Key Points
FXR transcriptionally represses the expression of hepatic PAI-1.
FXR plays a novel role in regulating DVT, linking metabolism to thrombus formation.
Visual Abstract
Obesity is a major health issue and a risk factor for venous thromboembolic disease. Plasminogen activator inhibitor 1 (PAI-1), encoded by the gene SERPINE1, is a negative regulator of fibrinolysis and has been associated with obesity. The liver, which senses obesity-induced metabolic stress, is a key determinant of circulating PAI-1 levels. However, the mechanisms underlying the increased PAI-1 expression in obesity are unclear. This study investigated the upstream regulation of PAI-1 and its role in fibrinolysis and deep vein thrombosis (DVT). Compared with lean mice, diet-induced obesity mice presented significantly shorter fibrinolysis times and larger venous thrombi, largely due to increased hepatocyte expression of PAI-1. A publicly available single-cell RNA sequence data set from the livers of individuals with obesity suggested that increased PAI-1 expression may be related to reduced hepatocyte farnesoid X receptor (FXR) signaling. FXR activation also suppressed Serpine1 mRNA and PAI-1 protein expression levels in both mice and primary mouse hepatocytes (MPHs), but a decrease in PAI-1 in MPHs of Fxr-null mice after FXR activation was not observed. Both Fxr-null mice and Fxrfl/fl mice with AAV8-TBG-Cre exhibited significantly elevated plasma PAI-1, resulting in further impaired fibrinolysis and increased DVT burden. Dual-luciferase reporter assays and chromatin immunoprecipitation suggested that FXR activation directly represses Serpine1 transcription. Importantly, tropifexor treatment of obese mice lowered plasma PAI-1 levels and further alleviated fibrinolysis and the DVT load. These findings suggest that targeting FXR in hepatocytes may improve fibrinolysis and reduce DVT risk.
Introduction
Venous thromboembolic disease (VTE) is a major contributor to global morbidity and mortality.1,2 The prevalence of VTE is further exacerbated by the global pandemic of obesity with its well-documented increased risk of deep vein thrombosis (DVT).3-5 A major contributor to increased thrombosis in obesity is a reduction in fibrinolysis,6,7 the primary mechanism that dissolves blood clots.8 Multiple studies have also detected abnormal expression of proteins9-13 that regulate fibrinolysis in obese humans and mice, including fibrin degradation products (FDPs), tissue plasminogen activator (tPA), plasminogen activator inhibitor-1 (PAI-1), and α2-antiplasmin (α2-AP).
Increased plasma levels of PAI-1 have been demonstrated in both humans and mice with obesity.9,14-16SERPINE1 is a member of the serine protease inhibitor superfamily encoding fibrinolytic plasminogen activator inhibitor 1 (PAI-1). PAI-1 has long been known as a potential target for DVT, but no inhibitors of PAI-1 have yet been approved for antithrombotic therapy. Thus, the reasons for the notable increase in PAI-1 in obesity deserve attention, which raises questions regarding the sources and regulatory mechanisms of PAI-1 in obesity. One source of PAI-1 is adipocytes, and elevated adipocyte PAI-1 was proposed as the dominant source for increased PAI-1 and the consequent fibrinolysis defect in obesity.7,17,18 However, when blood from subcutaneous adipose arteries and veins was assayed for PAI-1 protein and activity in obese humans, there was no PAI-1 arteriovenous gradient.19 These data raise the possibility that 1 or more other sources of circulating PAI-1 may be important in obesity. Interestingly, hepatocytes are sensitive to the metabolic stress that occurs in obesity and hepatocytes can produce PAI-1.20-22 A study revealed that ∼70% of the plasma PAI-1 derived from in diet-induced obese (DIO) mice is produced by hepatocytes.16 Together, evidence for a dominant contribution of hepatocytes to PAI-1 production in obesity seems to be more direct and robust.
The mechanisms underlying obesity induced alterations in PAI-1 expression remain uncharacterized. In this study, we show that hepatocyte-derived PAI-1 is negatively regulated by the nutrient-sensing nuclear receptor farnesoid X receptor (FXR), as a novel regulator of PAI-1, plays important roles in fibrinolysis and DVT.
Methods
IVC stenosis models
Inferior vena cava (IVC) stenosis was induced in mice as previously described.23-26 After 24 hours, the IVC was harvested and inspected for thrombus formation. The thrombus was then measured and weighed to evaluate the DVT burden. The detailed description of the method can be found in the supplemental Methods, available on the Blood website.
Mouse tail-bleeding assay
Mice were anesthetized with isoflurane and positioned horizontally on a platform that allowed the tail to descend. A distal tail segment was transected with a number 11 surgical scalpel to induce a wound ∼2 mm in diameter. Bleeding time was monitored by gently dabbing the tail tip on Whatman paper every 10 seconds until bleeding ceased.27 The bleeding time was defined as the interval from incision to stable cessation of bleeding, with no rebleeding for at least 60 seconds. Bleeding exceeding 15 minutes was stopped by applying pressure.
Mouse plasma collection and analyses
Blood obtained by IVC puncture into 10% volume of sodium citrate (3.8%, w/v) was centrifuged for 20 minutes at 2300g, and the plasma was carefully collected from the supernatant fraction. The plasma samples were divided into aliquots, snap-frozen, and stored at −80°C until analysis. The total plasma antigen levels of FDP, tPA, PAI-1, α2-AP, and plasminogen (Plg) were measured by ELISA (enzyme-linked immunosorbent assay) using kits according to the manufacturer’s instructions.
Fibrin formation and lysis
TF, phospholipids, and recombinant tissue plasminogen activator (rtPA) (10 μL of 1 pM, 4 μM, 1.25 μg/mL, final, respectively) were added to a 96-well plate.13 Diluted plasma (40 μL of 1:3 dilution) was then added, and the reactions were initiated by the addition of 10 μL of CaCl2 (16.6 mM, final). Clot formation and lysis were monitored at 405 nm using a Synergy 2 microplate reader (BioTek Instruments) for 1 hour at 37°C. The data were analyzed as previously described.28 The clot lysis time was defined as the time from a 50% increase in turbidity to a 50% turbidity decrease in turbidity.
Mouse primary hepatocytes experiments
Primary mouse hepatocytes (MPHs) were isolated from WT, HFD, or Fxr-null mice (all on a C57BL/6J background) as described previously.29 Cells were cultured in DMEM supplemented with 10% fetal bovine serum at 37°C in a humidified atmosphere of 5% CO2. For subsequent experiments, cells were switched to serum-free medium prior to harvesting for immunoblotting or mRNA quantification. Culture supernatants were collected after centrifugation at 2000g for 10 minutes, snap-frozen in liquid nitrogen, and stored at −80°C until processing.
Luciferase reporter assays
Cells were transfected with the appropriate plasmids in 24-well plates. After 48 hours, cells were harvested and lysed for luciferase assays using the Dual-Luciferase Reporter Assay System (Promega, WI) according to the manufacturer's protocol. Firefly luciferase activity was normalized to Renilla luciferase as an internal control. All transfections were performed in triplicate for each construct. Detailed procedures are provided in the supplemental Methods.
Ch-IP assay
Chromatin immunoprecipitation (Ch-IP) assays were conducted with the Pierce Agarose Ch-IP Kit (Thermo Scientific) following the manufacturer’s protocol. Four primer pairs targeting predicted FXR binding sites in the PAI-1 promoter, as identified by the JASPAR database (https://jaspar.genereg.net), were designed, including a distal control (Ch-IP1). Ch-IP primer sequences and corresponding PAI-1 promoter amplified sequences are listed in supplemental Table 1. Cells were crosslinked with 1% formaldehyde for 10 minutes at room temperature and quenched with glycine. Chromatin was then sheared by sonication and immunoprecipitated using an FXR antibody (Cell Signaling Technology). Precipitated DNA was analyzed by PCR to amplify regions containing FXR binding sites. Detailed procedures are described in the supplemental Methods.
Platelet aggregation assay
Mice were anesthetized, and whole blood was collected into tubes containing 10% (v/v) sodium citrate (3.8% w/v). Platelet-rich plasma (PRP) was obtained by centrifugation at 160g for 10 minutes at room temperature. Platelet-poor plasma (PPP) was prepared by further centrifuging PRP at 2000g for 10 minutes. PRP platelet counts were adjusted to 500 × 109/L using PPP. Platelet aggregation was assessed by optical turbidimetry at 37°C using 2.5 μM ADP as the agonist, with real-time monitoring for 6 minutes. Detailed methodology is described in the supplemental Methods.
Plasmin generation assay
Trigger solution (0.5 pM TF, 4 μM phospholipid, 0.31 μg/mL rtPA) was added to assay wells. Calibration wells received α2-macroglobulin-plasmin complex. Diluted plasma (40 μL of 1:3 dilution) was then added, and the reactions were initiated by adding 10 μL of substrate/calcium solution. After shaking, fluorescence (excitation/emission 390/460 nm) was monitored every 50 seconds for 60 minutes. The detailed description of the method can be found in the supplemental Methods.
Data acquisition
Single-cell RNA sequencing (scRNA-seq) and single-nuclei RNA sequencing (snRNA-seq) data from healthy and obese human livers were retrieved from the Gene Expression Omnibus (GEO) database (accession number GSE192740). Additionally, transcriptome microarray data from liver samples of healthy and obese humans (accession number GSE130991) and bulk RNA sequencing (RNA-seq) data from baboons on a high-fat diet (accession number GSE139981) were obtained. All data sets were accessed via the GEO database (https://www.ncbi.nlm.nih.gov/geo/). The detailed description of the method can be found in the supplemental Methods.
Statistical analysis
Statistical analyses were performed using R version 4.2.0 (R Foundation for Statistical Computing). Data visualization was generated with the ggplot2 package. Concentrated and discrete trends for continuous variables are expressed as the mean ± standard errors. Depending on normality, the unpaired t test was used to compare 2 groups, and the 1-way analysis of variance followed by Tukey-adjusted post hoc comparisons was used to compare multiple groups. Two-sided P < .05 indicated that the results were statistically significant.
Results
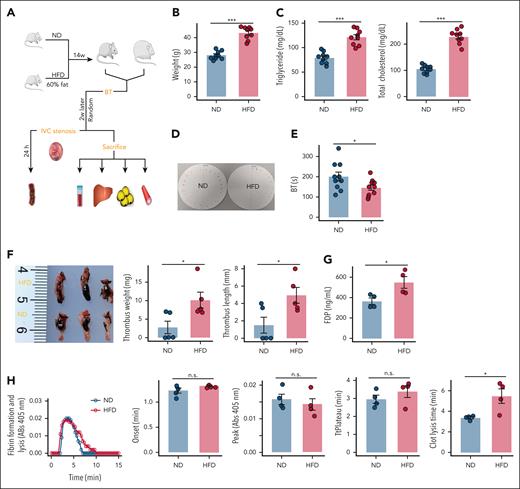
HFD mice have a shortened fibrinolysis time and exacerbated DVT load
To evaluate the progression of the obese phenotype in mice, we assessed hematologic and metabolic outcomes in male C57BL/6J mice that were fed either an high-fat diet (HFD) or normal diet (ND) for a duration of 16 weeks (Figure 1A). As expected, the HFD mice presented an increase in body weight (Figure 1B) and elevated levels of plasma triglycerides and cholesterol compared with the lean mice (Figure 1C). Moreover, a significantly shorter tail-bleeding time and greater thrombus load (assessed by both thrombus length and weight) were observed in HFD mice (Figure 1D-F). Most importantly, HFD mice had increased FDP (Figure 1G), indicating endogenous fibrin formation and lysis. These mice were then used to test the hypothesis that HFD alters plasma fibrin formation and lysis. The combined functional effects of obesity on plasma fibrin clot formation and lysis were revealed in turbidity assays, in which plasma samples from HFD mice presented normal fibrin formation kinetics but significantly prolonged clot lysis times (Figure 1H). Collectively, these findings suggest that the effect of HFD on DVT load is due in part to a decreased fibrinolytic response.
HFD delays fibrinolysis and exacerbates the DVT load in mice. (A) Schematic of the experimental model. The mice were fed an ND or an HFD for 4 months, after which tail bleeding assays were performed. After 2 weeks of recovery, each group of mice was randomly selected to evaluate the DVT burden. The remaining mice were euthanized, and sodium citrate anticoagulated blood and tissues were collected (N = 9). (B) Mean body weight (N = 9). (C) Plasma triglyceride and cholesterol levels (N = 9). (D-E) Tail bleeding time (N = 9). (F) Thrombus weight and length (N = 5). (G) Plasma FDP levels (N = 4). (H) Representative curves for plasma fibrin formation and lysis tests and parameters of the curves for fibrinolysis (N = 4). The data are represented as the mean ± standard error of the mean (SEM), n.s. P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 vs ND group. BT, bleeding time; n.s., no significance.
HFD delays fibrinolysis and exacerbates the DVT load in mice. (A) Schematic of the experimental model. The mice were fed an ND or an HFD for 4 months, after which tail bleeding assays were performed. After 2 weeks of recovery, each group of mice was randomly selected to evaluate the DVT burden. The remaining mice were euthanized, and sodium citrate anticoagulated blood and tissues were collected (N = 9). (B) Mean body weight (N = 9). (C) Plasma triglyceride and cholesterol levels (N = 9). (D-E) Tail bleeding time (N = 9). (F) Thrombus weight and length (N = 5). (G) Plasma FDP levels (N = 4). (H) Representative curves for plasma fibrin formation and lysis tests and parameters of the curves for fibrinolysis (N = 4). The data are represented as the mean ± standard error of the mean (SEM), n.s. P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 vs ND group. BT, bleeding time; n.s., no significance.
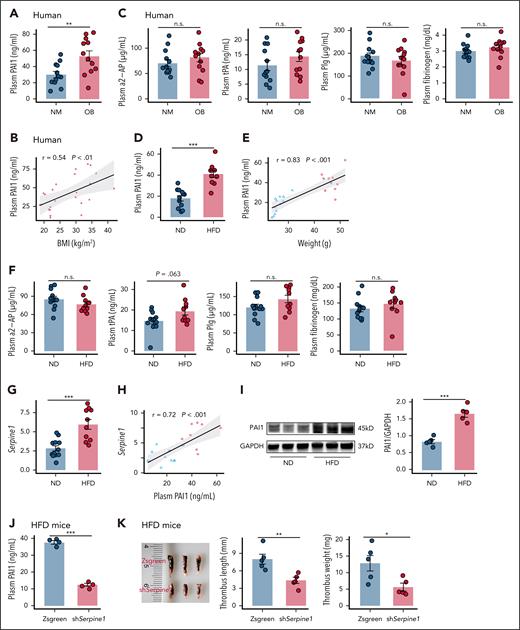
Hepatic PAI-1 overexpression mediates obesity-induced impaired fibrinolysis
To identify the molecular mechanisms of low fibrinolytic activity in obesity, circulating components already known to affect the fibrinolytic system were measured. Plasma PAI-1 was markedly elevated in individuals with obesity and positively correlated with body mass index (BMI) (Figure 2A-B). Sex-stratified analysis showed slightly lower plasma PAI-1 levels in women compared to men (supplemental Figure 2). Similarly, obese mice exhibited increased plasma PAI-1 (Figure 2D-E). In contrast, the concentrations of tPA, Plg, α2-AP, and fibrinogen showed no significant differences between lean and obese states in either humans (Figure 2C) or mice (Figure 2F). To further elucidate the cellular source of increased plasma PAI-1 in HFD mice, the expression levels of PAI-1 in mouse adipose tissue, blood vessel and the liver were analyzed. Consistent with previous studies, PAI-1 protein and Serpine1 mRNA levels in the liver significantly increased, without notably changing the levels of PAI-1 expression in two other tissues of HFD mice (Figure 2G,I; supplemental Figure 1). In addition, the plasma PAI-1 protein level was strongly correlated with the liver Serpine1 mRNA level in mice (Figure 2H). Moreover, others reported a decrease of ∼70% in the plasma PAI-1 protein in Serpine1-silenced hepatocytes of HFD mice.16 To directly assess the role of hepatic PAI-1 in thrombosis, we administered liver-targeted AAV8-TBG-shSerpine1 in HFD mice, followed by IVC stenosis. This intervention significantly reduced plasma PAI-1 levels and decreased thrombus length and weight (Figure 2J-K; supplemental Figure 3). In summary, obesity is associated with increased hepatic PAI-1 expression, which contributes to impaired plasma fibrinolysis.
Obesity elevates hepatic and circulating PAI-1 levels in mice. (A-B) Plasma PAI-1 levels (A) and their associations with BMI (B) among individuals (N = 12). (C) Plasma α2-AP, tPA, Plg and fibrinogen levels in individuals (N = 12). (D-E) Plasma PAI-1 levels (D) and their association with weights (E) in mice fed an ND or an HFD for 4 months. (F) Plasma α2-AP, tPA, Plg and fibrinogen levels in mice (N = 12). (G-H) Liver Serpine1 mRNA levels (G) and their associations with plasma PAI-1 (H) levels (N = 12) in mice. (I) Livers of mice were assayed for PAI-1 protein and the GAPDH loading control by immunoblotting with densitometric quantification shown (N = 5). (J-K) C57BL/6J mice were fed an HFD for 4 months, followed by injection with either AAV8-TBG-shSerpine1 or AAV8-TBG-Zsgreen: (J) plasma PAI-1 levels (N = 4); (K) thrombus weight and length (N = 5). The data are represented as the mean ± SEM, n.s. P > .05; ∗∗P < .01; ∗∗∗P < .001 vs ND group or HFD mice with AAV8-TBG-Zsgreen group. BMI, body mass index; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NM, normal weight; n.s., no significance; OB, obese.
Obesity elevates hepatic and circulating PAI-1 levels in mice. (A-B) Plasma PAI-1 levels (A) and their associations with BMI (B) among individuals (N = 12). (C) Plasma α2-AP, tPA, Plg and fibrinogen levels in individuals (N = 12). (D-E) Plasma PAI-1 levels (D) and their association with weights (E) in mice fed an ND or an HFD for 4 months. (F) Plasma α2-AP, tPA, Plg and fibrinogen levels in mice (N = 12). (G-H) Liver Serpine1 mRNA levels (G) and their associations with plasma PAI-1 (H) levels (N = 12) in mice. (I) Livers of mice were assayed for PAI-1 protein and the GAPDH loading control by immunoblotting with densitometric quantification shown (N = 5). (J-K) C57BL/6J mice were fed an HFD for 4 months, followed by injection with either AAV8-TBG-shSerpine1 or AAV8-TBG-Zsgreen: (J) plasma PAI-1 levels (N = 4); (K) thrombus weight and length (N = 5). The data are represented as the mean ± SEM, n.s. P > .05; ∗∗P < .01; ∗∗∗P < .001 vs ND group or HFD mice with AAV8-TBG-Zsgreen group. BMI, body mass index; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NM, normal weight; n.s., no significance; OB, obese.
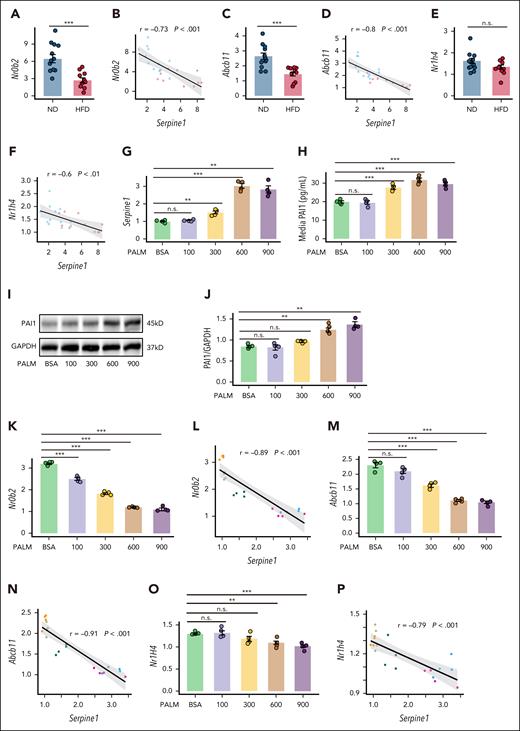
Transcriptomic and targeted bile acid metabolomic responses to obesity suggest altered FXR signaling
Analysis of a public single-cell RNA-seq data set (GSE192740) from obese human livers revealed that Serpine1 was predominantly expressed in hepatocytes (Figure 3A-B). Differential genes in the individuals with obesity were mapped onto Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. Among the top 10 significant pathways, bile acid (BA) secretion was enriched (Figure 3C), with the differentially expressed genes being primarily direct or indirect targets of FXR (Figure 3D). Similar pathway enrichment was observed in public transcriptomic data from obese individuals (GSE130991; Figure 3E) and in livers of baboon after long-term HFD feeding (GSE139981; Figure 3F). Importantly, hepatic expression of the direct FXR (NR1H4) targets, ABCB11 and NR0B2, were sharply decreased in both obese individuals (Figure 3D,G) and baboons (Figure 3H), suggesting decreased FXR signaling. Furthermore, strong negative correlations between of SERPINE1 with NR1H4, NR0B2, and ABCB11 were also identified in the baboons (GSE139981; Figure 3I-K).
Prediction of the upstream regulator of PAI-1 in obesity. (A) UMAP plots of cell populations in the human liver dataset (GSE192740). (B) Expression of Serpine1 across different cell types in the human liver dataset (GSE192740). (C) KEGG pathway analysis of differentially expressed liver genes in human livers (GSE192740). (D) FXR related genes in the BA pathway in hepatocytes (GSE192740). (E) KEGG analysis of liver differential genes in obese and normal weight individuals (GSE130991). (F) KEGG analysis of liver differential genes in baboons before and after high-fat feeding in GSE139981. (G) Heat map showing expression of PAI-1 (Serpine1), FXR (NR1H4), and FXR target genes (NR0B2 and ABCB11) in human livers from 737 non-statin-treated patients, stratified by BMI (GSE130991). (H) The expression of PAI-1, FXR and FXR direct targets in baboon liver samples (GSE139981) at 0 weeks, 7 weeks and 2 years post HFD. (I-K) Correlation coefficients of PAI-1 with FXR and FXR direct target genes in baboon livers (GSE139981). UMAP, uniform manifold approximation and projection.
Prediction of the upstream regulator of PAI-1 in obesity. (A) UMAP plots of cell populations in the human liver dataset (GSE192740). (B) Expression of Serpine1 across different cell types in the human liver dataset (GSE192740). (C) KEGG pathway analysis of differentially expressed liver genes in human livers (GSE192740). (D) FXR related genes in the BA pathway in hepatocytes (GSE192740). (E) KEGG analysis of liver differential genes in obese and normal weight individuals (GSE130991). (F) KEGG analysis of liver differential genes in baboons before and after high-fat feeding in GSE139981. (G) Heat map showing expression of PAI-1 (Serpine1), FXR (NR1H4), and FXR target genes (NR0B2 and ABCB11) in human livers from 737 non-statin-treated patients, stratified by BMI (GSE130991). (H) The expression of PAI-1, FXR and FXR direct targets in baboon liver samples (GSE139981) at 0 weeks, 7 weeks and 2 years post HFD. (I-K) Correlation coefficients of PAI-1 with FXR and FXR direct target genes in baboon livers (GSE139981). UMAP, uniform manifold approximation and projection.
Our experimental data from obese mice recapitulated these findings (Figure 4A-F). To further investigate obesity-associated hepatic pathology in vitro, we treated MPHs with palmitic acid, a saturated fatty acid that recapitulates key features of hepatocyte dysfunction in obesity.30-33 After palmitic acid treatment of MPHs, the mRNA level of PAI-1 (Serpine1), its protein level, and the content of PAI-1 in the culture supernatant increased significantly with increasing palmitic acid concentration (Figure 4G-J). The greatest increase in Serpine1 expression was observed at 12 h after palmitic acid intervention (supplemental Figure 4A). We also successfully isolated primary hepatocytes from HFD mice, as confirmed by glycogen and Oil Red O staining (supplemental Figure 4B-C), and demonstrated that these cells secreted significantly higher levels of PAI-1 in the supernatant than did wild-type hepatocytes (supplemental Figure 4D). Furthermore, palmitic acid treatment resulted in decreases in the mRNA levels of FXR and FXR direct target genes, including Nr0b2 and Abcb11, which were negatively correlated with the levels of Serpine1 (Figure 4K-P). In summary, obesity is associated with attenuated FXR signaling and increased synthesis and secretion of PAI-1 in hepatocytes.
PAI-1 expression is strongly correlated with the expression of direct FXR target genes in the liver. (A-F) Expression levels of FXR and FXR direct target genes and their association with PAI-1 in the livers of mice fed an ND or an HFD for 4 months (N = 12). Relative Serpine1 mRNA (G) and PAI-1 protein levels (H) in the supernatant of cultured medium, and (I-J) PAI-1 protein levels in MPHs treated with different concentrations of palmitic acid for 12 hours (N = 4). (K-P) mRNA expression levels of FXR and FXR direct target gene mRNAs and their associations with Serpine1 mRNA after treatment of MPHs and different concentrations of palmitic acid for 12 hours (N = 4). The data are represented as the mean ± SEM, n.s. P > .05; ∗∗P < .01; ∗∗∗P < .001 vs the BSA group. BSA, bovine serum albumin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; n.s., no significance.
PAI-1 expression is strongly correlated with the expression of direct FXR target genes in the liver. (A-F) Expression levels of FXR and FXR direct target genes and their association with PAI-1 in the livers of mice fed an ND or an HFD for 4 months (N = 12). Relative Serpine1 mRNA (G) and PAI-1 protein levels (H) in the supernatant of cultured medium, and (I-J) PAI-1 protein levels in MPHs treated with different concentrations of palmitic acid for 12 hours (N = 4). (K-P) mRNA expression levels of FXR and FXR direct target gene mRNAs and their associations with Serpine1 mRNA after treatment of MPHs and different concentrations of palmitic acid for 12 hours (N = 4). The data are represented as the mean ± SEM, n.s. P > .05; ∗∗P < .01; ∗∗∗P < .001 vs the BSA group. BSA, bovine serum albumin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; n.s., no significance.
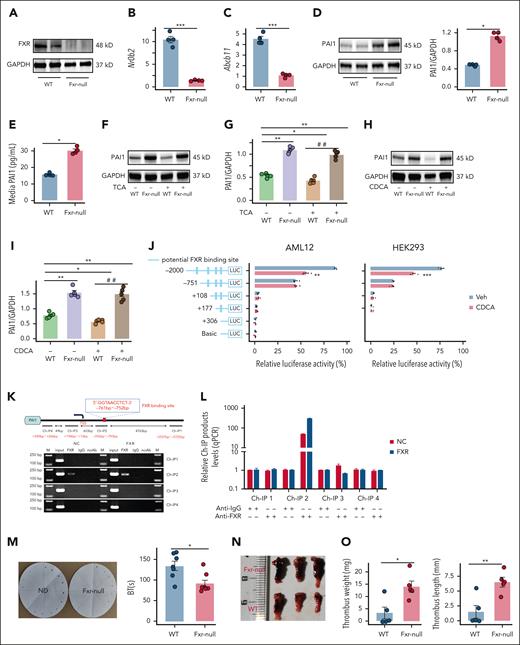
FXR activation lowers hepatocyte PAI-1 in mouse primary hepatocytes and mouse plasma by repressing PAI-1 transcription
To investigate the effect of FXR activation on hepatocyte PAI-1 expression, MPHs derived from WT male mice were treated with the endogenous ligands CDCA or TCA. PAI-1 protein and mRNA levels in cells, as well as PAI-1 levels in the culture medium, were significantly decreased after incubation with CDCA or TCA (supplemental Figure 5A; Figure 5A-G). In WT mice treated with CDCA or TCA by oral gavage, both agents significantly reduced plasma PAI-1 levels and hepatic Serpine1 mRNA expression (Figure 5L-O). Importantly, this PAI-1 suppression was recapitulated in a pathophysiological context. TCA administration in HFD mice also markedly lowered PAI-1 levels (supplemental Figure 5B).
PAI-1 expression may be modulated by FXR. (A-C) MPHs were treated with different concentrations of CDCA for 6 hours: (A) the amount of PAI-1 in the supernatant of the culture medium and (B-C) the protein expression levels of PAI-1 in the MPHs (N = 4). (D-G) MPHs were treated with different concentrations of TCA for 6 hours: (D-E) the protein and (F) relative Serpine1 mRNA levels of PAI-1 in MPHs (N = 4); (G) the amount of PAI-1 in the supernatant of the culture medium (N = 4). (H-K) MPHs were treated with different concentrations of DY268 for 6 hours: (H) the relative Serpine1 mRNA and (I) PAI-1 protein levels in the supernatant of the culture medium; (J-K) the PAI-1 protein level in the MPHs (N = 4). (L-M) C57BL/6J mice treated with 50 mg/kg per day CDCA for 4 days: (L) liver Serpine1 mRNA (N = 5); (M) plasma PAI-1 levels (N = 5). (N-O) C57BL/6J mice treated with 200 mg/kg per day TCA for 4 days: (N) liver C57BL/6J mRNA (N = 5); (O) plasma PAI-1 levels (N = 5). The data are represented as the mean ± SEM, n.s. P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 vs the lowest concentration of FXR ligands or Veh group. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; n.s., no significance; Veh, vehicle.
PAI-1 expression may be modulated by FXR. (A-C) MPHs were treated with different concentrations of CDCA for 6 hours: (A) the amount of PAI-1 in the supernatant of the culture medium and (B-C) the protein expression levels of PAI-1 in the MPHs (N = 4). (D-G) MPHs were treated with different concentrations of TCA for 6 hours: (D-E) the protein and (F) relative Serpine1 mRNA levels of PAI-1 in MPHs (N = 4); (G) the amount of PAI-1 in the supernatant of the culture medium (N = 4). (H-K) MPHs were treated with different concentrations of DY268 for 6 hours: (H) the relative Serpine1 mRNA and (I) PAI-1 protein levels in the supernatant of the culture medium; (J-K) the PAI-1 protein level in the MPHs (N = 4). (L-M) C57BL/6J mice treated with 50 mg/kg per day CDCA for 4 days: (L) liver Serpine1 mRNA (N = 5); (M) plasma PAI-1 levels (N = 5). (N-O) C57BL/6J mice treated with 200 mg/kg per day TCA for 4 days: (N) liver C57BL/6J mRNA (N = 5); (O) plasma PAI-1 levels (N = 5). The data are represented as the mean ± SEM, n.s. P > .05; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 vs the lowest concentration of FXR ligands or Veh group. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; n.s., no significance; Veh, vehicle.
We next determined the effects of FXR inhibition, or FXR deficiency on hepatocyte PAI-1 expression. In MPHs treated with the FXR antagonist, the PAI-1 protein and mRNA levels in cells, as well as the PAI-1 levels in the culture medium, were significantly increased after incubation with DY268 (Figure 5H-K). In addition, MPHs derived from male Fxr-null mice were utilized, and the expression levels of FXR and its direct target genes in these cells confirmed the successful construction of the cellular model (Figure 6A-C). PAI-1 protein levels in both cells and the culture medium were significantly increased in Fxr-null MPHs (Figure 6D-E). Furthermore, the loss of FXR prevented the decrease in PAI-1 induced by the FXR agonists CDCA and TCA (Figure 6F-I). Dual-luciferase reporter assays revealed that PAI-1 was regulated by FXR through binding sites located between −2000 and −751 bp (Figure 6J). Furthermore, we performed Ch-IP assays. The 4 Ch-IP target regions span the predicted FXR binding sites within the PAI-1 promoter. The locations of Ch-IP1 (−5725 to −5557 bp), Ch-IP2 (−793 to −592 bp), Ch-IP3 (+13 to +196 bp), and Ch-IP4 (+246 to +390 bp) relative to the transcription start site (TSS) were shown in Figure 6K. The results demonstrated that the FXR binding site within the PAI-1 promoter was located in the sequence amplified by the Ch-IP2 primers (−793 bp to −592 bp) (Figure 6K-L). Based on predictions from the JASPAR database (supplemental Table 2), the FXR binding site within the PAI-1 promoter was ultimately mapped to the region spanning −761 bp to −752 bp (Figure 6K). In summary, FXR inhibits hepatocyte PAI-1 expression at the transcriptional level.
Role of FXR in regulating PAI-1 expression and its impact on plasma fibrinolysis and DVT. (A-I) MPHs derived from WT or Fxr-null mice (FXRKO): (A) western blot analysis confirmed the knockout of FXR (N = 4); (B) Nr0b2 mRNA (N = 4); (C) Abcb11 mRNA (N = 4); (D) PAI-1 protein (N = 4); (E) PAI-1 concentration in medium (N = 4); (F-G) PAI-1 protein from TCA-treated MPH (N = 4); (H-I) PAI-1 protein from CDCA-treated MPHs (N = 4). (J) Serially truncated PAI-1 promoter constructs were cloned and inserted into pGL3-luciferase reporter plasmids and transfected into AML12 and HEK293 cells. Four hours after transfection, the cells were treated with CDCA (25 μM) for 24 hours and the relative luciferase activities were determined 72 hours after CDCA treatment. (K) A Ch-IP assay demonstrated the direct binding of FXR to the PAI-1 promoter in AML12 cells. (L) RT-qPCR of the Ch-IP products validated the binding capacity of FXR to the PAI-1 promoter. (M-O) WT and Fxr-null mice were randomly selected for DVT modeling or other assays: (M) tail bleeding time (N = 9); (N-O) thrombus weight and length (N = 5). (P) Representative curves for plasma fibrin formation and lysis tests and parameters of the curves for clot lysis time (N = 4). (Q-S) Fxr-null mice were injected with AAV8-TBG-Zsgreen or AAV8-TBG-shSerpine1: (Q) PAI-1 levels (N = 4); (R-S) thrombus length and weight (N = 6). WT mice were injected with AAV8-TBG-Zsgreen, and Fxr-null mice were injected with AAV8-TBG-Zsgreen or AAV8-TBG-shSerpine1: (T) representative curves for plasmin generation assay and parameters of the curves for EPP. (U-V) Fxrfl/fl mice were injected with AAV8-TBG-Cre or AAV8-TBG-Zsgreen: (U) plasma PAI-1 levels (N = 4); (V) thrombus weight and length (N = 5). The data are represented as the mean ± SEM, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 vs WT, Fxr-null and Fxrfl/fl mice with AAV8-TBG-Zsgreen or Veh group. ##P < .01 vs WT& FXR agonist (TCA or CDCA) group. BT, bleeding time; EPP, endogenous plasmin potential; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LUC, luciferase; NC, normal control; RT-qPCR, reverse transcription quantitative polymerase chain reaction.
Role of FXR in regulating PAI-1 expression and its impact on plasma fibrinolysis and DVT. (A-I) MPHs derived from WT or Fxr-null mice (FXRKO): (A) western blot analysis confirmed the knockout of FXR (N = 4); (B) Nr0b2 mRNA (N = 4); (C) Abcb11 mRNA (N = 4); (D) PAI-1 protein (N = 4); (E) PAI-1 concentration in medium (N = 4); (F-G) PAI-1 protein from TCA-treated MPH (N = 4); (H-I) PAI-1 protein from CDCA-treated MPHs (N = 4). (J) Serially truncated PAI-1 promoter constructs were cloned and inserted into pGL3-luciferase reporter plasmids and transfected into AML12 and HEK293 cells. Four hours after transfection, the cells were treated with CDCA (25 μM) for 24 hours and the relative luciferase activities were determined 72 hours after CDCA treatment. (K) A Ch-IP assay demonstrated the direct binding of FXR to the PAI-1 promoter in AML12 cells. (L) RT-qPCR of the Ch-IP products validated the binding capacity of FXR to the PAI-1 promoter. (M-O) WT and Fxr-null mice were randomly selected for DVT modeling or other assays: (M) tail bleeding time (N = 9); (N-O) thrombus weight and length (N = 5). (P) Representative curves for plasma fibrin formation and lysis tests and parameters of the curves for clot lysis time (N = 4). (Q-S) Fxr-null mice were injected with AAV8-TBG-Zsgreen or AAV8-TBG-shSerpine1: (Q) PAI-1 levels (N = 4); (R-S) thrombus length and weight (N = 6). WT mice were injected with AAV8-TBG-Zsgreen, and Fxr-null mice were injected with AAV8-TBG-Zsgreen or AAV8-TBG-shSerpine1: (T) representative curves for plasmin generation assay and parameters of the curves for EPP. (U-V) Fxrfl/fl mice were injected with AAV8-TBG-Cre or AAV8-TBG-Zsgreen: (U) plasma PAI-1 levels (N = 4); (V) thrombus weight and length (N = 5). The data are represented as the mean ± SEM, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 vs WT, Fxr-null and Fxrfl/fl mice with AAV8-TBG-Zsgreen or Veh group. ##P < .01 vs WT& FXR agonist (TCA or CDCA) group. BT, bleeding time; EPP, endogenous plasmin potential; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LUC, luciferase; NC, normal control; RT-qPCR, reverse transcription quantitative polymerase chain reaction.
Mice lacking FXR have impaired fibrinolysis and increased DVT burden
To directly assess the influence of FXR deficiency on plasma fibrinolysis and DVT, we used Fxr-null male mice. The expression of direct target genes of FXR in the liver confirmed the successful construction of the model (supplemental Figure 6A-B). In Fxr-null mice, the PAI-1 mRNA and protein levels in the liver, as well as plasma PAI-1 levels, were significantly increased (supplemental Figure 6C-E). Additionally, compared with WT mice, Fxr-null mice presented significantly shorter bleeding times (Figure 6M), larger thrombus indices (weight and length, Figure 6N-O), and longer clot lysis times (Figure 6P). No significant differences in fibrin formation kinetics were observed between Fxr-null mice and WT mice (supplemental Figure 7A). These findings indicate that FXR plays a crucial role in regulating plasma fibrinolysis and DVT. To confirm the causal role of PAI-1 in FXR-mediated thrombosis, we injected Fxr-null mice with liver-targeted AAV-TBG-shSerpine1, which significantly lowered plasma PAI-1 levels and liver Serpine1 expression (Figure 6Q; supplemental Figure 7B). In the IVC stenosis model, these mice exhibited reduced thrombus length and weight (Figure 6R-S). To assess the impact of PAI-1 modulation on plasminogen-to-plasmin conversion, we employed a plasmin generation assay. Fxr-null mice exhibited significantly reduced endogenous plasmin potential (EPP), prolonged lag time, and decreased peak levels compared to wild-type controls (Figure 6T; supplemental Figure 7C-D). Notably, AAV8-TBG-shSerpine1-mediated PAI-1 silencing markedly restored these parameters in Fxr-null mice, increasing EPP and peak levels while shortening lag time to near wild-type levels (Figure 6T; supplemental Figure 7C-D). However, time to peak (TtPeak) remained unchanged across all groups (supplemental Figure 7D).
Since Moraes et al have reported effects of FXR in platelets, we therefore performed platelet aggregation assays in Fxr-null mice. Consistent with their findings,34 we reproduced enhanced platelet aggregation in Fxr-null mice within our experimental setting (supplemental Figure 7F). To isolate the effects of platelet FXR from those of hepatocyte FXR, we employed a hepatocyte-targeted gene silencing approach using AAV8-TBG-Cre in Fxrfl/fl mice. This approach selectively silenced FXR expression in hepatocytes (supplemental Figure 7E), while control Fxrfl/fl mice were injected with AAV8-TBG-Zsgreen. Platelet aggregation assays, conducted three weeks post-injection, revealed no significant changes in platelet aggregation in response to ADP in Fxrfl/fl mice with AAV8-TBG-Cre (supplemental Figure 7G). However, these mice exhibited significantly elevated plasma PAI-1 levels (Figure 6U), and thrombus weight and length were markedly increased compared to controls (Figure 6V).
The FXR agonist tropifexor improved diet-induced low fibrinolytic activity and high DVT load in mice
To mimic the role of an FXR agonist in diet-induced low fibrinolytic activity in vitro, MPHs were treated with FXR agonists (GW4064, CDCA, tropifexor) after palmitic acid induction. As expected, the PAI-1 protein and mRNA levels in cells, as well as the PAI-1 levels in the culture medium, were significantly reduced after coincubation with these FXR agonists (Figure 7A-D; supplemental Figure 8A-D). To verify the role of an FXR agonist in alleviating diet induced high DVT load in vivo, we fed mice an HFD for 12 weeks to induce obesity and administered either an HFD or HFD and tropifexor for another 4 weeks. Compared with HFD mice receiving the control treatment, in HFD mice treated with tropifexor, borderline increases in the expression of FXR direct target genes, and significant reductions in liver Serpine1 and plasma PAI-1 expression were detected (Figure 7E-H). Tropifexor-treated HFD mice presented prolonged bleeding time (Figure 7I-J), accelerated fibrin formation and lysis dynamics (Figure 7N-R), and diminished thrombus parameters (weight/length; Figure 7K-M). Furthermore, to exclude potential sex-related confounding effects, we conducted additional experiments in female HFD mice. Notably, compared with lean controls, female HFD mice also presented significantly increased body weight, elevated plasma PAI-1 levels, and heightened thrombus indices (weight and length), consistent with observations in males (supplemental Figure 9A-E). Similarly, LJN452 (tropifexor) treatment reduced PAI-1 levels by ∼40% (supplemental Figure 9B) and attenuated the thrombus indices (supplemental Figure 9C-E). These data revealed that impaired fibrinolysis and a heavy DVT load can be partially reversed in HFD mice after FXR activation and that novel therapeutic drugs for nonalcoholic fatty liver disease could improve fibrinolysis and reduce the risk of DVT.
FXR agonists improve obesity-induced fibrinolysis impairment and high DVT burden. (A-H) MPHs were treated with palmitic acid for 6 hours and subsequently coincubated with FXR agonists (CDCA and tropifexor) for the next 6 hours: (A) Serpine1 mRNA (N = 5); (B) PAI-1 concentration in medium (N = 5); (C-D) PAI-1 protein (N = 5). (E-R) Obese mice treated with tropifexor were randomly selected for DVT modeling or other assays (N = 4): (E) liver Nr0b2 mRNA (N = 4); (F) liver Abcb11 mRNA (N = 4); (G) liver Serpine1 mRNA (N = 4); (H) plasma PAI-1 levels (N = 4); (I-J) tail bleeding time (N = 9); (K-M) thrombus weight and length (N = 5); (N-R) representative curves for plasma fibrin formation and lysis tests and parameters of the curves for fibrinolysis (N = 4). The data are represented as the mean ± SEM, n.s. P > .05; ∗P < .05; ∗∗P < .01 vs PA600 or HFD group. BSA, bovine serum albumin; BT, bleeding time; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LJN452, tropifexor; n.s., no significance; PA, palmitic acid.
FXR agonists improve obesity-induced fibrinolysis impairment and high DVT burden. (A-H) MPHs were treated with palmitic acid for 6 hours and subsequently coincubated with FXR agonists (CDCA and tropifexor) for the next 6 hours: (A) Serpine1 mRNA (N = 5); (B) PAI-1 concentration in medium (N = 5); (C-D) PAI-1 protein (N = 5). (E-R) Obese mice treated with tropifexor were randomly selected for DVT modeling or other assays (N = 4): (E) liver Nr0b2 mRNA (N = 4); (F) liver Abcb11 mRNA (N = 4); (G) liver Serpine1 mRNA (N = 4); (H) plasma PAI-1 levels (N = 4); (I-J) tail bleeding time (N = 9); (K-M) thrombus weight and length (N = 5); (N-R) representative curves for plasma fibrin formation and lysis tests and parameters of the curves for fibrinolysis (N = 4). The data are represented as the mean ± SEM, n.s. P > .05; ∗P < .05; ∗∗P < .01 vs PA600 or HFD group. BSA, bovine serum albumin; BT, bleeding time; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LJN452, tropifexor; n.s., no significance; PA, palmitic acid.
Discussion
Obesity has reached epidemic proportions worldwide and is a major contributor to a number of widespread chronic diseases, notably type 2 diabetes, nonalcoholic steatohepatitis (NASH), and cardiovascular disease.35,36 Among the more serious consequences of obesity, one that contributes to cardiovascular disease, is an increased risk of thrombosis,4,6,37 which causes 1 in 4 deaths worldwide.1 Most research studies centered on coagulation perturbations in obesity have focused on altered procoagulant factors in patients with obesity and related experimental settings. In fact, impaired fibrinolysis is a significant contributor to obesity-associated thrombosis,6,14,17,38-41 but the underlying molecular mechanisms linking obesity to defects in fibrinolysis have remained largely unknown.
Compared with lean mice, DIO mice had significantly reduced fibrinolysis and increased DVT burden largely because of increased hepatocyte expression of PAI-1. Increased plasma concentrations of PAI-1 have been documented in previous studies of obese humans and mice,9,11,14,16 and PAI-1 has emerged as a potential target for DVT. However, no inhibitors of PAI-1 have yet been approved for antithrombotic therapy. Thus, the reasons for the notable increase in PAI-1 in obese individuals deserve attention. The first critical gap in this area of research is the cellular source of PAI-1 that contributes to the low fibrinolytic activity in obesity. The role of adipose tissue as a key determinant of circulating PAI-1 levels in obesity has been reported, as evidenced by the analysis of Serpine1 mRNA and PAI-1 protein levels across different tissues.7,17,18 However, contradictory findings have been reported, indicating the absence of an arteriovenous gradient for the PAI-1 protein and activity in adipose tissue among obese individuals.19 Thus, the current study analyzed the levels of PAI-1 in adipose tissue, blood vessels and the liver, which express the Serpine1 gene. Consistent with previous studies, PAI-1 protein and Serpine1 mRNA levels in the liver were significantly increased, and liver Serpine1 mRNA was strongly correlated with plasma PAI-1.16,42 Additionally, hepatocyte-specific silencing of PAI-1 in obese mice, decreased liver Serpine1 mRNA by ∼10-fold and plasma PAI-1 protein by ∼70%.16 These findings provide robust evidence for a dominant contribution of the liver to plasma PAI-1 in obesity.
The second critical gap is the mechanism by which obesity alters PAI-1 expression. As hepatocytes can be considered a key intersection in the sensing of metabolic stress induced by obesity and the regulation of fibrinolysis, the present study focused on identifying upstream regulatory factors at the crossroad of metabolism and thrombosis regulation. In the DIO mouse model, loss of FXR signaling was observed, which may be related to the levels and composition of bile acids. FXR, which is known primarily for its role in regulating bile acid homeostasis, has been implicated in various physiological and pathophysiological processes, including liver metabolism, inflammation, and cardiovascular diseases.43-46 The role of FXR in the phenotypes of obesity induced venous thrombosis was investigated in the present study, revealing that FXR activation can decrease Serpine1 mRNA and PAI-1 protein expression in mice and that Fxr-null mice have significantly increased plasma PAI-1 levels as well as further impaired fibrinolysis and increased DVT load. In vitro, palmitic acid treatment of mouse primary hepatocytes (MPHs) models obesity-related hepatocyte dysfunction, including lipid accumulation,30-33 insulin resistance,47 ER stress,48 and proinflammatory responses.49-51 We found FXR activation also suppressed PAI-1, reducing its secretion, protein, and mRNA levels. Hepatocyte FXR has emerged as an important link between obesity-associated metabolic stress, impaired fibrinolysis, and DVT.
The specific targeting of FXR is considered a promising approach for the treatment of various diseases, including metabolic disorders and tumors,52,53 and the FXR agonist obeticholic acid is used in the clinic for the treatment of primary biliary cholangitis, whereas other FXR agonists are drug candidates that are in undergoing clinical trials for the treatment of NASH. Notably, tropifexor, a highly effective nonsteroidal FXR agonist,54 has demonstrated favorable tolerability for NASH in a phase 1 study, with only minimal and temporary increases in serum ALT levels, and it was demonstrated in vivo and in vitro that tropifexor can reduce plasma levels of PAI-1, thus improving obesity-induced fibrinolytic impairment and high DVT load. Given the extensive clinical trials conducted with FXR agonists, tropifexor, and other FXR agonists hold promise as potential drugs for long-term prophylactic antithrombotic treatment in individuals at high risk of DVT due to obesity. These findings may provide new insights for long-term prophylactic antithrombotic treatment in the clinical setting.
The hepatocyte FXR-PAI-1 pathway revealed here is unique in the pathophysiology of clotting disorders induced by obesity. Although FXR plays a crucial role in platelet activity,34 both DIO and Fxr-null mice in our study exhibited shortened bleeding times, indicating enhanced platelet function. Additionally, Fxr-null mice demonstrated increased platelet aggregation. However, no significant change in ADP-induced platelet aggregation was observed in Fxrfl/fl mice following AAV8-TBG-Cre injection, suggesting that platelet FXR has a limited role. Hepatocytes act as a link between sensing obesity-induced metabolic stress and regulating fibrinolysis, which can offer potential innovative strategies to increase basal fibrinolysis in obese individuals before injury occurs. However, other obesity-related perturbations, including alterations in coagulation and platelet function, also contribute to the increased risk of thrombotic disorders in individuals with obesity. Therefore, a holistic understanding of these interconnected processes is necessary to fully grasp the pathophysiology of thrombotic disease in obesity and to develop effective therapeutic interventions.
Although our findings first reported hepatocyte FXR-PAI-1 pathway, several limitations warrant consideration. First, our human cohort showed no significant difference in fibrinogen levels between obese and healthy individuals (Figure 2C), but another large independent cohort (N = 18 587), known as the Xifei Prospective Cohort Study, found slightly higher fibrinogen levels in obesity (supplemental Figure 10A). A weak positive correlation was also observed between body mass index and fibrinogen (supplemental Figure 10B). However, both our data and previous studies indicate that fibrinogen levels remain relatively stable in high-fat diet–fed mice, which limits direct translation of turbidity changes to human obesity.13 Furthermore, structural divergences in fibrin clots (eg, altered internal fiber density from LDL-C differences55,56) and absent comorbidities (hypertension, dyslipidemia) further constrain clinical extrapolation. Despite these limitations, murine models remain invaluable for isolating conserved pathways like PAI-1-driven fibrinolysis impairment. Future studies should integrate humanized models to better mimic obesity-associated thrombosis.
In conclusion, for the first time, we show that FXR regulation of PAI-1 has an important role in fibrinolysis and DVT. Investigational drugs targeting FXR may have therapeutic efficacy for improving fibrinolysis and lowering DVT risk.
Acknowledgments
The authors thank all the patients who participated in this study. They extend their gratitude to Bin Du for his valuable contributions to technical support in this research.
This work was supported by the National Key R&D Program of China (2024YFA1307004, 2021YFA1301200) and the National Natural Science Foundation of China (82425004).
Authorship
Contribution: Y.W., T.L., and B.L. conceptualized the study; B.L., H.F., H.W., Y.Z., N.D., P.L., Q.W., and M.C. were responsible for methodology; Y.W., B.L., T.L., H.F., J.L., Z.M., and X.F. contributed to formal analysis; H.W., Z.M., X.F., X.Z., W.W., Y.X., K.D., J.L., and Y.W. were responsible for investigation; Y.W. and J.L. supervised the study; B.L. and H.F. wrote the original draft; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yue Wu, Department of Cardiology, The First Affiliated Hospital of Xi’an Jiaotong University, No. 76, Yanta West Rd, Xi’an 710061, China; email: yue.wu@xjtu.edu.cn; Junhui Liu, Department of Clinical Laboratory, The First Affiliated Hospital of Xi’an Jiaotong University, No. 76, Yanta West Rd, Xi’an 710061, China; email: liu1109@xjtu.edu.cn; and Ting Li, Department of Cardiology, The First Affiliated Hospital of Xi’an Jiaotong University, No. 76, Yanta West Rd, Xi’an 710061, China; email: li.ting@xjtu.edu.cn.
References
Author notes
B.L., H.F., and H.W. are joint first authors.
Additional information, resources, and reagents are available from the corresponding author, Yue Wu (yue.wu@xjtu.edu.cn), on request.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal