-hit lymphoma. This suggests that the OHI identifies a high-risk factor currently overlooked in standard assessments. Over the past decade, elevated sCD25 has been established as an independent predictor of outcomes across various HMs.30-34 Similarly, ferritin has shown prognostic value both in B- and T-cell lymphoma.35-38 Although sCD25 is commonly elevated in patients with lymphoma39 and elevated ferritin may be attributed to underlying HMs, blood transfusions, or other medical conditions,40 the synergy captured by the OHI aligns with HLH pathogenesis. This involves activation of both adaptive and innate immune cells with sCD25 serving as a marker of activated T cells41 and ferritin being produced by activated macrophages.42 The OHI’s ability to integrate these markers provides a more comprehensive assessment of the inflammatory state, potentially offering improved risk stratification and guiding more tailored treatment approaches for patients with high-risk lymphoma.
It is essential to recognize that MOF in OHI+ patients may stem from the lymphoma itself, and the significantly poorer prognosis observed in OHI+ patients suggests a more aggressive disease phenotype or potential resistance to standard chemotherapy. In our cohort, patients had decreased EFS and inferior responses to first-line therapy, supporting a contribution of resistant malignancies. Distinguishing MOF caused by disease progression from other etiologies is challenging, yet the consistently worse outcomes in this group highlight the need for alternative treatment strategies. OHI+ status may indicate a requirement for more intensive or novel therapies to address both hyperinflammation and the aggressive nature of the lymphoma. Future research should focus on elucidating the mechanisms behind these poor outcomes and exploring targeted interventions for this high-risk population.
Although our study yields valuable insights into lymphoma-associated hyperinflammation, it is essential to acknowledge its inherent limitations, primarily stemming from its retrospective design. Our findings may be susceptible to selection bias because HLH was clinically suspected in some of the study patients. To address this limitation, we are conducting a prospective study to assess the diagnostic capability of the OHI index and its prognostic significance in patients with newly diagnosed HMs (ClinicalTrials.gov identifier: NCT05882175). Another limitation is the relatively small sample size, which may have led to an underestimation of the significant differences between the groups. In addition, although the IPI is primarily validated for use in DLBCL, its relevance in T-cell lymphomas may be limited. Nonetheless, it was included as a reference point in this analysis. Finally, analyzing the effects of the treatments our patients received for lymphoma and for HLH on OS is challenging because of their marked variability and the influence of confounding factors. Despite these deficiencies, this analysis demonstrates the severity and uniqueness of the OHI+ patients’ disease course.
To our knowledge, this study represents the first description of the causes of death among patients with a positive hyperinflammatory index associated with lymphoma. Our findings indicate that these patients most often die from proximate causes that are related to detrimental inflammation. Our finding that incorporating etoposide into the malignancy-directed treatment regimen warrants prospective validation in future studies. Nonetheless, early identification of such patients using sCD25 and ferritin levels as defined for the OHI index may be a first step toward altering their disease trajectory.
Acknowledgments
This work was supported by National Institutes of Health/National Cancer Institute grant R21CA256390 (M.B.J.), an American Society of Hematology Global Research award (A.Z.-L.), a Conquer Cancer–Israel Cancer Research Fund Career Development award (A.Z.-L.), and the Varda and Boaz Dotan Foundation of Tel Aviv University (A.Z.-L.).
Authorship
Contribution: A.Z.-L. and M.B.J. initiated the project, designed the research, analyzed the data, and wrote the manuscript with inputs from other authors; M.E. contributed to writing the manuscript; J.M., I.C., S.M., A.E.H.N., H.L.C., and S.W. collected the data; R.G., G.I., P.R., J.Y., A.N., S.I., S.N., N.D., and N.B. edited the manuscript and provided essential inputs; and G.G. helped to design the data collection tool.
Conflict-of-interest disclosure: A.Z.-L. and M.B.J. report consulting fees from Sobi. R.G. reports consulting fees from Roche, Takeda, Novartis, Gilead Sciences, and AbbVie, none of which is directly related to the content of this study. J.M. reports consulting fees from Chugai Pharmaceutical for work that was not directly related to the content of this article. The remaining authors declare no competing financial interests.
Correspondence: Michael B. Jordan, Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH, 45229; email: michael.jordan@cchmc.org; and Adi Zoref-Lorenz, Hematology Institute, Meir Medical Center, 59 Tchernichovsky St, Kfar Saba 4428164, Israel; email: adi.zoref.lorenz@cchmc.org.
References
Author notes
Original data are available on request from the corresponding author, Adi Zoref-Lorenz (adi.zoref.lorenz@cchmc.org).
The full-text version of this article contains a data supplement.