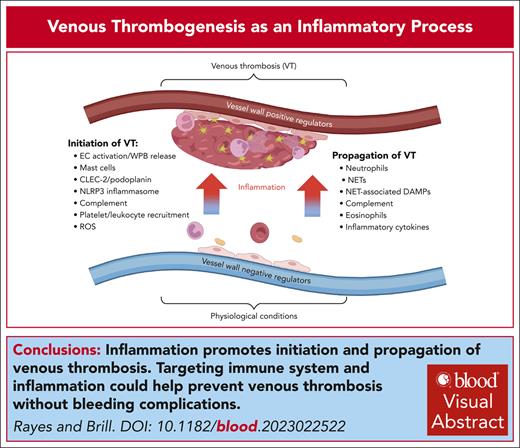
Visual Abstract
Venous thrombosis (VT) is a serious medical condition in which a blood clot forms in deep veins, often causing limb swelling and pain. Current antithrombotic therapies carry significant bleeding risks resulting from targeting essential coagulation factors. Recent advances in this field have revealed that the cross talk between the innate immune system and coagulation cascade is a key driver of VT pathogenesis, offering new opportunities for potential therapeutic interventions without inducing bleeding complications. This review summarizes and discusses recent evidence from preclinical models on the role of inflammation in VT development. We highlight the major mechanisms by which endothelial cell activation, Weibel-Palade body release, hypoxia, reactive oxygen species, inflammasome, neutrophil extracellular traps, and other immune factors cooperate to initiate and propagate VT. We also review emerging clinical data describing anti-inflammatory approaches as adjuncts to anticoagulation in VT treatment. Finally, we identify key knowledge gaps and future directions that could maximize the benefit of anti-inflammatory therapies in VT. Identifying and targeting the inflammatory factors driving VT, either at the endothelial cell level or within the clot, may pave the way for new therapeutic possibilities for improving VT treatment and reducing thromboembolic complications without increasing bleeding risk.
Introduction
Venous thrombosis (VT), together with its major complication, pulmonary embolism, designated as venous thromboembolism (VTE), is a major health issue and economic burden. Current prevention strategies focus on targeting clotting factors, which inevitably leads to bleeding in some patients, especially vulnerable groups like elderly individuals or patients with kidney or liver insufficiency. VT can be provoked by certain diseases (eg, cancer), genetic predisposition (eg, prothrombin mutation), infection, and other conditions, whereas VT not associated with a clear causative factor is designated as unprovoked.1 Blood flow stagnancy is 1 of the major causes of VT; it can be associated with prolonged immobilization resulting from major surgery, paralysis, or long-haul flights. It is a general assumption that VT initiation occurs in the valvular pockets, an hypothesis stemming from autopsy data, hypoxia observed in the valvular pockets in dogs,2 and different expression of prothrombotic and antithrombotic molecules compared with the luminal endothelium.3 However, we were unable to find direct evidence proving that VT originate from the valvular pockets. It cannot be excluded that thrombus formation starts upstream of the valve and grows downstream, reaching the valve, preventing its progression. The lack of valves in mouse inferior vena cava (IVC) precludes verification of the thrombus initiation site in the commonly used preclinical models of VT based on IVC ligation in rodents. Another limitation is the lack of vasa vasorum in the IVC in rodents, which can lead to more severe local hypoxia compared with humans. Rodents live in the horizontal plane, whereas humans live in both horizontal and vertical planes, making the effects of gravity on venous hemodynamics substantially different. Finally, different experimental settings may induce VT through dissimilar mechanisms, limiting the extrapolation of data across models. For example, VT induced by IVC stenosis (partial occlusion) likely recapitulates features of local sterile inflammation (although a role for tissue factor [TF] in myeloid cells has been reported as well), whereas in the complete ligation model, VT is mostly driven by TF in the vessel wall.4-6 It is still to be determined what the relative impact of these mechanisms is in human VT developed under various conditions. Although most of our knowledge of the mechanisms regulating VT arises from preclinical models, extrapolation of these data to humans should be applied with caution.7-9 It is however encouraging that some antithrombotic targets initially identified in rodents are also beneficial in humans, supporting the importance of preclinical studies in the field.
Over the past decades, multiple immune and inflammatory mechanisms triggering VT have been identified, which justified introducing the term “immunothrombosis.”10 A recent study identified changes in the levels of multiple immune- and inflammation-related proteins in the venous wall and blood in experimental VT.11 In VT modeled by IVC ligation, higher vessel wall levels of neutrophils but lower levels of other immune and stromal cells were reported.12 Most upregulated genes were associated with inflammation, hypoxia, and apoptosis, whereas the most downregulated genes encoded extracellular matrix proteins. Moreover, ∼30 inflammation-related molecules have been reported to modulate thrombosis in preclinical models of VT.13 This offers a tempting window of opportunity to develop new strategies for tackling VT through modulation of the immune system, thereby reducing the risk of bleeding complications. The number of regulatory entities involved is surprisingly high, making it challenging to comprehend how they intermingle to effectively regulate VT. Here, we discuss new advances in understanding the contribution of inflammatory mechanisms to VT, speculate about the role of new factors, and a potential hierarchy of the mechanisms controlling its development. Most of the discussed data have been obtained from preclinical models (predominantly mice), whereas the chapter on clinical perspectives highlights the axis that has been validated, at least in part, in humans.
EC activation in VT
In the absence of an obvious trigger, such as cancer or genetic predisposition, reduced and/or perturbed blood flow becomes the leading cause of VT. Flow stagnancy contributes to the initiation of a cascade of events converging on endothelial cell (EC) activation controlled by several regulatory circuits. These pathways operate in parallel ensuring redundancy of the regulatory mechanisms and explaining why targeting multiple molecules leads to a similar outcome, induction of or protection against VT. Normal flow maintains EC quiescence mainly through the release of nitric oxide (NO) and prostacyclin and the expression of anti-inflammatory and antithrombotic molecules (Figure 1A). Activation of ECs leads to downregulation of these pathways, release of Weibel-Palade bodies (WPBs; Table 1), and upregulation of adhesion receptors, resulting in the recruitment of platelets and immune cells and promoting thrombosis (Figure 1B). Each component in this inflammatory cascade participates in VT initiation and/or propagation.14,15 These factors are temporally and spatially interconnected, forming multiple positive feedback loops driving thrombus development. Thus, targeting EC activation and WPB release represents a potential strategy for preventing VT initiation, whereas focusing on upstream pathways, which converge on EC activation, is likely to provide only partial protection because of built-in redundancy of these regulatory mechanisms.
EC quiescence and its dysfunction in VT. (A) Under physiological conditions, laminar flow maintains EC quiescence by releasing NO and PGI2. The antithrombotic phenotype of the ECs is supported by activating protein C and NO biosynthesis stimulated through multiple pathways including AdipoQ and HDL/apoA–SR-BI, and mechanosensors through PI3K/Akt-, cAMP-, and PKA-dependent eNOS activation. eNOS activation, supported by SIRT1, inhibits WPB release. Transcription factors KLF2, KLF4, KLF11, and NRF2 support the transcription of cytoprotective and antioxidant molecules (eg, HO-1, SOD, and GST), which inhibit ROS. KLF11 inhibits the expression of TF. Cilium and the glycocalyx induce NRF2 expression and protect the endothelial surface by masking adhesion molecules and limiting immune cell adhesion. VWF multimers are rapidly cleaved by ADAMTS-13, limiting its thrombotic functions. In the VT setting (B), hypoxia (low O2) favors a proinflammatory and prothrombotic environment. Hypoxia inhibits NRF2, KLF2, and other cytoprotective molecules and increases ROS generation. Hypoxia and ROS increase the degradation of the glycocalyx, thrombomodulin shedding, and CAM exposure, favoring immune cell recruitment. Hypoxia and ROS also upregulate receptors for DAMPs and promote cell activation. ROS induce fibrinogen oxidation, which potentiates fibrin generation. Reduction of eNOS supports WPB release including VWF and P-selectin, supporting cell recruitment. Piezo1 activation stimulates ATP production and entry, activation of NLRP3 assembly, pyroptosis, and release of IL-1β. IL-1β, activated and secreted by NLRP3 inflammasome, promotes VWB release and expression of CAMs. Gab-2 supports IL-1β–induced WPB release through the CARMA3–BCL10–MALT1 signalosome, NF-κB activation, neutrophil adhesion, and TF expression. ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; adipoQ, adiponectin; APC, activated protein C; apo-AI, apolipoprotein-AI; Ca2+, calcium; CAMs, nuclear cell adhesion molecules; Fg, fibrinogen; Gab2, Grb2-associated binder 2; GPCR, G-protein coupled receptor; GST, glutathione S-transferase; HDL, high-density lipoprotein; HO-1 heme-oxygenase-1; ICAM-1, intercellular adhesion molecule 1; IL1R, interleukin-1 receptor; PGI2, prostacyclin; PI3K, phosphoinositide 3-kinase; SR-B1, scavenger receptor class B type I; T, thrombin; TFP1, tissue factor pathway inhibitor; TM, thrombomodulin; VCAM-1, vascular cell adhesion molecule 1. Figure created with BioRender.com.
EC quiescence and its dysfunction in VT. (A) Under physiological conditions, laminar flow maintains EC quiescence by releasing NO and PGI2. The antithrombotic phenotype of the ECs is supported by activating protein C and NO biosynthesis stimulated through multiple pathways including AdipoQ and HDL/apoA–SR-BI, and mechanosensors through PI3K/Akt-, cAMP-, and PKA-dependent eNOS activation. eNOS activation, supported by SIRT1, inhibits WPB release. Transcription factors KLF2, KLF4, KLF11, and NRF2 support the transcription of cytoprotective and antioxidant molecules (eg, HO-1, SOD, and GST), which inhibit ROS. KLF11 inhibits the expression of TF. Cilium and the glycocalyx induce NRF2 expression and protect the endothelial surface by masking adhesion molecules and limiting immune cell adhesion. VWF multimers are rapidly cleaved by ADAMTS-13, limiting its thrombotic functions. In the VT setting (B), hypoxia (low O2) favors a proinflammatory and prothrombotic environment. Hypoxia inhibits NRF2, KLF2, and other cytoprotective molecules and increases ROS generation. Hypoxia and ROS increase the degradation of the glycocalyx, thrombomodulin shedding, and CAM exposure, favoring immune cell recruitment. Hypoxia and ROS also upregulate receptors for DAMPs and promote cell activation. ROS induce fibrinogen oxidation, which potentiates fibrin generation. Reduction of eNOS supports WPB release including VWF and P-selectin, supporting cell recruitment. Piezo1 activation stimulates ATP production and entry, activation of NLRP3 assembly, pyroptosis, and release of IL-1β. IL-1β, activated and secreted by NLRP3 inflammasome, promotes VWB release and expression of CAMs. Gab-2 supports IL-1β–induced WPB release through the CARMA3–BCL10–MALT1 signalosome, NF-κB activation, neutrophil adhesion, and TF expression. ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; adipoQ, adiponectin; APC, activated protein C; apo-AI, apolipoprotein-AI; Ca2+, calcium; CAMs, nuclear cell adhesion molecules; Fg, fibrinogen; Gab2, Grb2-associated binder 2; GPCR, G-protein coupled receptor; GST, glutathione S-transferase; HDL, high-density lipoprotein; HO-1 heme-oxygenase-1; ICAM-1, intercellular adhesion molecule 1; IL1R, interleukin-1 receptor; PGI2, prostacyclin; PI3K, phosphoinositide 3-kinase; SR-B1, scavenger receptor class B type I; T, thrombin; TFP1, tissue factor pathway inhibitor; TM, thrombomodulin; VCAM-1, vascular cell adhesion molecule 1. Figure created with BioRender.com.
WPB constituents and their link with VT
| WPB constituent . | Effect on VT . | Mechanism . | Phenotype in mutant mice . | Reference . |
|---|---|---|---|---|
| VWF | ↑ | Mediates recruitment of leukocytes and platelets to the site of thrombosis. At low shear rate, recruits leukocytes through PSGL-1 and β2-integrin and supports firm adhesion. Binds to macrophages leading to modified expression of over a thousand inflammation-related genes, which could contribute to additional endothelial activation and leukocyte recruitment to the site of thrombosis. | Confirmed | 14-19 |
| P-selectin | ↑ | Mediates leukocyte recruitment to the thrombosis site. Reportedly anchors released VWF multimers to the endothelial surface, although this was disputed in another study. | Confirmed | 15,20,21 |
| Eotaxin | ↑ | Chemoattractant for eosinophils, increases the permeability and oxidative stress in ECs, supports inflammation through eosinophil recruitment, MC degranulation, and blood clotting. Eosinophils stimulate the release of VWF from ECs leading to recruitment and activation of platelets by major basic protein. Hypereosinophilia is a risk factor for VTE. | Not confirmed | 22-25 |
| Angiopoietin-2 | ↑ | Increases thrombus size after 3-d IVC ligation. Reduces plasma levels of activated protein C. Hypoxia strongly enhances the expression of Ang-2 messenger RNA in human endothelium, and plasma levels correlate with the severity of pulmonary embolism. | Confirmed | 26-28 |
| Endothelin-1 | TBD | Induces vasoconstriction and stimulates the production of NO and PGI2. | Not confirmed | 29 |
| Osteoprotegerin | TBD | Binds to A1 VWF domain and prevents platelet adhesion to VWF strings. | Not confirmed | 30,31 |
| WPB constituent . | Effect on VT . | Mechanism . | Phenotype in mutant mice . | Reference . |
|---|---|---|---|---|
| VWF | ↑ | Mediates recruitment of leukocytes and platelets to the site of thrombosis. At low shear rate, recruits leukocytes through PSGL-1 and β2-integrin and supports firm adhesion. Binds to macrophages leading to modified expression of over a thousand inflammation-related genes, which could contribute to additional endothelial activation and leukocyte recruitment to the site of thrombosis. | Confirmed | 14-19 |
| P-selectin | ↑ | Mediates leukocyte recruitment to the thrombosis site. Reportedly anchors released VWF multimers to the endothelial surface, although this was disputed in another study. | Confirmed | 15,20,21 |
| Eotaxin | ↑ | Chemoattractant for eosinophils, increases the permeability and oxidative stress in ECs, supports inflammation through eosinophil recruitment, MC degranulation, and blood clotting. Eosinophils stimulate the release of VWF from ECs leading to recruitment and activation of platelets by major basic protein. Hypereosinophilia is a risk factor for VTE. | Not confirmed | 22-25 |
| Angiopoietin-2 | ↑ | Increases thrombus size after 3-d IVC ligation. Reduces plasma levels of activated protein C. Hypoxia strongly enhances the expression of Ang-2 messenger RNA in human endothelium, and plasma levels correlate with the severity of pulmonary embolism. | Confirmed | 26-28 |
| Endothelin-1 | TBD | Induces vasoconstriction and stimulates the production of NO and PGI2. | Not confirmed | 29 |
| Osteoprotegerin | TBD | Binds to A1 VWF domain and prevents platelet adhesion to VWF strings. | Not confirmed | 30,31 |
Ang-2, angiopoietin-2; PGI2, prostaglandin I2; TBD, to be determined.
The main natural antagonist for WPB release is NO, synthesized by endothelial NO synthase (eNOS).32,33 Various strategies altering NO levels showed beneficial effects in VT. For example, apolipoprotein A-I, the main protein component of high density lipoprotein (“good cholesterol”), prevents VT through a scavenger receptor class B, type 1–eNOS-NO–dependent axis.34 Adiponectin, a hormone predominantly produced by adipocytes, also inhibits thromboinflammation by stimulating eNOS through an adenosine 5′-monophosphate (AMP)-activated kinase.35
Initial triggers of VT: hypoxia and perturbed hemodynamics
Because veins receive most of oxygen from blood in their lumen, flow stagnancy–induced local hypoxia of all vessel wall layers is considered a major trigger of VT.36 Because of the low number of mitochondria, the major energy-producing mechanism in ECs is glycolysis, irrespective of the oxygen tension.37 A low level of oxidative phosphorylation likely constitutes a natural antioxidant mechanism, minimizing reactive oxygen species (ROS) generation and rendering ECs less susceptible to hypoxia. However, a substantial reduction in blood flow can trigger hypoxia in the endothelium, inducing oxidative stress and promoting WPB release.38 ROS also induce nicotinamide adenine dinucleotide phosphate-dependent TF expression in ECs,39 monocytes and smooth muscle cells,40 and inactivate anticoagulant proteins like protein C41 and thrombomodulin.
Altered blood flow is another potential trigger of thrombosis. ECs express multiple mechanosensors including cilia, the glycocalyx, membrane receptors (eg, platelet EC adhesion molecule 1), and ion channels (eg, Piezo1; transient receptor potential vanilloid 4 channel; and the inwardly rectifying potassium channel, Kir2.1), which regulate NO production.42 Unidirectional laminar flow detected by cilia, a special organelle on ECs, leads to elevated intracellular calcium and NO production43 and protects ECs from oxidative damage through nuclear factor-E2–related factor 2 (Nrf2), an effect that could be lost in flow stagnancy.44 In a rat model of VT, Nrf2 expression in the IVC is decreased, alongside other cytoprotective enzymes, including heme-oxygenase 1 and superoxide dismutase (SOD).45 Heme-oxygenase 1 exerts anti-inflammatory functions by preserving the inactivation of NF-κB, whereas SOD acts as an antioxidant mitigating vascular dysfunction. SOD-1 deficiency promotes VT, at least partially through thrombomodulin oxidation, limiting its anticoagulant activity.46 ROS-dependent fibrinogen oxidation favors its conversion to fibrin and reducing its interaction with the anticoagulant system.47
Hypoxia also induces shedding of another mechanosensor, the glycocalyx,48 and degradation of its components, hyaluronic acid, heparan sulfate, and chondroitin sulfate; unmasking adhesion molecules, increasing leukocyte recruitment, and reducing NO availability.49 Disturbed shear stress and hypoxia contribute to thromboinflammation in VT by altering the expression of microRNAs, a class of noncoding small RNAs that posttranscriptionally suppress target genes.50 Thus, ECs integrate diverse input signals and, based on this processed information, generate output cues initiating thrombosis, positioning ECs among the central mediators of VT initiation.
Krüppel-like factors (KLFs): linking shear stress and inflammation
Physiological flow induces the expression of KLF2, a shear stress–induced subclass of the zinc finger of DNA-binding transcription factors, which exerts anti-inflammatory and antithrombotic effects in ECs.51 KLF2 activates thrombomodulin and reduces plasminogen activator inhibitor 1 expression52 whereas overexpression of KLF2 inhibits cytokine-driven TF expression and prolongs clotting time on ECs under basal and inflammatory conditions.52 Disturbed shear stress, typical for VT, reduces KLF2 expression in vivo.53 KLF2 also suppresses proinflammatory activities of neutrophils, monocytes, and macrophages.54 Loss of KLF2 in neutrophils increases experimental VT, associated with higher levels of myeloperoxidase and neutrophil elastase, whereas neutralization of myeloperoxidase negates the prothrombotic environment associated with neutrophil KLF2 deficiency.55 Neutrophils lacking KLF2 are more prone to adhere, migrate, and express higher levels of TF. Besides its role in clotting, TF mediates inflammation by interacting with multiple protease-activated receptors, increasing adhesion molecules, and the release of inflammatory mediators. Low-level expression of TF in mice protects against lethality and bleeding while reducing inflammatory mediators in lipopolysaccharide-induced endotoxemia. Targeting TF or preventing the incorporation of TF-bearing microparticles into a thrombus was predicted to be a promising strategy against VT.5,56 Moreover, neutrophil KLF2 deficiency increases neutrophil extracellular trap (NET) formation (NETosis) in vitro, and thrombi from these mice exhibit higher NET content.55 In immune cells, KLF2 inhibits hypoxia-inducible factor 1α (HIF-1α) and NF-κB signaling and maintains cell quiescence. Neutrophil KLF2 deficiency also increases P-selectin glycoprotein ligand 1 (PSGL-1) clustering, supports neutrophil accumulation at the thrombosis site, increases NETosis and thrombosis in response to angiotensin-2.57 The expression of KLF2 is regulated by inflammatory cytokines, which exacerbate immunothrombosis. Interleukin-1β (IL-1β) inhibits the expression of KLF2, whereas overexpression of KLF2 activates eNOS and reduces the expression of adhesion molecules on ECs in response to inflammatory stimuli.58 Another transcription factor from this family, KLF11, attenuates VT by reducing TF production by ECs.59 Conversely, the expression of KLF15, which reduces eNOS activity in vitro, increases in VT.60 The critical role of KLF in VT positions this protein family as new targets to combat VT.
Neutrophils, NETs, and inflammasomes: drivers of VT propagation
NETs
Upon activation, ECs recruit immune cells and platelets, which support thrombosis via releasing NETs and supporting nucleotide-binding domain, leucine-rich–containing family, pyrin domain-containing-3 (NLRP3) inflammasome assembly. Chromatin decondensation, the initial step of NETosis, is mediated by peptidyl arginine deiminase 4 (PAD4) and leads to the release of DNA strands decorated with antimicrobial proteins and enzymes.61 Upon discovery, NETs were attributed the function of antimicrobial defense, but thereafter their role in thrombosis was discovered.62 NETs represent an adhesive and activating surface for platelets, trigger thrombin generation, inhibit fibrinolysis, and recruit red blood cells, which may be crucial for VT, because venous thrombi are essentially red (Figure 2).62,63 NETs are found in murine and human venous thrombi, whereas destruction of NETs by DNase I or prevention of their formation by genetic inhibition or pharmacological ablation of PAD4 reduces experimental VT.4,64 Whole blood clots, resistant to lysis by either tissue plasminogen activator (tPA) or DNase I separately, are completely digested by a combination of both.63 Thus, NETs likely form an additional scaffold besides fibrin, thereby consolidating the clot.
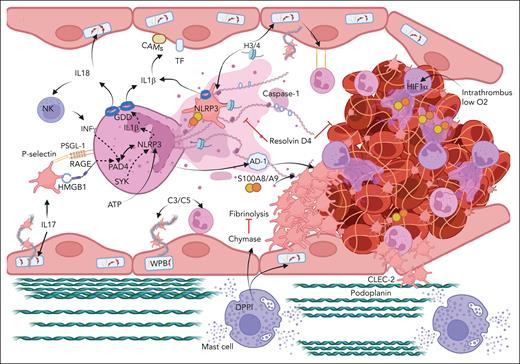
Cooperation between immune cells and factors in VT. In the VT setting, the release of WPBs, the expression of cell adhesion molecules, and inflammasome activation initiate thrombosis. MC degranulation supports VT, at least in part, through MC-derived chymase. The upregulation of podoplanin in the IVC triggers VT through CLEC-2 on platelets. Complement system components C3 and C5 activation supports platelet and neutrophil recruitment. Activated platelets support NETosis through HMGB1-RAGE and P-selectin–PSGL-1 leading to PAD4-dependent release of NETs. NLRP3 activation in neutrophils can be triggered by different mechanisms including SYK and PAD4 axis. NLRP3 activation leads to GDD pore formation and release of IL-1β and IL-18. IL-18 supports NK cell recruitment, release of IFN-γ, and NETosis. Platelet NLRP3 is also activated in VT. NET-associated histones and S100A8/A9 support thrombosis. Hypoxia inside thrombus induces HIF-1α in neutrophils and supports NETosis. AD-1 makes fibrin fibers thicker and protected from fibrinolysis. IL-17 activates ECs and neutrophils and promotes NETosis. Conversely, resolvin D4 prevents thrombus propagation. CAMs, cell adhesion molecules; CLEC-2, C-type lectin like-receptor 2; DPPI, dipeptidyl peptidase I; H3/4, histone H3 and H4. Figure created with BioRender.com.
Cooperation between immune cells and factors in VT. In the VT setting, the release of WPBs, the expression of cell adhesion molecules, and inflammasome activation initiate thrombosis. MC degranulation supports VT, at least in part, through MC-derived chymase. The upregulation of podoplanin in the IVC triggers VT through CLEC-2 on platelets. Complement system components C3 and C5 activation supports platelet and neutrophil recruitment. Activated platelets support NETosis through HMGB1-RAGE and P-selectin–PSGL-1 leading to PAD4-dependent release of NETs. NLRP3 activation in neutrophils can be triggered by different mechanisms including SYK and PAD4 axis. NLRP3 activation leads to GDD pore formation and release of IL-1β and IL-18. IL-18 supports NK cell recruitment, release of IFN-γ, and NETosis. Platelet NLRP3 is also activated in VT. NET-associated histones and S100A8/A9 support thrombosis. Hypoxia inside thrombus induces HIF-1α in neutrophils and supports NETosis. AD-1 makes fibrin fibers thicker and protected from fibrinolysis. IL-17 activates ECs and neutrophils and promotes NETosis. Conversely, resolvin D4 prevents thrombus propagation. CAMs, cell adhesion molecules; CLEC-2, C-type lectin like-receptor 2; DPPI, dipeptidyl peptidase I; H3/4, histone H3 and H4. Figure created with BioRender.com.
In the sterile experimental VT setting, NETosis can be triggered by activated platelet and/or endothelial P-selectin or platelet high mobility group box 1 protein (HMGB1) through PSGL-1 or the receptor for advanced glycation end products (RAGE), respectively.65,66 Platelets express HMGB1 and facilitate monocyte recruitment, which, upon activation, induce oxidation of HMGB-1, thereby supporting NETosis and promoting VT.67 The effects of HMGB-1 could be further amplified by free heparan sulfate, which acts as a cofactor for HMGB-1 to activate RAGE.68,69 Both soluble and platelet-expressed P-selectin induce expression of its own receptor, PSGL-1, on neutrophils and stimulate NETosis through the PSGL1-spleen tyrosine kinase (Syk)–calcium–PAD4 axis.66,70 Interestingly, the oxygen tension inside a 1-day-old thrombus is 90% lower than in the surrounding venous blood.71 This leads to accumulation of HIF-1α in neutrophils inside the thrombus, which could facilitate NETosis and promote thrombosis. Indeed, under hypoxic conditions, neutrophil-derived ROS are essential to the stabilization of HIF-1α,72 which is associated with NETosis. Deletion of HIF-1α reduces NETosis,73 whereas hypoxic microenvironment promotes it.74
Besides DNA strands, NETs promote VT through proteins decorating NETs, most of which act as damage-associated molecular patterns (DAMPs). NET-associated histones H3 and H4 promote thrombosis by supporting platelet and EC activation, pyroptosis, fibrin generation, and inhibition of fibrinolysis.75-78 The polyanionic agent defibrotide reduces VT in the IVC stenosis model, partly by inhibiting histone H4–induced secretion of IL-1β and IL-18 from ECs.76 Interestingly, although infusion of free histones exacerbates VT, inhibition of nucleosomes (complexes of DNA with histones) reduces thrombosis. Degradation of NETs by DNase I that leads to the formation of circulating nucleosomes, or inhibition of NETosis by hydroxychloroquine, reduces VT.4,79-81 This might be explained by lower activity of nucleosome-bound histones than free histones, although the precise mechanisms of this phenomenon remain unclear.
NLRP3 inflammasome and IL-1β
NLRP3 inflammasome is a molecular complex cleaving and activating IL-1β and IL-18 through caspase-1 and eventually leading to their release through gasdermin D (GDD) pores. Although NLRP3 was initially found in immune cells, its presence has also been reported in platelets.82 The role of NLRP3 was initially demonstrated in hypoxia-associated VT.83 In this study, hypoxia upregulates the expression of NLRP3 and IL-1β, orchestrated by HIF-1α, whereas NLRP3 knockdown reduces murine VT. Inhibition of IL-1β or its receptor also attenuates VT in mice.84 The assembly of NLRP3 is supported by adenosine triphosphate (ATP), a DAMP released from damaged cells, whereas CD39 expressed on leukocytes and endothelium, cleaves ATP and forms an antithrombotic regulatory loop.84 Genetic ablation of CD39 promotes experimental VT, leukocyte recruitment, NET formation, and fibrin deposition. Interestingly, the prothrombotic effect of adenosine diphosphate ([ADP], a platelet agonist), a product of CD39-mediated ATP cleavage, is apparently outweighed by the prevention of ATP-induced NLRP3 activation, rendering the overall effect of CD39 antithrombotic. ADP is converted by CD39 to AMP and further hydrolyzed by CD73 to adenosine, which could explain elimination of ADP from the thrombosis site. Removal of adenosine potentiates the production of resolvin D4, a molecule involved in the resolution of acute inflammation, during blood clotting.85 Resolvin D4 was recently shown to reduce VT propagation, decrease intrathrombus neutrophil numbers, and limit NETosis.86 Piezo1 mediates calcium influx and liberation of NO and ATP,87 suggestive of a potential role as a molecular switch between prothrombotic and antithrombotic pathways depending on shear stress. Thus, the CD39–CD73 axis and Piezo1 could be important checkpoints orchestrating thromboinflammation in the VT setting.
Recently, a new regulatory pathway of VT has been identified, involving IL-1β–initiated Gab2-dependent assembly of CAM (CARMA3-BCL-10-MALT1) signalosome in ECs.88 This signalosome induces Rho-mediated WPB release and NF-κB–mediated expression of TF and vascular cell adhesion molecule 1, leading to neutrophil and monocyte recruitment. Disruption of this signaling chain reduces VT in mice. Another signaling route involved in NLRP3 assembly,89 NETosis, and neutrophil activation, is Syk kinase.90 A central role for Syk in VT corroborates the role in VT of the platelet hemi-ITAM receptor C-type lectin-like receptor 2 (CLEC-2),91 whose signaling involves Src-Syk-PLCγ2 activation. IVC ligation enhances subendothelial expression of podoplanin, the major CLEC-2 ligand, and penetration of platelets into the vessel wall. Neutralization of podoplanin or CLEC-2 reduces thrombosis, suggesting that the interaction between platelet CLEC-2 and podoplanin supports VT.91 Tumor necrosis factor α and IL-1β, both involved in VT, upregulate podoplanin on fibroblasts,92,93 making fibroblasts a potential source of podoplanin in the VT setting, although the exact source of podoplanin in the IVC remains to be identified.
We have recently shown that NETs and the NLRP3 system cooperate to promote VT.94 Notably, NETs constitute a platform for caspase-1, which originates, at least in part, from platelets, and targeting caspase-1 reduces VT in mice. Our findings corroborate another report showing that PAD4 is involved in NLRP3 inflammasome assembly and NLRP3 signaling in neutrophils, which in return, supports NETosis.95 Extracellular histones, a part of NETs, also activate NLRP3 inflammasome assembly and enhance intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 expression in ECs on their surface. NLRP3 deficiency results in lower NET density in experimental VT.95 The specific role of caspase-1, but not caspase-11, in VT has been confirmed, along with reduced thrombosis in GDD-deficient mice.96 Overall, these findings imply that NETs and NLRP3 operate in concert forming a positive inflammatory feedback loop supporting thrombosis.
IL-18: novel regulator of VT
In addition to IL-1β, caspase-1 in the NLRP3 complex also activates IL-18, and human ECs express functional IL-18 and IL-18 receptors. Experimental VT increases plasma levels of IL-18 in rats, and overexpression or inhibition of IL-18 in vivo results in increased and reduced thrombosis, respectively.97,98 Plasma von Willebrand factor (VWF) levels mirror this trend, suggesting EC activation by IL-18. These findings are corroborated in vitro, with treatment of HUVECs with IL-18 inducing surface expression of VWF and P-selectin while reducing the expression of tPA.97 Moreover, IL-18 stimulates the production of interferon gamma (IFN-γ) by immune cells.99 Specifically, IFN-γ released from natural killer (NK) cells supports VT by facilitating NETosis.100 NK cell depletion, IFN-γ inhibition, or ablation of the transcription factor T-box in T cells, necessary for IFN-γ synthesis, attenuates VT in mice. Conversely, administration of IFN-γ or adoptive transfer of wild-type NK cells restores thrombosis. Notably, lipopolysaccharide-primed mast cells (MCs) also stimulate IFN-γ production by NK cells in an OX40 ligand–dependent manner.101 The pathophysiological role of the NLRP3–IL-18–IFN-γ axis in driving NETosis and VT warrants further investigation.
MCs as initiators of VT
MCs contain granules enriched in antithrombotic (eg, heparin and tPA) and proinflammatory (eg, histamine and tumor necrosis factor α) mediators, whose degranulation is controlled, in part, by HIF-1α.102 In the VT setting, MCs could potentially become activated by the local inflammatory milieu, including complement, thrombin, factor Xa, hypoxia, IL-18, and other factors.103-107 Genetic ablation or pharmacological inhibition of MC degranulation reduces thrombosis, suggesting that MC proinflammatory/prothrombotic effect predominates in the VT setting.108 Recently, an essential role of MC chymase (murine monocyte chemoattractant protein 4) in the prothrombotic effect of MCs was reported.109 Genetic depletion or pharmacological inhibition of chymase reduces experimental VT. This chymase effect is mediated by its ability to limit plasmin activity. Another MC enzyme, dipeptidyl peptidase I, is a critical activator of MC chymase.110 Chymase inhibition does not affect hemostasis, which makes it, along with dipeptidyl peptidase I, potentially attractive targets for antithrombotic therapies. MCs also stimulate the respiratory burst and phagocytic activity of neutrophils,111 which could potentiate neutrophil recruitment and support VT.
Differential roles of monocytes, macrophages, and eosinophils in VT
Monocytes, although present in lower numbers than neutrophils, likely contribute to VT15 because depletion of mononuclear cells reduces thrombosis.96 VWF induces the accumulation of IL-1β messenger RNA and its protein precursor in macrophages, with subsequent IL-1β release occurring only after exposure to both VWF and ADP, a classic activator of NLRP3 assembly.16 In a sepsis model, inflammasome activation in macrophages triggers TF expression and blood clotting.112 In the IVC stenosis model, inflammatory Ly6Chi monocytes promote VT, whereas TF-positive Ly6Chi monocytes restore thrombosis in aged CCR2−/− mice.113 However, the contribution of monocytes to VT is disputed in some studies. For example, depletion of CD11b+Ly6C+ monocytes/macrophages does not alter thrombosis 48 hours after IVC ligation.114 Therefore, monocytes are likely involved in VT, but their relative importance and precise mechanism of impact are yet to be elucidated. Eosinophils are also involved in VT initiation. Thrombosis is substantially reduced in 2 strains of eosinophil-deficient mice,115 with eosinophils promoting VT at least partially by increasing thrombin generation. Eosinophil cationic protein, a plasma marker of eosinophil activation, is an independent biomarker for cardiovascular events in patients.
Blood-borne mechanism of regulation
Complement
Complement activation occurs through 3 pathways, classical, lectin, and alternative, leading to the formation of C3- and C5-convertases and the release of the potent anaphylatoxins C3a and C5a, and the formation of the membrane attack complex C5b-9. Complement components differentially alter platelet activation and fibrin generation in VT. Deficiency in either C3 or C5 reduces fibrin generation and VT, through different mechanisms: C3 supports platelet but not leukocyte recruitment, whereas C5 exerts an opposite effect. In the VT setting, C5 can support fibrin generation independently of platelets, likely through myeloid cell phosphatidylserine.116 The mechanisms driving complement activation in VT remain elusive, although P-selectin on activated platelets and possibly ECs may contribute.117,118 Plasma VWF inhibits complement activation,119 potentially depicting a self-limiting regulatory function of VWF in VT. A similar negative regulatory effect is exerted by thrombomodulin, downregulated in VT, which enhances complement activation.120 Thrombin can cleave C5 and supports the release of inflammatory mediators,121 whereas plasmin directly contributes to C5 cleavage and the release of C5a in VT.122 The abundance of plasminogen in murine venous thrombi123 renders this pathway potentially important for thrombus formation. Moreover, C5a promotes neutrophil recruitment, IL-8 and monocyte chemoattractant protein 1 production,122 and TF expression by neutrophils and ECs.124,125 C5a also induces WPB release and increases neutrophil adherence to ECs, with all these effects potentially supporting VT.126 C5a might be involved also in VT propagation because NETs can activate the complement system and vice versa,127,128 suggesting a positive feedback between these 2 inflammatory mechanisms.
Neutrophil-derived antimicrobial proteins
The role of various antimicrobial proteins in VT has been reported. α-defensin-1 (AD-1), an antimicrobial protein stored in neutrophil azurophilic granules, emerges as a novel regulator of VT. AD-1 is released from neutrophils during blood clotting activation via the contact/intrinsic pathway,129 a surface for which could be provided by NETs.130 Once released, AD-1 incorporates the thrombus, in which it facilitates fibrin polymerization, resulting in thicker fibers that are less susceptible to fibrinolysis. Although mice lack AD-1, animals expressing human AD-1 (Def+/+) produce larger heparin-resistant venous thrombi. The anti-inflammatory drug colchicine reduces plasma AD-1 levels and thrombus size in Def+/+ mice and decreases the dose of heparin required to prevent VT.
Another platelet- and NET-associated protein, MRP14 (S100A9), also supports experimental VT. S100A9 deficiency reduces NETosis and VT in mice, and transfusion of wild-type platelets or neutrophils into S100A9-deficient mice restores thrombosis.131 S100A9 originates from multiple cells including neutrophils during NETosis, and its release is driven by NLRP3 inflammasome activation.132,133 S100A8/A9 supports NETosis in Mac-1– and platelet-dependent manner by inducing platelet pyroptosis through the ROS–NLRP3–caspase-1 axis.134 GDD-dependent pore formation in platelets promotes the release of oxidized mitochondrial DNA, amplifying NETosis and further exacerbating inflammation. Although TLR4 plays a role in S100A9-mediated neutrophil recruitment and activation, global TLR4 deficiency does not affect the early stages of VT.135 S100A8/A9 may potentially support VT by inducing the formation of procoagulant platelets, a mechanism dependent on GPIb and CD36.136 Therefore, a cooperation between NETosis and EC and platelet activation likely generates an inflammatory milieu that facilitates VT.
PCSK9
Proprotein convertase subtilisin kexin 9 (PCSK9), a hepatocyte-derived protease, facilitates low-density lipoprotein (“bad cholesterol”) receptor degradation, thereby increasing low-density lipoprotein levels in the blood. In the IVC stenosis model, PCSK9 deficiency reduces leukocyte recruitment to the vessel wall and thrombosis.17 Low shear, typical for the VT setting, induces PCSK9 expression in smooth muscle cells and ECs in a nicotinamide adenine dinucleotide phosphate-ROS–dependent manner.137 PCSK9 exerts proinflammatory and prothrombotic activities independently of its lipid-related function.138 For example, PCSK9 enhances agonist-dependent platelet activation, increases expression of inflammatory cytokines such as IL-1β and IL6 in macrophages, and initiates mitochondrial DNA damage thereby activating the NLRP3 inflammasome.139,140 NLRP3 activation and IL-1β, in turn, induce secretion of PCSK9.141 Overall, inhibition of PCSK9 leads to multiple anti-inflammatory effects including decreased vessel wall inflammation, inhibition of blood coagulation, NETosis, as well as platelet activation, making PCSK9 a promising target to prevent VT.
IL-17: biomarker and driver of VT
IL-17, a cytokine secreted from different immune cells, has recently emerged as an early diagnostic marker for VT as well as a drug target to reduce thrombosis. Inhibition of IL-17 reduces experimental VT by decreasing platelet activation and neutrophil function.142,143 Injection of recombinant IL-17A promotes VT associated with increased platelet and EC activation, neutrophil recruitment, and NET formation. IL-17 induces S100A8/A9 release from neutrophils,144 supporting local thrombo-inflammation and forming another “vicious circle” promoting VT.
Sirtuin-1: a negative regulator for VT
Nicotinamide adenosine dinucleotide (NAD)-dependent deacetylase sirtuin-1 is involved in deacetylation of transcription factors. A protective role of sirtuin-1 in VT was recently demonstrated in rats.11 In this study, sirtuin-1 agonist SRT1720 reduces thrombosis, leukocyte recruitment, and expression of adhesion receptors in the VT setting, whereas sirtuin-1 knockdown exerts the opposite effect. Sirtuin-1 levels in rat IVC rapidly decrease after stenosis application.11,145 Sirtuin-1 suppresses inflammation by stimulating the Nrf2 antioxidant pathway, deacetylating NF-κB, and activating eNOS thereby limiting WPB release.146,147 Sirtuin-1 also deacetylates HMGB-1, inhibiting its release,148,149 thereby removing 1 of the major NETosis activators from the VT milieu. Overall, sirtuin-1 exerts multiple anti-inflammatory effects and attenuates VT.
Clinical perspectives of targeting inflammation for VT prevention
Modulating the immune system and inflammation offers a promising potential intervention to target VT, especially given the low likelihood of their interfering with hemostasis. Although clinically proven anti-inflammatory medications to prevent VTE are currently lacking, a growing volume of preclinical data supports evaluation of this approach in clinical settings. Targeting multiple inflammatory pathways simultaneously or combining anti-inflammatory strategies with interventions targeting coagulation could be more efficient and/or enable lower anticoagulant/fibrinolytic doses, reducing bleeding risk. Preclinical data suggest that inhibiting WPB release and targeting VWF, platelet/endothelial (P/E)-selectins,150,151 or the NLRP3 inflammasome are probably the most promising approaches to prevent VT initiation, whereas targeting NETs, their components, or complement could potentially prevent thrombosis propagation or both processes. Hydroxychloroquine, an immunomodulatory drug, has shown promise in reducing thromboembolic events in patients.152 Apolipoprotein A-I or agonists of scavenger receptor class B type 1 might exert an antithrombotic effect in VT,34 which corroborates reduction in symptomatic VTE prevalence in humans by statins, cholesterol-lowering drugs with multiple anti-inflammatory effects.153,154 Inhibition of PCSK9 also reduces VTE risk in patients with atherosclerosis and hyperlipidemia.155
Enhancing the bioavailability of NO presents another clinically promising anti-inflammatory approach. Targeting a newly discovered route of eNOS regulation, the endothelial AMP-activated kinase–PI3K–Akt–eNOS axis, for example, by small molecule modulators, could be beneficial in VT prevention.156 Recently, Ferraro et al described an interesting approach to limit the prothrombotic potential of ECs by reducing WPB length and subsequently decreasing VWF multimer size and activity.157
Another interesting antithrombotic option is omega-3 fatty acids, known to increase the production of NO and reported to reduce the risk of VTE in elderly patients after surgery.158 Moreover, omega-3 fatty acids decrease platelet aggregation and blood clotting without increasing the risk of bleeding.159 This makes omega-3 fatty acids a promising tool for VTE prophylaxis, combining anti-inflammatory and anticoagulant effects.
The NLRP3 inflammasome complex emerges as an antithrombotic target. Blocking components of the NLRP3 complex may offer effects dissimilar to neutralizing each cytokine separately especially considering caspase-1’s direct support of blood clotting, NLRP3’s promotion of platelet aggregation, and the susceptibility of IL-1β to activation through caspase-1–independent mechanisms.160,161 Long-term blockade of IL-1β may render patients more susceptible to infection, whereas targeting NLRP3 upstream could block pathological mature IL-1β production while preserving some protective baseline IL-1β activity. Consequently, targeting the NLRP3 complex could potentially have fewer undesirable effects, for example, severe infection and sepsis.162 Targeting other components of the immune system, such as NETs, complement, histones, S100A8/A9, or IL-17, may be considered for VT prevention, whereas including DNase-I in thrombolysis mixtures may aid in removing existing thrombus. However, the clinical utility of targeting these and other inflammatory molecules in VT prevention requires further validation. Moreover, although some inflammatory biomarkers (eg, C-reactive protein or white blood cell counts) correlate with VTE, their specificity is limited.163,164
Interestingly, the activity of coagulation factor XI, involved in regulating various aspects of inflammation,165 has been associated with immune system components in patients with VTE.166 Given that factor XI inhibition efficiently prevents VT without inducing bleeding,167 it could be expected to protect against thrombosis via combined anticoagulant and anti-inflammatory properties.
In conclusion, comprehending the inflammatory processes involved in VT represents a crucial area for future research and drug development to devise new treatment approaches that offer optimal antithrombotic effects while minimizing bleeding risks.
Major gaps in the thromboinflammation field and future research directions
Despite progress in understanding thrombosis–inflammation interplay in VTE, significant knowledge gaps persist, necessitating future research efforts. One critical area is elucidating the hierarchical sequence of molecular events driving thrombus initiation and propagation. Currently, the multitude of inflammatory pathways participating in thrombogenesis poses challenges in distinguishing critical drivers from auxiliary contributors. Furthermore, effectively translating emerging mechanistic insights from preclinical animal models into clinical practice has proven challenging, likely because of inherent physiological discrepancies with humans; potential comorbidities; genetic heterogeneity; and influences of sex, ethnicity, socioeconomics, and environment on thromboinflammation regulation.
Additional critical areas include defining the cross talk between immune cells and coagulation factors; determining the contributions of DAMPs and microbiome perturbations to pathological thromboinflammation; and exploring mechanisms underlying interindividual variability in disease predisposition, severity, and resolution. Thrombi developed in different circumstances (eg, sterile vs septic conditions) may have distinct structures and thus require specific treatment approaches. Developing improved research models that incorporate coagulation–inflammation interactions while better mimicking human VT will aid in addressing these gaps. Evaluation of thrombus susceptibility to lysis, for example, by tPA or DNase I, and assessment of fibrin deposition in the lungs in the VT setting are currently overlooked important readouts of rodent models. Ultimately, refining our understanding of the pathways governing thrombosis initiation, propagation, revascularization, and resolution in various clinical settings is essential for identifying optimal therapeutic windows and targets. This will facilitate the emergence of personalized interventions capable of safely and effectively preventing and treating deleterious thromboinflammation in patients.
Acknowledgments
J.R. is supported by British Heart Foundation Intermediate Fellowship (FS/IBSRF/20/25039). A.B. is supported by British Heart Foundation Senior Basic Science Research Fellowship (FS/19/30/34173). The National Institute for Health and Care Research (NIHR) Birmingham Biomedical Research Centre (NIHR203326) and the British Heart Foundation Accelerator (AA/18/2/34218) have supported the University of Birmingham Institute of Cardiovascular Sciences where this research is based.
The opinions expressed in this paper are those of the authors and do not represent any of the listed organizations.
Authorship
Contribution: J.R. and A.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexander Brill, Institute of Cardiovascular Sciences, College of Medical and Dental Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom; email: a.brill@bham.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal