Key Points
CD20/CD3 bispecific antibody enhances the efficacy of CD19–directed CAR T cells.
Combination treatment improved survival in the Eμ-TCL-1 mouse model.
Visual Abstract
Relapse after anti-CD19 chimeric antigen receptor (CD19-CAR) occurs in a substantial proportion of patients with lymphoid malignancies. We assessed the potential benefits of co-administering CD20-targeting bispecific antibodies (CD20-BsAbs) with CD19-CAR T cells with the aim of enhancing immunotherapeutic efficacy. Addition of CD20-BsAbs to cocultures of CD19-CARs and primary samples of B-cell malignancies, comprising malignant B cells and endogenous T cells, significantly improved killing of malignant cells and enhanced the expansion of both endogenous T cells and CD19-CAR T cells. In an immunocompetent mouse model of chronic lymphocytic leukemia, relapse after initial treatment response frequently occurred after CD19-CAR T-cell monotherapy. Additional treatment with CD20-BsAbs significantly enhanced the treatment response and led to improved eradication of malignant cells. Higher efficacy was accompanied by improved T-cell expansion with CD20-BsAb administration and led to longer survival with 80% of the mice being cured with no detectable malignant cell population within 8 weeks of therapy initiation. Collectively, our in vitro and in vivo data demonstrate enhanced therapeutic efficacy of CD19-CAR T cells when combined with CD20-BsAbs in B-cell malignancies. Activation and proliferation of both infused CAR T cells and endogenous T cells may contribute to improved disease control.
Introduction
Chimeric antigen receptor (CAR) T cells targeting the B-cell lineage marker CD19 have greatly improved the outcome in patients with relapsed/refractory B-cell malignancies, which led to the approval of several CAR T-cell therapies for these patients.1-3 However, patients who had incomplete responses after CAR T-cell therapy are especially prone to disease progression or relapse.4,5 This failure to respond has been linked to molecular alterations within the tumor, for example, loss of target antigen expression.6,7 Moreover, limited persistence of CAR T cells, for instance, because of T-cell exhaustion, has been associated with an inferior clinical response.8,9
Previous studies suggested that there were potential benefits to cotargeting multiple tumor-associated antigens to prevent antigen escape. Although CAR T cells that target both CD19 and CD20 have demonstrated efficacy in vitro10,11 and in vivo,12,13 limited CAR T-cell expansion remains associated with poor outcomes.12,13 An alternative strategy to dual targeting might be the combination of CAR T cells with bispecific antibodies (BsAbs). Sequencing these 2 treatment strategies is currently being tested for patients with relapsed/refractory multiple myeloma or diffuse large B-cell lymphoma in several clinical trials and is showing meaningful clinical benefit with durable response rates.14,15 Because BsAbs that target CD20 and CD3 (CD20-BsAb) induce killing of B cells by recruiting autologous infiltrating T cells,16 we hypothesized that the addition of CD20-BsAbs will complement treatment with CD19-targeting CAR T cells (CD19-CAR) by promoting CAR T-cell expansion and simultaneously exploiting the activity of endogenous T cells.
Study design
A detailed description is provided in the supplemental Materials, available on the Blood website.
CAR T-cell and BsAb combination in human in vitro and ex vivo models
Human B-cell lymphoma lines or primary lymph node–derived samples (supplemental Tables 1 and 2) were cocultured with third-generation CD19-CAR17 or nontransduced (NT) T cells. For the combination experiments with CD20-BsAbs (Genentech), the indicated concentrations of CD20-BsAb or solvent control were added to the cocultures. Cell suspensions were analyzed using flow cytometry.
CAR T-cell and BsAb combination in the murine Eμ-TCL1 model
The fully immunocompetent Eμ-TCL118 adoptive transfer model of chronic lymphocytic leukemia (CLL)19 was used to evaluate murine second-generation anti-CD19 CAR T cells20 in combination with CD20-BsAbs (bAb0185, absolute antibody) in vivo. After engraftment of TCL1-CLL cells and partial lymphodepletion through sublethal irradiation, mice were intravenously injected with CAR or NT T cells. CD20-BsAbs were administered weekly by intravenous injections starting 1 week after CAR T-cell injection. Disease progression was monitored in peripheral blood and lymphoid tissues by flow cytometry.
This study was approved by the ethics committee of the University of Heidelberg and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients in advance.
Results and discussion
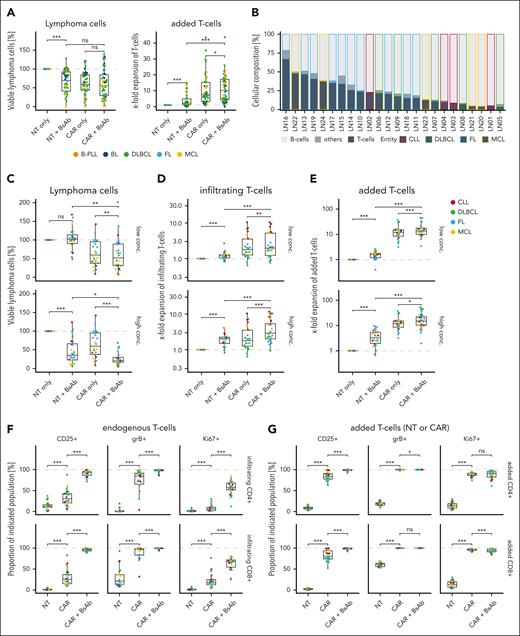
We evaluated the efficacy of third-generation CD19-CAR T cells, which showed in vivo activity that was comparable with second-generation CD19-CAR T cells,17 and combined them with CD20-BsAbs in vitro. Therefore, we cocultured 46 human B-cell lymphoma lines from different entities (supplemental Figure 1A,C; supplemental Table 1) with either CD19-CAR T cells or NT control T cells derived from 1 healthy donor with or without CD20-BsAbs. CD20-BsAbs increased the expansion of CD19-CAR T cells, however, the killing of lymphoma cells was not significantly affected. In contrast, CD20-BsAbs induced killing of lymphoma cells in cocultures with NT T cells while enhancing their expansion, indicating that CD20-BsAbs successfully activated non-CAR–transduced T cells (Figure 1A). Considering the cellular makeup of lymphomas, which encompasses both malignant B cells and endogenous T cells, we hypothesized that CD20-BsAbs could complement CAR T-cell–mediated killing by inducing antitumor cytotoxicity, mediated by endogenous T cells, in an autologous ex vivo setting.21 We therefore assessed the combination of CD19-CAR T cells and CD20-BsAbs in 24 primary lymph node–derived samples of different B-cell malignancies comprising different proportions of endogenous T cells that were collected either at initial diagnosis (n = 14) or at relapse (n = 10) (Figure 1B; supplemental Figure 1B,D; supplemental Table 2). In cocultures with CD19-CAR T cells, CD20-BsAbs significantly improved the killing of malignant B cells (Figure 1C) while enhancing the expansion of both endogenous T cells and CAR T cells (Figure 1D-E). Of note, irrespective of the presence of CD20-BsAbs, expansion of endogenous T cells was higher in cocultures with CAR T cells than with NT T cells, indicating that the presence of CAR T cells provided a benefit to endogenous T cells.
CD20-BsAbs increase killing by expansion of CD19-CAR T cells in vitro. (A) The percentage of viable lymphoma cells and x-fold expansion of T cells in cocultures of B-cell lymphoma cell lines and CD19-CAR or NT T cells in the presence or absence of 100 ng/mL CD20-BsAb are shown for 46 different cell lines. (B) The cellular composition is shown for primary B-cell malignancy samples from patients as determined by flow cytometry. Samples are ordered by decreasing T-cell content (n = 24 samples). The percentage of viable lymphoma cells (C), x-fold expansion of endogenous T cells (D), and x-fold expansion of added CD19-CAR or NT T cells (E) in cocultures with primary B-cell malignancy samples in the presence or absence of 100 ng/mL (low concentration) or 1000 ng/mL (high concentration) CD20-BsAb are shown for n = 24 samples. Cocultures were performed using an effector (E) to target (T) cell ratio of 0.2:1. The percentage CD25+, GrB+, and Ki67+ T cells among CD4+ or CD8+ endogenous (F) or CD19-CAR or NT (G) T cells in the presence or absence of 1000 ng/mL CD20-BsAb in n = 24 samples. P values were calculated using the 2-sided, paired Wilcoxon test (A,C-G). ∗∗∗P ≤ .001, ∗∗P ≤ .01, ∗P ≤ .05. ns, not significant.
CD20-BsAbs increase killing by expansion of CD19-CAR T cells in vitro. (A) The percentage of viable lymphoma cells and x-fold expansion of T cells in cocultures of B-cell lymphoma cell lines and CD19-CAR or NT T cells in the presence or absence of 100 ng/mL CD20-BsAb are shown for 46 different cell lines. (B) The cellular composition is shown for primary B-cell malignancy samples from patients as determined by flow cytometry. Samples are ordered by decreasing T-cell content (n = 24 samples). The percentage of viable lymphoma cells (C), x-fold expansion of endogenous T cells (D), and x-fold expansion of added CD19-CAR or NT T cells (E) in cocultures with primary B-cell malignancy samples in the presence or absence of 100 ng/mL (low concentration) or 1000 ng/mL (high concentration) CD20-BsAb are shown for n = 24 samples. Cocultures were performed using an effector (E) to target (T) cell ratio of 0.2:1. The percentage CD25+, GrB+, and Ki67+ T cells among CD4+ or CD8+ endogenous (F) or CD19-CAR or NT (G) T cells in the presence or absence of 1000 ng/mL CD20-BsAb in n = 24 samples. P values were calculated using the 2-sided, paired Wilcoxon test (A,C-G). ∗∗∗P ≤ .001, ∗∗P ≤ .01, ∗P ≤ .05. ns, not significant.
To determine whether the observed improvement in tumor cell killing by CD20-BsAbs was because of endogenous T cells or CAR T cells or both, we quantified the proportions of activated, endogenous, or added CD4+ and CD8+ T cells by measuring the frequencies of CD25+, CD69+, granzyme B (GrB)+, and Ki67+ T cells (Figure 1F-G; supplemental Figure 2). Interestingly, the mere presence of CAR T cells increased the proportion of CD25+, GrB+, and Ki67+ endogenous CD4+ and CD8+ T cells. Together with the observed increase in endogenous T-cell expansion, these results are in line with previous findings that demonstrated activation of endogenous non-CAR–transduced T cells in patients with diffuse large B-cell lymphoma following CAR T-cell transfusion.22 More importantly, CD20-BsAbs further increased the proportion of activation and proliferation marker-positive endogenous T cells, suggesting that they complemented CAR T-cell–mediated killing in combination therapy (Figure 1F). In case of CAR T cells, the percentage of GrB+ and Ki67+ CAR T cells was close to 100%, indicating strong activation in the monotherapy. Consequently, CD20-BsAbs did not further enhance the expression of these markers and only led to a significant increase in the proportion of CD25+ and CD69+ CAR T cells (Figure 1G; supplemental Figure 2B). Combining CD19-BsAbs with CD19-CAR T cells led to similar beneficial effects (supplemental Figure 3), emphasizing the importance of enhancing endogenous T-cell activation instead of targeting 2 antigens. Nevertheless, targeting different antigens may have additional positive effects, such as reducing the risk for treatment resistance caused by antigen loss.23
Collectively, these data suggest that CD20-BsAbs complement CAR T-cell–mediated killing of malignant B cells ex vivo by (1) recruiting and activating endogenous T cells and (2) supporting activation and proliferation of CAR T cells.
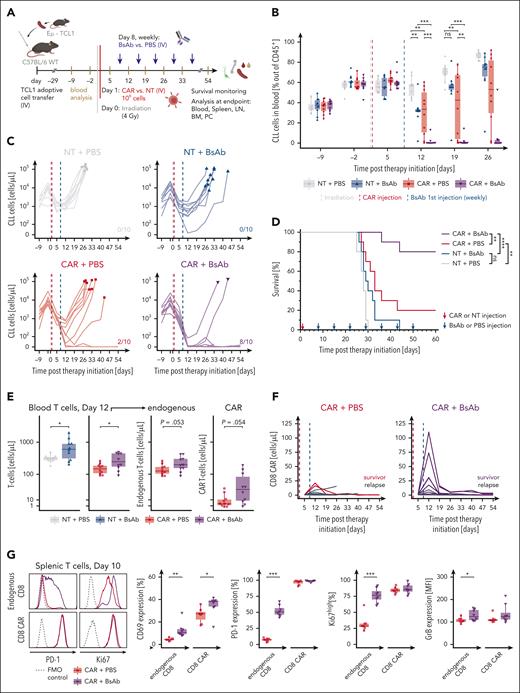
We subsequently evaluated the combination of CD19-CAR T cells and CD20-BsAbs in vivo in the fully immunocompetent Eμ-TCL1 mouse model of CLL.18 The efficacy of murine CD19-CAR T cells and CD20-targeting BsAbs was first confirmed in vitro (supplemental Figure 4). The combination therapy in vivo was then compared with both monotherapies and with a control group injected with NT T cells (Figure 2A). After the initial reduction in CD5+CD19+ malignant cells in the blood owing to the sublethal irradiation required for conditioning mice to CAR T-cell therapy, both CD19-CAR T-cell and CD20-BsAb monotherapy significantly reduced the CLL cell counts in the blood when compared with the control. By using suboptimal treatment conditions, most mice in both monotherapy groups rapidly relapsed and reached high CLL frequencies in the blood within 2 to 5 weeks after therapy initiation (Figure 2B-C; supplemental Figure 5A). Response in the CAR T-cell monotherapy group was heterogeneous and resembled the clinical performance of CD19-CAR T-cell therapy in patients with CLL.24
CD20-BsAb enhance in vivo antitumor efficacy of CD19-CAR T cells in the Eμ-TCL1 mouse model of CLL. (A) A schematic overview of the in vivo study is presented. C57BL/6 mice (n = 10 mice per group) were transplanted with 107 Eμ-TCL1 splenocyte–derived CLL cells intravenously, and disease progression was monitored through weekly blood withdrawals. After CLL establishment (median of 60% CLL cells of CD45+ leukocytes and >1000 CLL cells per μL in blood), the mice were conditioned with sublethal 4 Gy irradiation (day 0). Injection with 106 CD19-CAR or NT T cells was performed 24 hours later (day 1). Weekly CD20-BsAb or phosphate-buffered saline (PBS) therapy was initiated the following week (day 8). Summarized frequency in blood (B) and absolute concentration (C) of CD5+ CD19+ CLL cells per mouse before and during treatment. In panel B, 3 mice reached end point criteria before the day 26 analysis (compare panel F) and the remaining mice are summarized without statistical analyses. In panel C, the symbols represent end point analysis. The number of surviving mice 60 days after therapy initiation in each treatment group are indicated on the respective graphs. The absolute concentrations are shown on pseudo-log scale to allow the plotting of mice without detectable cancer cells. (D) The Kaplan-Meier survival curves of mice treated with CD19-CAR T-cell and CD20-BsAb combination therapy, the respective monotherapies, and the NT T cells with PBS controls are shown. (E) The effects of CD20-BsAb treatment start (day 8) on total T-cell concentration are shown. The absolute T-cell concentrations in the blood of NT and CAR T-cell injected mice are depicted for day 12 (left). In addition, the endogenous and endothelial growth factor receptor+ CAR T-cell expansion in blood are shown separately for CAR T-cell injected mice (right). (F) CD19-CAR T-cell engraftment and expansion as monotherapy (CAR + PBS) and in combination with CD20-BsAb. The concentration of CD8+ CAR in blood is shown over time with relapsing mice shown in black and cured mice shown in the respective colors. (G) A second independent treatment study was performed with n = 7 CAR with PBS and n = 8 CAR with BsAb animals following the same timeline and treatment schedule as outlined in panel A. The initial response of T cells to BsAb therapy was assessed by analysis of blood and lymphoid organs 2 days after the first BsAb treatment dose (day 10) (supplemental Figure 7). Expression of CD69 (activation), PD-1 (activation/exhaustion), Ki67 (proliferation), and GrB (degranulation) in endogenous CD8+ and CD8+ CAR T cells were analyzed by flow cytometry. Exemplary histograms (left) and frequencies of marker-positive cells (right) are shown. For panel B, the 1-way analysis of variance with Tukey’s honest significant difference multiple comparison test was used. For panel D, the Mantel-Cox log-rank test was used. For panels F-G, unpaired t tests were used. In panel G, ns is not shown. ∗∗∗P ≤ .001, ∗∗P ≤ .01, ∗P ≤ .05. ns, not significant.
CD20-BsAb enhance in vivo antitumor efficacy of CD19-CAR T cells in the Eμ-TCL1 mouse model of CLL. (A) A schematic overview of the in vivo study is presented. C57BL/6 mice (n = 10 mice per group) were transplanted with 107 Eμ-TCL1 splenocyte–derived CLL cells intravenously, and disease progression was monitored through weekly blood withdrawals. After CLL establishment (median of 60% CLL cells of CD45+ leukocytes and >1000 CLL cells per μL in blood), the mice were conditioned with sublethal 4 Gy irradiation (day 0). Injection with 106 CD19-CAR or NT T cells was performed 24 hours later (day 1). Weekly CD20-BsAb or phosphate-buffered saline (PBS) therapy was initiated the following week (day 8). Summarized frequency in blood (B) and absolute concentration (C) of CD5+ CD19+ CLL cells per mouse before and during treatment. In panel B, 3 mice reached end point criteria before the day 26 analysis (compare panel F) and the remaining mice are summarized without statistical analyses. In panel C, the symbols represent end point analysis. The number of surviving mice 60 days after therapy initiation in each treatment group are indicated on the respective graphs. The absolute concentrations are shown on pseudo-log scale to allow the plotting of mice without detectable cancer cells. (D) The Kaplan-Meier survival curves of mice treated with CD19-CAR T-cell and CD20-BsAb combination therapy, the respective monotherapies, and the NT T cells with PBS controls are shown. (E) The effects of CD20-BsAb treatment start (day 8) on total T-cell concentration are shown. The absolute T-cell concentrations in the blood of NT and CAR T-cell injected mice are depicted for day 12 (left). In addition, the endogenous and endothelial growth factor receptor+ CAR T-cell expansion in blood are shown separately for CAR T-cell injected mice (right). (F) CD19-CAR T-cell engraftment and expansion as monotherapy (CAR + PBS) and in combination with CD20-BsAb. The concentration of CD8+ CAR in blood is shown over time with relapsing mice shown in black and cured mice shown in the respective colors. (G) A second independent treatment study was performed with n = 7 CAR with PBS and n = 8 CAR with BsAb animals following the same timeline and treatment schedule as outlined in panel A. The initial response of T cells to BsAb therapy was assessed by analysis of blood and lymphoid organs 2 days after the first BsAb treatment dose (day 10) (supplemental Figure 7). Expression of CD69 (activation), PD-1 (activation/exhaustion), Ki67 (proliferation), and GrB (degranulation) in endogenous CD8+ and CD8+ CAR T cells were analyzed by flow cytometry. Exemplary histograms (left) and frequencies of marker-positive cells (right) are shown. For panel B, the 1-way analysis of variance with Tukey’s honest significant difference multiple comparison test was used. For panel D, the Mantel-Cox log-rank test was used. For panels F-G, unpaired t tests were used. In panel G, ns is not shown. ∗∗∗P ≤ .001, ∗∗P ≤ .01, ∗P ≤ .05. ns, not significant.
Importantly, combining CD19-CAR and CD20-BsAbs led to improved eradication of malignant cells and was significantly more effective than either of the monotherapies alone. In 80% of mice in the combination group and in 20% of the CD19-CAR T-cell monotherapy–treated animals, the malignant cell population was permanently depleted under therapy, suggesting cure in this murine CLL model (Figure 2B-C; supplemental Figure 5B). Accordingly, combining CD19-CAR T-cell therapy with BsAb therapy significantly enhanced the survival of mice when compared with either monotherapy (Figure 2D). Of note, monotherapy with BsAbs led to a relative redistribution of cancer cells toward lymph nodes (supplemental Figure 5E-F), suggesting lower efficacy of CD20-BsAbs in this tissue.
To further elucidate the mode of action, we quantified T-cell abundance in the blood after initiation of CD20-BsAb therapy. Irrespective of CAR T-cell injection, the administration of BsAbs induced initial expansion of total T cells when compared with the corresponding phosphate-buffered saline controls (Figure 2E; supplemental Figure 5C). CAR T-cell abundance in peripheral blood peaked within 2 weeks after infusion. BsAb therapy initiated within this timeframe supported the expansion of not only endogenous T cells but also CAR T cells with higher CAR abundance, especially in the CD8+ T-cell compartment (Figure 2E-F; supplemental Figure 5D). Mice that experienced relapse later during therapy tended to show low CAR T-cell frequencies in the blood at early time points, underlining the importance of in vivo CAR expansion for therapeutic efficacy.8 Of interest, survivor mice were resistant to rechallenge with CLL irrespective of BsAb continuation (supplemental Figure 6).
We performed phenotypic and functional analysis of T cells 2 days after the initial BsAb treatment (supplemental Figure 7A-C). CD20-BsAbs increased the expression of activation markers (CD69, CD25) on both CD8+ endogenous T cells and CAR T cells. It induced upregulated activation or exhaustion marker expression and supported the proliferation and cytotoxicity of endogenous T cells, whereas CAR T cells already induced high expression levels of PD-1, CD39, and Ki67 as monotherapy (Figure 2G; supplemental Figure 7D-G). Ex vivo treatment revealed strong CD20-BsAb–induced degranulation and cytokine production in endogenous CD8+ T cells (supplemental Figure 8), emphasizing the potential of CD20-BsAbs to support CAR T-cell therapy by harnessing endogenous T cells.
In summary, our work showed that CAR T cells and BsAb–directed endogenous T cells significantly potentiated each other to eradicate malignant B cells. Improved CAR T-cell expansion and persistence have been shown to correlate with improved outcomes in patients with B-cell lymphoma and might be a considerable treatment alternative for treatment-refractory patients with CLL. Our results therefore suggest that combination treatment with CAR T cells and BsAbs may warrant clinical exploration.
Acknowledgments
The authors thank the Fluorescence Activated Cell Sorting core facility of the University Hospital Heidelberg with Volker Eckstein and the Flow Core Facility at European Molecular Biology Laboratory Heidelberg for their excellent (technical) assistance. The authors also thank the Central Animal Laboratory and Flow Cytometry Core Facilities at the Deutsches Krebsforschungs Zentrum. The authors are grateful to Verena Kalter, Sibylle Ohl, Lavinia Arseni, Julia Hartmann, Hannah Briesch, Covadonga Castellanos Gonzalez, Liesa-Marie Pilger, Nicolas Aubert, Daniel Medina Gil, and Eric Konrath for their support with experimental work.
S.D. was supported by a grant from the Hairy Cell Leukemia Foundation, the Heidelberg Research Centre for Molecular Medicine, the e:med junior group grant SYMPATHY of the German Federal Ministry of Education and Research (Bundesministerium für Forschung und Wissenschaft [BMBF], 01ZX1506), the BMBF ERA PerMed grant SYMMETRY (01KU2210), the European Research Area Net Transcan grant BIALYMP (01KT2311), the Else Kroener Fresenius Professorship, the German Research Foundation through the SFB873 (project B7), and funds from the state of Baden-Wuerttemberg within the Centers for Personalized Medicine Baden-Wuerttemberg. T.R. was supported by a fellowship of the BMBF and a physician scientist fellowship of the Medical Faculty of the University of Heidelberg. A.F. and M.C. were supported by Helmholtz International Graduate School stipends awarded by the German Cancer Research Center. N.L. was supported by a Heidelberg School of Oncology (HSO2) fellowship from the National Center for Tumor Diseases, Heidelberg. P.M.R. was funded by a fellowship of the Deutsches Krebsforschungs Zentrum Clinician Scientist Program, supported by the Dieter Morszeck Foundation. M.S. was supported by the German Cancer Aid and the German José Carreras Foundation (grant 03 R/2021).
Schematics were created using BioRender.com.
Authorship
Contribution: S. Dietrich conceptualized the study; B.J.B., A.F., T.R., N.M., M.C., and N.L. performed data curation; B.J.B. and A.F. conducted formal analysis; S. Dietrich and M. Seiffert performed funding acquisition; B.J.B., A.F., T.R., P.M.R., M.C., and C.S. were responsible for the investigation; B.J.B., A.F., T.R., D.H.B., P.M.R., P.-M.B., S. Dötsch, and M. Schmitt were responsible for methodology; S. Dietrich and M. Seiffert provided resources; S. Dietrich and M. Seiffert provided supervision; B.J.B., A.F., and C.S. were responsible for visualization; B.J.B., A.F., S. Dietrich, and M. Seiffert wrote the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sascha Dietrich, Universitätsklinikum Düsseldorf, MNR Klinik, Moorenstraße 5, 40225 Düsseldorf, Germany; email: sascha.dietrich@embl.de; and Martina Seiffert, German Cancer Research Center, B060, Im Neuenheimer Feld 580, 69120 Heidelberg, Germany; email: m.seiffert@dkfz.de.
References
Author notes
B.J.B., A.F., and C.S. contributed equally to this study.
M. Seiffert and S. Dietrich are senior authors who contributed equally to this study.
The authors declare that they make renewable materials, data sets, and protocols available to other investigators without unreasonable restrictions.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal