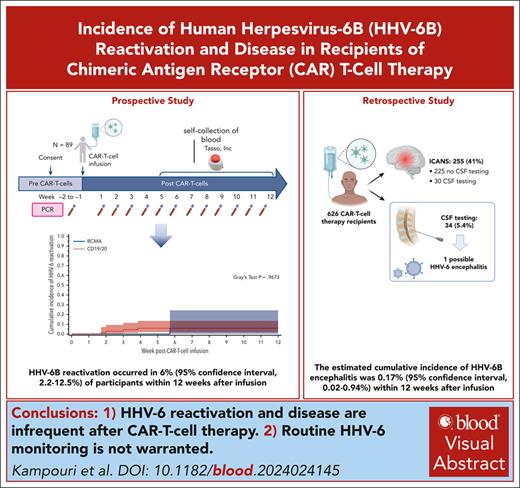
Key Points
HHV-6 reactivation in plasma occurred in 6% and possible HHV-6 encephalitis in 0.2% of patients within 12 weeks after CARTx.
HHV-6 reactivation and disease are infrequent after CARTx, and routine HHV-6 monitoring is not warranted.
Visual Abstract
Human herpesvirus 6B (HHV-6B) reactivation and disease are increasingly reported after chimeric antigen receptor (CAR) T-cell therapy (CARTx). HHV-6 reactivation in the CAR T-cell product was recently reported, raising questions about product and patient management. Because of overlapping manifestations with immune effector cell–associated neurotoxicity syndrome, diagnosing HHV-6B encephalitis is challenging. We provide 2 lines of evidence assessing the incidence and outcomes of HHV-6B after CARTx. First, in a prospective study with weekly HHV-6B testing for up to 12 weeks after infusion, HHV-6B reactivation occurred in 8 of 89 participants; 3 had chromosomally integrated HHV-6 and were excluded, resulting in a cumulative incidence of HHV-6B reactivation of 6% (95% confidence interval [CI], 2.2-12.5). HHV-6B detection was low level (median peak, 435 copies per mL; interquartile range, 164-979) and did not require therapy. Second, we retrospectively analyzed HHV-6B detection in the blood and/or cerebrospinal fluid (CSF) within 12 weeks after infusion in CARTx recipients. Of 626 patients, 24 had symptom-driven plasma testing, with detection in 1. Among 34 patients with CSF HHV-6 testing, 1 patient had possible HHV-6 encephalitis for a cumulative incidence of 0.17% (95% CI, 0.02-0.94), although symptoms improved without treatment. Our data demonstrate that HHV-6B reactivation and disease are infrequent after CARTx. Routine HHV-6 monitoring is not warranted.
Introduction
Reactivation of latent viral infections causes substantial morbidity in patients who are immunocompromised.1 Human herpesvirus 6B (HHV-6B) is well established as the most frequent infectious cause of encephalitis after allogeneic hematopoietic cell transplant (HCT), resulting in high mortality and frequent long-term sequelae.2 In addition, HHV-6B detection in the blood after allogeneic HCT is associated with delirium and neurocognitive decline.3 The epidemiology and clinical significance of HHV-6B reactivation is not well elucidated after chimeric antigen receptor (CAR) T-cell therapy (CARTx). Cases of HHV-6B encephalitis after CARTx have been reported but estimates of the incidence are unknown.4-9 Overlapping clinical manifestations between viral encephalitis and immune effector cell–associated neurotoxicity syndrome (ICANS)5 make diagnosis challenging without systematic testing. Importantly, the frequent occurrence of ICANS in 40% to 77% of CARTx recipients10 raises suspicion for a link with HHV-6B reactivation and associated neurologic dysfunction in this population.3
Recently, the potential role of cellular therapies as a source of viral infection in recipients who are immunocompromised was suggested.11 Using single-cell sequencing, Lareau et al identified a rare population of cells with high HHV-6B transcriptional activity in CD4+ T cells from in vitro cultures of preinfusion CAR T-cells in addition to in vivo cultures from post-infusion patient blood. These data have important implications for manufacturing and screening of T-cell therapeutics, as well as monitoring of patients. Considering these results and given the potential impact of this ubiquitous lymphotropic and neurotropic virus on ICANS, there is renewed interest to explore the kinetics and clinical presentation of HHV-6B after CARTx. We herein provide 2 lines of compelling clinical evidence demonstrating infrequent detection of HHV-6 DNA in plasma and cerebrospinal fluid (CSF) after CARTx, with no temporal association with ICANS.
Study design
First, we conducted a prospective study to assess the incidence and kinetics of, and risk factors for HHV-6B reactivation in adults receiving CARTx for hematologic malignancies from August 2021 through March 2023 at Fred Hutchinson Cancer Center (FHCC). We obtained plasma once before lymphodepleting chemotherapy and weekly after CARTx for up to 12 weeks. For samples after week 4, participants had the option to use a novel device (Tasso, Inc; Seattle, WA) for home-based self-collection of blood and shipment to our center (supplemental Information, available on the Blood website). We tested for HHV-6 using polymerase chain reaction (PCR)12; HHV-6 species and inherited chromosomally integrated HHV-6 (iciHHV-6) were determined by droplet digital PCR.13 iciHHV-6 occurs when HHV-6 integrates into the chromosome of a germ cell that is subsequently fertilized, resulting in a copy of the HHV-6 genome in every nucleated cell. As a result, all cellular samples (or samples contaminated by cell lysis, eg, plasma) will have detection of HHV-6 DNA that is not indicative of viral replication (supplemental Information).14 HHV-6 detection was defined as ≥1 results of >50 copies per mL, the lower limit of detection of the assay.12 Next, to estimate the incidence of HHV-6 encephalitis after CARTx, we retrospectively analyzed clinical test results for HHV-6B in blood and/or CSF within 12 weeks after CARTx in adults receiving CARTx for hematologic malignancies at FHCC from July 2013 to January 2023. The studies were approved by the FHCC Institutional Review Board, and all participants provided written informed consent.
Cytokine release syndrome and ICANS were graded according to the American Society for Transplantation and Cellular Therapy Consensus Guidelines15 (or Common Terminology Criteria for Adverse Events criteria version 4.03 for CARTx before 201916). We calculated the cumulative incidence of HHV-6B reactivation and encephalitis within 12 weeks after CARTx, treating death and subsequent CARTx or HCT as competing risks. We stratified cumulative incidence curves by predefined baseline variables and used the Gray test to compare between groups. Analyses were performed using Stata/SE (version 17.0, StataCorp LLC) and SAS version (9.4 TS1M3; SAS Institute).
Results and discussion
HHV-6 reactivation is infrequent after CARTx
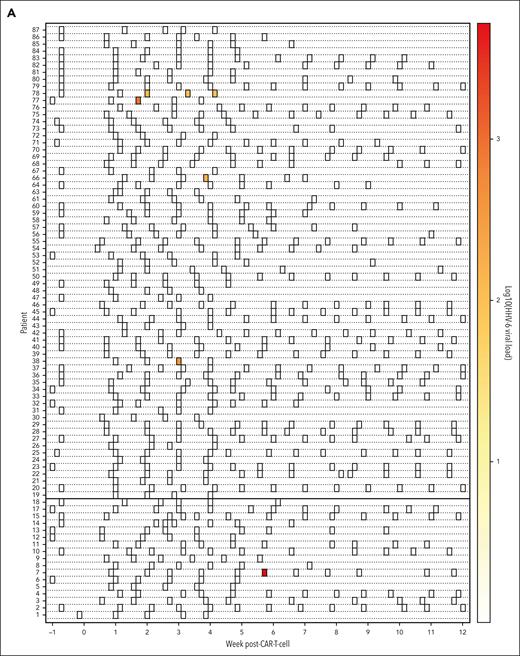
In the prospective study, we enrolled 84 participants receiving 89 CAR T-cell infusions (supplemental Table 1). HHV-6 viremia occurred in 8 participants, 3 of whom (38%) had iciHHV-6 and were excluded from analyses, resulting in a cumulative incidence of HHV-6 (all species B) reactivation of 6% (95% confidence interval [CI], 2.2-12.5) within 12 weeks after CARTx (Figure 1A-B). All HHV-6B detection occurred 2 to 6 weeks after infusion (median, 21 days). Four of 5 participants had a single positive result; the remaining participant had HHV-6B detection at weeks 2 to 4 but no subsequent testing. The median peak viral load was 435 copies per mL (interquartile range, 164-979).
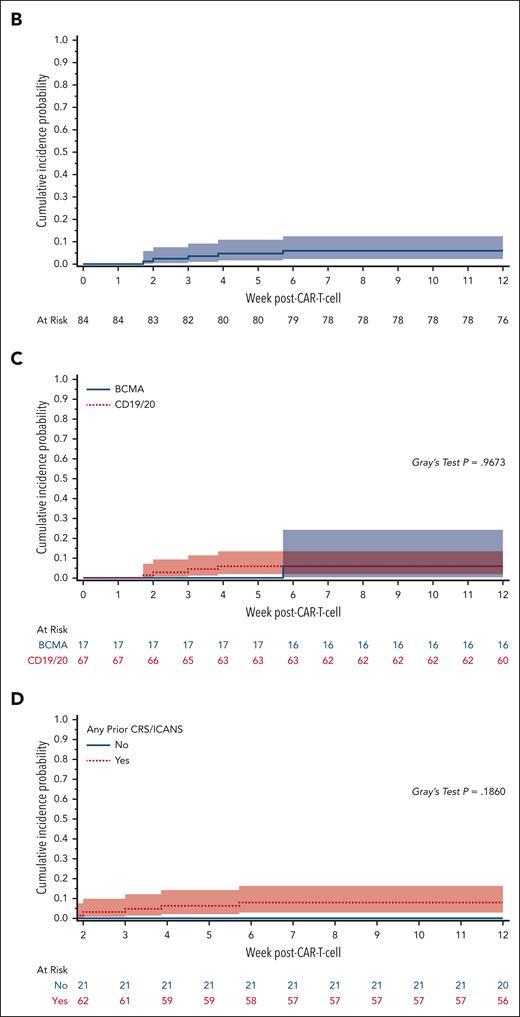
Cumulative incidence and kinetics of HHV-6B detection within 12 weeks after CAR T-cell infusion. (A) Heat map of HHV-6B reactivation kinetics after CARTx. Three participants with iciHHV-6 had HHV-6 detection at every tested time point and were excluded from this plot. Each row represents a patient, and each square represents a plasma sample. The intensity of color represents the viral load (negative samples are depicted in white). BCMA CARTx recipients are depicted at the bottom and CD19/CD20 CARTx recipients at the top of the heat map. (B) Cumulative incidence curve of HHV-6B reactivation within 12 weeks after CARTx: 6% (95% CI, 2.2-12.5). (C) Cumulative incidence of HHV-6B reactivation stratified by CAR T-cell target: BCMA, 6% (95% CI, 0.4-24.2) vs CD19/20, 6% (95% CI, 1.9-13.5). (D) Cumulative incidence of HHV-6B reactivation stratified by prior cytokine release syndrome (CRS) and/or ICANS, starting at week 2 after CAR T-cell infusion as a landmark analysis: prior CRS and/or ICANS, 7.9% (95% CI, 2.9-16.3) vs no prior CRS and/or ICANS, 0%. In all curves, death was treated as a competing risk. The Gray test was used for statistical comparisons.
Cumulative incidence and kinetics of HHV-6B detection within 12 weeks after CAR T-cell infusion. (A) Heat map of HHV-6B reactivation kinetics after CARTx. Three participants with iciHHV-6 had HHV-6 detection at every tested time point and were excluded from this plot. Each row represents a patient, and each square represents a plasma sample. The intensity of color represents the viral load (negative samples are depicted in white). BCMA CARTx recipients are depicted at the bottom and CD19/CD20 CARTx recipients at the top of the heat map. (B) Cumulative incidence curve of HHV-6B reactivation within 12 weeks after CARTx: 6% (95% CI, 2.2-12.5). (C) Cumulative incidence of HHV-6B reactivation stratified by CAR T-cell target: BCMA, 6% (95% CI, 0.4-24.2) vs CD19/20, 6% (95% CI, 1.9-13.5). (D) Cumulative incidence of HHV-6B reactivation stratified by prior cytokine release syndrome (CRS) and/or ICANS, starting at week 2 after CAR T-cell infusion as a landmark analysis: prior CRS and/or ICANS, 7.9% (95% CI, 2.9-16.3) vs no prior CRS and/or ICANS, 0%. In all curves, death was treated as a competing risk. The Gray test was used for statistical comparisons.
The incidence of HHV-6B reactivation was similar after CD19/CD20- vs B-cell maturation antigen (BCMA)-targeted CARTx (Figure 1C). All participants with HHV-6B reactivation had preceding cytokine release syndrome, and 3 had preceding or concomitant ICANS (Figure 1D; supplemental Table 1). HHV-6B reactivation occurred after ICANS onset in 2 participants, and the additional participant had a single positive test on the day of symptoms onset attributed to ICANS. Clinical courses are detailed in Figure 2 and the supplemental Information. No patients had evidence of HHV-6B encephalitis, and none received antiviral therapy active against HHV-6B. Among 3 individuals with iciHHV-6, none were diagnosed with ICANS (supplemental Information).
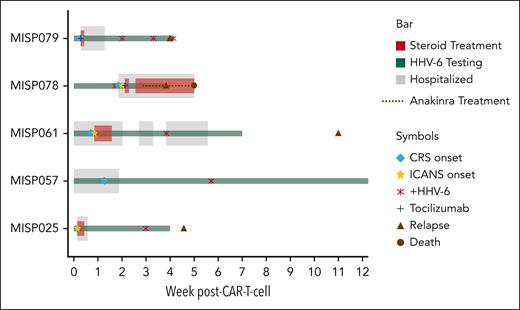
Clinical course of participants with HHV-6 reactivation after CARTx. Swimmer plot of clinical courses of participants with HHV-6 reactivation depicting clinical events (CRS and ICANS), management, HHV-6 detection in plasma, and outcomes. Each row depicts an individual participant.
Clinical course of participants with HHV-6 reactivation after CARTx. Swimmer plot of clinical courses of participants with HHV-6 reactivation depicting clinical events (CRS and ICANS), management, HHV-6 detection in plasma, and outcomes. Each row depicts an individual participant.
HHV-6 encephalitis is rare after CARTx
We retrospectively analyzed HHV-6B detection in blood and/or CSF testing within 12 weeks after CARTx in 626 adult CARTx recipients (supplemental Table 2). Overall, 255 of 626 patients (41%) had ICANS of any grade. Twenty-four patients (3.8% overall), including 19 (7.5%) of 255 patients with ICANS, had plasma HHV-6B testing, with detection in 1 patient. Altered mental status was the main indication for testing (50%; supplemental Table 3). Thirty-four patients (5.4% overall), including 30 (11.8%) of 255 patients with ICANS, had CSF tested with PCR for HHV-6B and other viruses in the context of neurologic symptoms (supplemental Table 4). CSF testing practices evolved over time: testing was performed more frequently for neurologic symptoms before 2018; after 2018, testing was limited to patients with ICANS grade ≥3 or atypical symptoms (supplemental Figure 1).
CSF testing detected HHV-6B in 1 patient 32 days after infusion with a viral load of 1100 copies per mL. This was also the 1 patient with HHV-6B detection in the plasma, who had 2 consecutive positive tests with a peak viral load of 1900 copies per mL. The patient was diagnosed with ICANS grade 4; HHV-6B encephalitis was considered but not treated given the absence of typical features of HHV-6B encephalitis and improvement with corticosteroids. Based on these data, the estimated cumulative incidence of HHV-6B encephalitis was 0.17% (95% CI, 0.02-0.94) including this possible case. Empiric treatment for HHV-6B in the absence of positive blood and/or CSF detection was not administered per standard practice at FHCC.
Our study provides 2 lines of compelling clinical evidence, demonstrating infrequent detection of HHV-6B DNA in plasma and CSF after CARTx, with no evident temporal association with ICANS. HHV-6B viremia was infrequent, low level, and transient without clear sequelae after CARTx. Furthermore, 38% of participants with HHV-6B detection on systematic screening had iciHHV-6, indicating that routine testing would be almost as likely to incidentally identify latent iciHHV-6, potentially leading to unnecessary treatment.17 Published guidelines do not recommend monitoring or preemptive therapy for HHV-6B after allogeneic HCT, even in the highest risk groups such as cord blood HCT recipients, in whom reactivation occurs in up to 90% and HHV-6B encephalitis in up to 10%.14,18 Although antivirals with activity against HHV-6B are available, they have toxicities that warrant careful stewardship, and preemptive strategies do not appear to prevent encephalitis.19-23 Thus, routine testing of CAR T-cell products and postinfusion monitoring for HHV-6B are not supported by clinical data.
The study by Lareau et al provides first evidence of reactivation of latent viruses in CAR T-cell products, although reactivation of HHV-6 and other viruses in cell cultures has been previously demonstrated.24 Taken alone, these data raise concern for viral transmission to vulnerable patients via CAR T-cell products and have potentially important implications for product manufacturing, regulatory requirements, and monitoring of treated individuals. In the patient samples studied by Lareau et al, it is not possible to determine whether the source of HHV-6B was the CAR-T cells or endogenous cells,25 but our data suggest that the clinical significance is limited.
To our knowledge, this is the first study to systematically evaluate HHV-6B reactivation and disease in CARTx recipients including both CD19- and BCMA-targeted CARTx. Innovative sampling methods for home-based self-collection of blood were used to support robust monitoring for up to 12 weeks after CARTx, establishing the feasibility of this emerging strategy in cellular therapy recipients. The retrospective assessment of HHV-6B detection in the blood and CSF in a large cohort offers further insights into the infrequent detection of HHV-6 and related complications in clinical practice. Study limitations include the single-center design and the lack of CSF testing in the large majority of patients with neurological symptoms precluding any estimate of the true incidence of HHV-6B encephalitis. Larger studies may reveal associations between HHV-6B reactivation and other outcomes after CARTx.14 No patients in our study received allogeneic CARTx, which may confer increased risk because of more intensive immunosuppression, highlighting the need to remain diligent in assessing infectious complications of novel therapies.
In conclusion, our findings suggest that HHV-6B reactivation and disease are infrequent after CARTx. Although routine monitoring is not supported by our findings, HHV-6B testing should be performed in patients with refractory or atypical neurologic symptoms.
Acknowledgments
The authors thank Alythia Vo, Winnie L. Liu, and Clementine Chalal (Fred Hutchinson Cancer Center [FHCC]) for data collection; Haiying Zhu and Tracy Santo (University of Washington) for performing laboratory testing of samples; Ryan S. Basom and Chris Davis (FHCC) for support with data extraction; and Cameron Turtle, Mazyar Shadman, Brian Till, Ryan D. Cassaday, Aude Chapuis, and David G. Maloney (FHCC) for expert input.
This work was supported by grants from the Swiss National Science Foundation (P500PM_202961 to E.K.); SICPA Foundation (to E.K.); Dharam Ablashi Research Fund, Pilot Grant by HHV-6 Foundation (to E.K. and J.A.H.); National Institutes of Health, National Cancer Institute (P30 CA015704-47); and research support from Merck (to J.A.H).
Figures in the visual abstract was created with BioRender.com.
Authorship
Contribution: E.K. and J.A.H. were responsible for the design of the study and interpretation of the data; E.K., J.A.H., E.M.K., and H.X. analyzed the data and created the figures; E.K., S.S.I., E.S.K., M.K.S., and J.A.H. enrolled participants, and collected samples and data; E.C.L., A.J.C., A.P., D.J.G., A.A., J.J.H., and J.G. collected data; A.C.P.-O. and K.R.J. supervised the laboratory work; E.K. and J.A.H. prepared the first draft of the manuscript; and all authors contributed to the writing and revision of the manuscript and approved the final version.
Conflict-of-interest disclosure: A.J.C. has served as consultant and participated in the advisory board, or steering committee for Janssen, Bristol Myers Squibb, Sebia, Sanofi, and Adaptive Biotechnologies; has received research funding from AbbVie, Sanofi, Janssen, Bristol Myers Squibb, Adaptive Biotechnologies, Nektar, Harpoon, Regeneron, Caelum, and IGM Biosciences. D.J.G. has served as an adviser and has received research funding and royalties from Juno Therapeutics, a Bristol Myers Squibb company; has served as an adviser and received research funding from Seattle Genetics; has served as an adviser for GlaxoSmithKline, Celgene, Ensoma, Janssen Biotech, and Legend Biotech; and has received research funding from SpringWorks Therapeutics, Sanofi, and Cellectar Biosciences. J.G. has served as ad hoc consultant and has received honoraria from Sobi, Legend Biotech, Janssen, Kite Pharma, and MorphoSys; research funding from Sobi, Juno Therapeutics (a Bristol Myers Squibb company), Celgene (a Bristol Myers Squibb company), and Angiocrine Bioscience; and has participated in the independent data review committee for Century Therapeutics. D.M.Z. has received research funding from Merck; and has served as a consultant for AlloVir for service on end point adjudication committees for 2 trials. M.J.B has served as consultant and has received research funding from Merck; has served as consultant for Symbio, Helocyte, Moderna, and AlloVir; has served as consultant and had option to acquire stock for EvrysBio. J.A.H. has served as a consultant for Moderna, AlloVir, Gilead, SentiBio, Modulus, and Allogene; and received research funding from AlloVir, Gilead, and Merck. The remaining authors declare no competing financial interests.
Correspondence: Joshua A. Hill, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, 1100 Fairview Ave N, Mail stop E4 100, Seattle, WA 98109; email: jahill3@fredhutch.org.
References
Author notes
The data sets generated and analyzed for this study are available after publication upon reasonable request from the corresponding author, Joshua A. Hill (jahill3@fredhutch.org) with investigator financial support, and with appropriate documentation of institutional review board approval and/or data access agreements, as applicable.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal