Key Points
In human T-ALL, a NOTCH1 dimer–HES4 axis primes an isoform switch in the TP53 gene that favors the antiapoptotic Δ133p53 isoform.
The NOTCH1 dimer signature links indirectly to the expression of proapoptotic genes and directly to markers of poor clinical outcome.
Visual Abstract
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive malignancy that is characterized by an expansion of T-cell progenitors and DNA mutations that lead to overactive NOTCH1 signaling in >50% of T-ALL cases. Using synthetic models of human T-ALL, we report that NOTCH1 dimeric signaling was crucial for the leukemogenesis of human hematopoietic stem/progenitor cells (HSPCs) from cord blood. We also identified a Notch dimerization–dependent gene signature, including the HES4 transcription factor, which induced a proliferative advantage in human HSPCs and in Notch dimerization–dependent, patient-derived xenografts of T-ALL. Interestingly, in human T-ALL cells, HES4 enforced the expression of the Δ133p53 isoform with the concomitant block of proapoptotic p53 target genes and the induction of BCL2L1 gene expression and antiapoptotic B-cell lymphoma extra-large protein. In addition, through an integrated experimental approach that included genetically modified cell lines, RNA/chromatin immunoprecipitation sequencing, and single-cell RNA sequencing profiles of primary T-ALL samples, we revealed cell subsets with Notch dimerization–dependent gene signatures, which indirectly correlated with proapoptotic genes and directly associated with cell markers of poor clinical outcome in primary T-ALL samples. Taken together, these findings highlight the crucial role of NOTCH1 dimeric signaling in human T-cell leukemogenesis and T-ALL maintenance, suggesting that a possible benefit can be obtained with a therapeutic strategy that target NOTCH1 dimer signaling or its downstream effectors.
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive malignancy that is characterized by an expansion of T-cell progenitors.1,2 T-ALL affects pediatric and adult patients who experience relapses or diseases refractory to standard treatments.2-4 More than 50% of T-ALL cases are associated with DNA alterations in the NOTCH1 gene5 or other genes that lead to enhanced activation of the NOTCH1 pathway.6-8 Moreover, the constitutive expression of the NOTCH1-ΔE variant, which lacks the extracellular domain, leads to overactive NOTCH1 signaling, thereby enforcing T-cell differentiation6 and the development of T-ALL–like disease.9-11
In the canonical NOTCH1 pathway, the intracellular domain of NOTCH1 receptor (ICN1) is proteolytically released from the membrane upon ligand binding. Subsequently, ICN1 translocates to the nucleus and promotes the transcription of target genes as a monomer or dimer when bound to so-called sequence paired sites.12-14 Specific mutations, such as the R1984A amino acid substitution in ICN1, abrogate the formation of the NOTCH1 dimeric complex and prevent the induction of T-ALL in mice.15,16 Nevertheless, the functional significance of NOTCH1 dimerization in human T-cell leukemogenesis is still poorly understood.
In this study, we evaluated the role of NOTCH1 dimerization in the T-cell leukemogenesis of human hematopoietic stem/progenitor cells from cord blood (CB). We found that NOTCH1 dimers promoted a dimerization-dependent gene signature, which included the transcription factor HES4. Interestingly, HES4 promoted an isoform switch in the P53 gene locus by inducing the expression of the Δ133p53 transcript with a concomitant block of the proapoptotic and full-length p53 transcript. In addition, gene expression data at the single-cell level of leukemic cells of patients with primary T-ALL revealed cell subsets with a Notch dimerization–dependent gene signature, which indirectly correlated with proapoptotic genes and directly associated with cell markers of poor clinical outcome.
Materials and methods
Isolation of human hematopoietic stem/progenitor cells
Samples of human CB were collected after informed consent was obtained in accordance with the protocols approved by the research ethics board of the Fondazione Casa Sollievo della Sofferenza–Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) in San Giovanni Rotondo, Italy. CD34+ CB cells were obtained as previously described9 and seeded into 96-well round bottom plates, followed by prestimulation in StemSpan-SFEMII (StemCell Technologies) with 10 ng/mL human stem cell factor (SCF), 20 ng/mL human thrombopoietin (TPO), 20 ng/mL human insulin-like growth factor 2 (IGF2), and 10 ng/mL human fibroblast growth factor α (FGFα) (PeproTech) for 16 hours.
Cell cultures and drug treatment
CB cells and explanted CB leukemia cells were cultured on OP9-DL1 cells in α minimum essential medium (αMEM) media supplemented with 20% fetal bovine serum (Gibco), 2 mM Gluta-MAX (Gibco), 1% penicillin/streptomycin (pen/strep) antibiotics (Gibco), 10 ng/mL SCF, 5 ng/mL FMS-like tyrosine kinase 3 ligand (FLT3L), and 3 ng/mL interleukin-7 (PeproTech). OP9-DL1, CUTLL1,17 RPMI-8402, ALLSIL, DND41, KOPTK1, HPBALL, and TALL-1 cells were obtained from A.P.W. The RPMI-8402 and CUTLL1 cell lines were maintained in RPMI-1640 media supplemented with 10% fetal bovine serum (Gibco), 2 mM Gluta-MAX (Gibco), and 1% Pen/Strep antibiotics (Gibco). Patient-derived xenografts (PDXs) were established in the laboratories of A.P.W. and V.G. as previously reported.18,19 Drug treatment with a γ-secretase inhibitor (GSI) was performed by adding Compound E (1 μM, Sigma-Aldrich) or dimethyl sulfoxide as mock control (1 μM) in αMEM media supplemented with 2 mM Gluta-MAX (Gibco), 1% pen/strep antibiotics (Gibco), 10 ng/mL SCF, 5 ng/mL FLT3L, and 3 ng/mL interleukin-7 (PeproTech) without OP9-DL1 feeder cells.
Well-initiation assay
The 96-well plates were coated using the StemSpan Lymphoid Differentiation Coating Material (StemCell Technologies) according to the manufacturer instructions. The CB cells were sorted in coated 96-well plates 3 days after transduction and allowed to grow in T-cell expansion media, obtained by supplementing StemSpan-SFEMII with StemSpan Lymphoid Progenitor Expansion Supplement (StemCell Technologies) for 21 days and supplementing with T-cell expansion media on day 10. Cells were acquired using an LSR-FortessaII (BD) equipped with a high-throughput sampler for automatic acquisition of 96-well plates. AccuCheck counting beads (catalog no. PCB100, ThermoFisher) were used for the absolute cell count in accordance with the manufacturer’s instructions. The frequency of well-initiating cells in limiting dilutions condition was evaluated using the extreme limiting dilution analysis.20
Results
NOTCH1 dimeric signaling in the leukemogenesis of human T-cell precursors
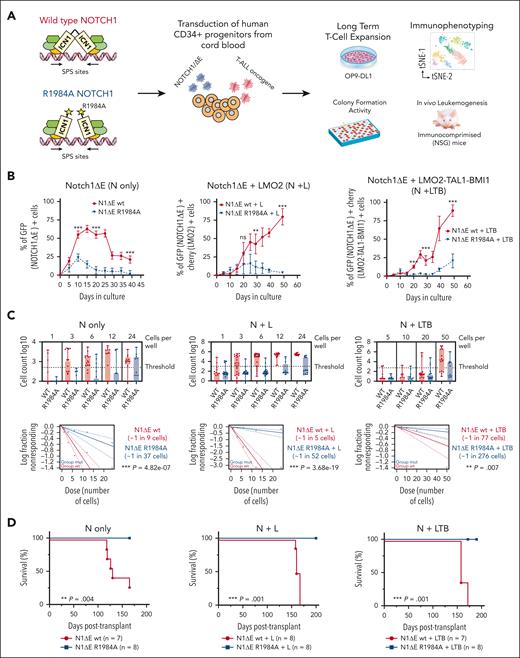
To assess the role of NOTCH1 dimers in the malignant transformation of human T-cell precursors, we employed 3 different synthetic models of T-ALL in which normal CD34+ cells, derived from human CB, were lentivirally transduced with the activated NOTCH1-ΔE isoform alone or in a combination with known T-ALL oncogenes, such as LMO2, TAL1, and BMI1.9,10 Specifically, to evaluate the impact of ICN1 dimerization on T-cell precursor expansion, we generated a lentivector that encoded either a dimerization-defective NOTCH1-ΔE-R1984A mutant15,16 or the wild type (wt) and transduced them into CB cells alone (N-only) or with LMO2 (N+L) or LMO2-TAL1-BMI1 (N+LTB) constructs (Figure 1A; supplemental Figure 1, available on the Blood website). The transduced cells were subsequently maintained on OP9-DL1 stromal feeders.21 As previously reported, the N-only cells exhibited an initial burst in growth, expanding in the first days only to regress some days later.9 In contrast, the N+L and N+LTB cells expanded in vitro and saturated the culture. Interestingly, these growth patterns were only observed with the NOTCH1-ΔE-wt construct. In the 3 systems, the NOTCH1-ΔE-R1984A cells did not achieve a sustained expansion (Figure 1B). Furthermore, the flow cytometry analysis of NOTCH1-ΔE-wt and NOTCH1-ΔE-R1984A cells revealed minimal immunophenotypic variations between the 2 cell conditions in all models (supplemental Figures 2-4). To assess the clonogenic capability of transduced cells, we also performed in vitro well-initiation assays and found an approximately threefold increased frequency in the N-only condition and an ∼10-fold and ∼3.6-fold increase in the N+L and N+LTB conditions, respectively (Figure 1C).
The dimerization-defective R1984A NOTCH1-ΔE mutant blocks the expansion and transformation of human HSPCs, mediated by the constitutive activation of NOTCH1 signaling. (A) Schematic overview of the experimental approach. Human CD34+ cells derived from CB were doubly transduced with NOTCH1-ΔE/GFP or NOTCH1-ΔE-R1984A/GFP dimer mutant lentiviruses alone (N-only) or in combination with LMO2/mCherry (N+L) or LMO2-TAL1-BMI1/mCherry (N+LTB) constructs on days 0 and 5. Transduced cells were plated on OP9-DL1 feeders for the in vitro long-term coculture assay and before transplantation by IV injection into immunodeficient NSG mice. (B) Flow cytometric analysis of single (GFP+) or doubly transduced (GFP+Cherry+) CB cells that were transduced with N-only, N+L, or N+LTB viruses and tracked at each serial passage onto OP9-DL1 feeders every 5 days. The mean values ± range for duplicate experiments are plotted. ∗∗P < .01; ∗∗∗P < .001 (Student t test). (C) Limiting dilution growth assays. CB cells were transduced with N-only, N+L, or N+LTB viruses on days 0 and cultured until day 3, followed by FACS into individual wells of a 96-well plate on precoated plastic in T-cell expansion media (StemCell Technologies) and culturing for 3 weeks. The entire content of each well was assayed by flow cytometry, and the frequency of well-initiating cells was calculated using the extreme limiting dilution analysis (ELDA) software. In each ELDA plot, the cell dose vs log fraction of negative cultures is represented. The dotted lines represent the confidence interval of 95%. The estimated frequencies are indicated in the box in the right side. The P values were calculated using the ELDA tool. (D) Kaplan-Meier survival curves for the primary recipient NSG mice that were injected with CB cells transduced with N-only, N+L, or N+LTB viruses and expanded for 10 days (N-only) or 15 days (N + T-ALL oncogenes) in vitro. The number of recipient animals for each cell type is indicated in parentheses. The data were pooled from 2 separate cohorts of animals. ∗∗∗P ≤ .001; log-rank Mantel-Cox test. GFP, green fluorescent protein; ns, not significant.
The dimerization-defective R1984A NOTCH1-ΔE mutant blocks the expansion and transformation of human HSPCs, mediated by the constitutive activation of NOTCH1 signaling. (A) Schematic overview of the experimental approach. Human CD34+ cells derived from CB were doubly transduced with NOTCH1-ΔE/GFP or NOTCH1-ΔE-R1984A/GFP dimer mutant lentiviruses alone (N-only) or in combination with LMO2/mCherry (N+L) or LMO2-TAL1-BMI1/mCherry (N+LTB) constructs on days 0 and 5. Transduced cells were plated on OP9-DL1 feeders for the in vitro long-term coculture assay and before transplantation by IV injection into immunodeficient NSG mice. (B) Flow cytometric analysis of single (GFP+) or doubly transduced (GFP+Cherry+) CB cells that were transduced with N-only, N+L, or N+LTB viruses and tracked at each serial passage onto OP9-DL1 feeders every 5 days. The mean values ± range for duplicate experiments are plotted. ∗∗P < .01; ∗∗∗P < .001 (Student t test). (C) Limiting dilution growth assays. CB cells were transduced with N-only, N+L, or N+LTB viruses on days 0 and cultured until day 3, followed by FACS into individual wells of a 96-well plate on precoated plastic in T-cell expansion media (StemCell Technologies) and culturing for 3 weeks. The entire content of each well was assayed by flow cytometry, and the frequency of well-initiating cells was calculated using the extreme limiting dilution analysis (ELDA) software. In each ELDA plot, the cell dose vs log fraction of negative cultures is represented. The dotted lines represent the confidence interval of 95%. The estimated frequencies are indicated in the box in the right side. The P values were calculated using the ELDA tool. (D) Kaplan-Meier survival curves for the primary recipient NSG mice that were injected with CB cells transduced with N-only, N+L, or N+LTB viruses and expanded for 10 days (N-only) or 15 days (N + T-ALL oncogenes) in vitro. The number of recipient animals for each cell type is indicated in parentheses. The data were pooled from 2 separate cohorts of animals. ∗∗∗P ≤ .001; log-rank Mantel-Cox test. GFP, green fluorescent protein; ns, not significant.
To evaluate the role of NOTCH1 dimeric signaling in the in vivo leukemogenesis of T-cell precursors, CB cells, transduced with NOTCH1-ΔE or NOTCH1-ΔE-R1984A lentiviruses alone or in combination with LMO2 (N+L) or LMO2-TAL1-BMI1 (N+LTB) constructs after 10 days of in vitro growth for N-only and after 15 days for the N+L or N+LTB conditions, were intravenously injected into sublethally irradiated immunocompromised (NSG) mice. Only NOTCH1-ΔE-wt cells generated T-ALL–like leukemias unlike the dimerization-defective NOTCH1-ΔE-R1984A mutant (Figure 1D; supplemental Tables 2-4). Clinically morbid animals generally showed signs of hepatosplenomegaly, enlarged lymph nodes and thymus masses, hypercellular bone marrow with extensive infiltration of leukemic blasts, and circulating leukemia cells.
To define the gene expression profiling modulated by NOTCH1 dimers in human T-cell precursors, we performed RNA sequencing (RNA-seq) on the N-only, N+L, and N+LTB CB cells that were expanded in vitro for up to 10 days for the N-only and for up to 15 days for the N+L and N+LTB cells. Using the top variable genes among the NOTCH1-ΔE-wt and NOTCH1-ΔE-R1984A samples in each cell condition and unsupervised hierarchical clustering, we ranked genes according to the expression (Figure 2A; supplemental Figure 5). Interestingly, the Gene Set Enrichment Analysis of the RNA-seq data highlighted the G2/M checkpoint, mTORC1 signaling, and MYC targets as the most enriched gene sets that were modulated by NOTCH1 dimers in the N-only and N+L cells (Figure 2B; supplemental Table 10). To identify target genes of NOTCH1 dimers, we focused on those with a documented sequence-paired binding site in the promoter region22 (Figure 2C) and, subsequently, on the top transcripts that were upregulated in NOTCH1-ΔE-wt cells when compared with NOTCH1-ΔE-R1984A cells. The identified NOTCH1 dimer target genes included HES4, ABCA1, CD300A, CR1, CR2, MYO7B, and PCGF5 with HES4 consistently having the lowest P adjusted value (Figure 2D; supplemental Table 11). Interestingly, the genomic region over the HES4 promoter is enriched in DNA binding sites for the NOTCH1 complex through recombination signal binding protein for immunoglobulin kappa J region (RPBJ) in the CUTLL-1 human T-ALL cell line17,23 and is associated with an active genomic region identified by the enhancer markers H3K4me1 and H3K4me323 (supplemental Figure 6). We validated the NOTCH1 dimer–specific modulation of the HES4 transcript in each cell condition at the messenger RNA level by quantitative reverse transcription polymerase chain reaction (Figure 2E).
The HES4 transcription factor is a direct transcriptional target of NOTCH1 dimers. (A) Heat map and hierarchical clustering based on normalized values of the gene expression RNA-seq data. Human CB cells with N-only, N+L, or N+LTB viruses, expanded in vitro for up to 10 days for N-only and up to 15 days for the other 2 cell conditions, and followed by GFP+Cherry+ FACS before RNA isolation and sequencing. The differentially expressed genes, scaled to mean = 0 and standard deviation (SD) = 1, are represented (adjusted P value ≤.1 and logFC >0.5). The highlighted genes have a documented sequence-paired binding site (SPS) in the promoter region. (B) Lollipop plots of the Hallmark GeneSets found to be enriched in human NOTCH1-ΔE (wt)- or NOTCH1-ΔE-R1984A (R1984A)-transduced CB cells for each condition (N-only, N+L, or N+LTB) by Gene Set Enrichment Analysis (GSEA). (C) Volcano plot of differentially expressed genes (adjusted P value <.05) in human NOTCH1-ΔE (wt)- vs NOTCH1-ΔE-R1984A (R1984A)-transduced cell subsets for each condition (N-only, N+L or N+LTB). (D) Venn diagram showing the up- and downregulated genes with a recognized NOTcH1 SPS in the promoter region in human NOTCH1-ΔE (wt)-transduced CB cells in comparison with NOTCH1-ΔE-R1984A (R1984A)-transduced cell subsets that were identified in the RNA-seq data sets and shared across all 3 conditions (N-only, N+L, and N+LTB). (E) HES4 messenger RNA (mRNA) expression level in human NOTCH1-ΔE (wt) or NOTCH1-ΔE-R1984A (R1984A) transduced CB cells for each condition (N-only, N+L, or N+LTB) as reported in panel A. The transduced cells were sorted using FACS before performing the TaqMan reverse transcriptase-digital droplet polymerase chain reaction assay. The graphs report the result of 3 independent experiments performed in triplicate. ∗∗∗P < .001 (Student t test). FDR, false discovery rate; Max, maximum; Min, minimum; NES, normalized enrichment score.
The HES4 transcription factor is a direct transcriptional target of NOTCH1 dimers. (A) Heat map and hierarchical clustering based on normalized values of the gene expression RNA-seq data. Human CB cells with N-only, N+L, or N+LTB viruses, expanded in vitro for up to 10 days for N-only and up to 15 days for the other 2 cell conditions, and followed by GFP+Cherry+ FACS before RNA isolation and sequencing. The differentially expressed genes, scaled to mean = 0 and standard deviation (SD) = 1, are represented (adjusted P value ≤.1 and logFC >0.5). The highlighted genes have a documented sequence-paired binding site (SPS) in the promoter region. (B) Lollipop plots of the Hallmark GeneSets found to be enriched in human NOTCH1-ΔE (wt)- or NOTCH1-ΔE-R1984A (R1984A)-transduced CB cells for each condition (N-only, N+L, or N+LTB) by Gene Set Enrichment Analysis (GSEA). (C) Volcano plot of differentially expressed genes (adjusted P value <.05) in human NOTCH1-ΔE (wt)- vs NOTCH1-ΔE-R1984A (R1984A)-transduced cell subsets for each condition (N-only, N+L or N+LTB). (D) Venn diagram showing the up- and downregulated genes with a recognized NOTcH1 SPS in the promoter region in human NOTCH1-ΔE (wt)-transduced CB cells in comparison with NOTCH1-ΔE-R1984A (R1984A)-transduced cell subsets that were identified in the RNA-seq data sets and shared across all 3 conditions (N-only, N+L, and N+LTB). (E) HES4 messenger RNA (mRNA) expression level in human NOTCH1-ΔE (wt) or NOTCH1-ΔE-R1984A (R1984A) transduced CB cells for each condition (N-only, N+L, or N+LTB) as reported in panel A. The transduced cells were sorted using FACS before performing the TaqMan reverse transcriptase-digital droplet polymerase chain reaction assay. The graphs report the result of 3 independent experiments performed in triplicate. ∗∗∗P < .001 (Student t test). FDR, false discovery rate; Max, maximum; Min, minimum; NES, normalized enrichment score.
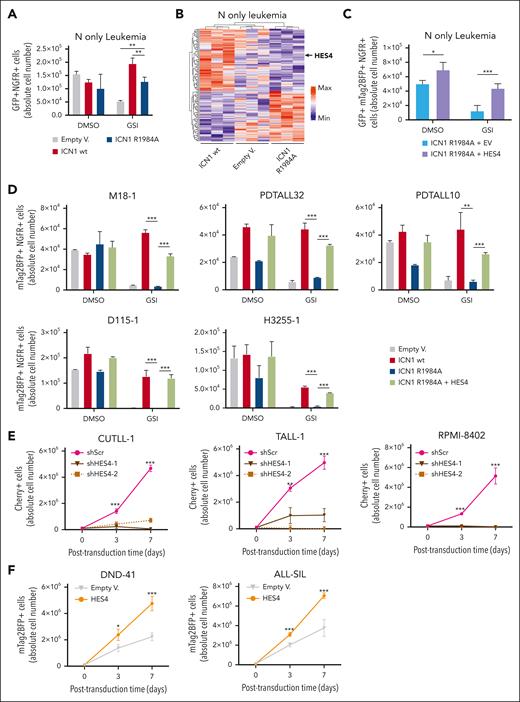
To address the relevance of HES4 in the transformation of human T-cell precursors, the CB cells were transduced with NOTCH1-ΔE lentivirus and short hairpin HES4 (shHES4) constructs to knock down HES4 or with a scramble control (supplemental Figure 7). The short hairpin RNA (shRNA)-mediated decrease in HES4 gene expression in N1ΔE-transduced CB cells inhibited the in vitro expansion when compared with the control cells (Figure 3A). Furthermore, the CB cells were doubly transduced with the dimerization-defective NOTCH1-ΔE-R1984A lentivector and a construct encoding a HES4 transcript or an empty vector (EV) as control. HES4 and NOTCH1-ΔE-R1984A double-transduced cells steadily progressed from the early days when compared with the control cells (Figure 3B-C). We also assessed the clonogenic capability of HES4-transduced CB cells using an in vitro well-initiation assay and observed that the overexpression of HES4 in CB cells increased the frequency of initiation of a colony by ∼2.4-fold when compared with the NOTCH1-ΔE-R1984A cells (Figure 3D). HES4-transduced cells were also IV injected into NSG mice after 10 days of in vitro growth. Interestingly, HES4 cells generated malignant leukemias with T-ALL–like features and at a similar frequency as the NOTCH1-ΔE cells (Figure 3E-F), suggesting that HES4 mimics the leukemogenic capacity of NOTCH1 dimeric signaling in human T-cell progenitors.
HES4 transcriptional expression confers growth advantage in the transformation of human T-cell progenitors. (A) Flow cytometric analysis of GFP+ and mCherry+ cell abundance in the CB cells doubly transduced, respectively, with NOTCH1-ΔE/GFP or shRNA/mCherry lentiviral constructs. Transduced cells were cultured in vitro on OP9-DL1 feeders and serially passaged every 5 days. GFP+ mCherry+ alive cells were measured at the indicated time points using flow cytometry for DRAQ7 exclusion. The graphs report the result of 2 independent experiments performed in triplicate. ∗∗∗P < .001 (2-way analysis of variance [ANOVA] with Dunnett test, comparing the sh-scramble (shScr) control mean with the other values). (B) Flow cytometric tracking of doubly transduced (GFP+mTagBFP2+) CB cells at each serial passage onto OP9-DL1 feeders every 5 days. The CB cells were transduced with lentiviral particles with NOTCH1-ΔE-R1984A/GFP dimer mutant lentiviruses, in addition to the HES4/mTagBFP2 construct or EV as control on days 0 and 5. The mean values ± range for duplicate experiments are plotted. ∗∗P < .01; ∗∗∗P < .001 (Student t test). (C) Flow cytometric tracking of mTagBFP2+ CB cells, transduced with HES4/mTagBFP2 lentivector or EV as control on days 0 and 5. The transduced cells were serially passaged onto OP9-DL1 feeders every 5 days. The mean values ± range for duplicate experiments are plotted. ∗∗∗P < .001 (Student t test). (D) Limiting dilution growth assays. CB cells were transduced with HES4/mTagBFP2 lentivector or EV as control on day 0, cultured until day 3, and then sorted using FACS into individual wells of a 96-well plate on precoated plastic in T-cell expansion media (StemCell Technologies) and maintained in vitro for 3 weeks. The entire content of each well was assayed by flow cytometry and the frequency of well-initiating cells was calculated using the ELDA software. In the ELDA plot, the cell dose vs the log fraction of negative cultures is represented. Dotted lines represent the confidence interval of 95%. The estimated frequencies are indicated in the box on the right side. P values were calculated using the ELDA tool. (E) t-distributed stochastic neighbour embedding (tSNE) plots based on the flow cytometric tracking of CB cells, singly transduced with NOTCH1ΔE/GFP or HES4/mTagBFP2 lentivectors at day 10 of in vitro coculture on OP9-DL1 feeders. (F) The Kaplan-Meier survival curves of primary NSG mice injected with CB cells transduced with NOTCH1-ΔE, NOTCH1-ΔE-R1984A, or HES4 lentiviruses after 10 days of in vitro growth. The survival curve of secondary recipient mice transplanted with primary HES4 CB leukemia is indicated with a dashed line. The number of recipient animals for each cell type is indicated in parentheses. Data are pooled from 2 separate cohorts of animals.
HES4 transcriptional expression confers growth advantage in the transformation of human T-cell progenitors. (A) Flow cytometric analysis of GFP+ and mCherry+ cell abundance in the CB cells doubly transduced, respectively, with NOTCH1-ΔE/GFP or shRNA/mCherry lentiviral constructs. Transduced cells were cultured in vitro on OP9-DL1 feeders and serially passaged every 5 days. GFP+ mCherry+ alive cells were measured at the indicated time points using flow cytometry for DRAQ7 exclusion. The graphs report the result of 2 independent experiments performed in triplicate. ∗∗∗P < .001 (2-way analysis of variance [ANOVA] with Dunnett test, comparing the sh-scramble (shScr) control mean with the other values). (B) Flow cytometric tracking of doubly transduced (GFP+mTagBFP2+) CB cells at each serial passage onto OP9-DL1 feeders every 5 days. The CB cells were transduced with lentiviral particles with NOTCH1-ΔE-R1984A/GFP dimer mutant lentiviruses, in addition to the HES4/mTagBFP2 construct or EV as control on days 0 and 5. The mean values ± range for duplicate experiments are plotted. ∗∗P < .01; ∗∗∗P < .001 (Student t test). (C) Flow cytometric tracking of mTagBFP2+ CB cells, transduced with HES4/mTagBFP2 lentivector or EV as control on days 0 and 5. The transduced cells were serially passaged onto OP9-DL1 feeders every 5 days. The mean values ± range for duplicate experiments are plotted. ∗∗∗P < .001 (Student t test). (D) Limiting dilution growth assays. CB cells were transduced with HES4/mTagBFP2 lentivector or EV as control on day 0, cultured until day 3, and then sorted using FACS into individual wells of a 96-well plate on precoated plastic in T-cell expansion media (StemCell Technologies) and maintained in vitro for 3 weeks. The entire content of each well was assayed by flow cytometry and the frequency of well-initiating cells was calculated using the ELDA software. In the ELDA plot, the cell dose vs the log fraction of negative cultures is represented. Dotted lines represent the confidence interval of 95%. The estimated frequencies are indicated in the box on the right side. P values were calculated using the ELDA tool. (E) t-distributed stochastic neighbour embedding (tSNE) plots based on the flow cytometric tracking of CB cells, singly transduced with NOTCH1ΔE/GFP or HES4/mTagBFP2 lentivectors at day 10 of in vitro coculture on OP9-DL1 feeders. (F) The Kaplan-Meier survival curves of primary NSG mice injected with CB cells transduced with NOTCH1-ΔE, NOTCH1-ΔE-R1984A, or HES4 lentiviruses after 10 days of in vitro growth. The survival curve of secondary recipient mice transplanted with primary HES4 CB leukemia is indicated with a dashed line. The number of recipient animals for each cell type is indicated in parentheses. Data are pooled from 2 separate cohorts of animals.
Dimeric NOTCH1 signaling in established human T-ALLs
To assess whether NOTCH1 dimers have a crucial role in established human T-cell leukemia, the N-only CB-derived leukemia cells were transduced using lentivectors that encoded wildtype ICN1, the ICN1-R1984A dimer mutant, or the EV as control. Subsequently, the transduced cells underwent fluorescence-activated cell sorting (FACS), followed by seeding in wells coated with lymphoid differentiation material and T-cell expansion media (StemCell Technologies) and treatment with GSI for 4 days to inhibit the signal induced by the NOTCH1-ΔE receptor that still requires a γ-secretase cleavage to release ICN1.24 ICN1-R1984A expression in the N-only CB-derived leukemia cells was less effective than ICN1-wt in rescuing the cell growth reduced by NOTCH1 inactivation following GSI treatment (Figure 4A). Similar results were also obtained for N-LTB CB-derived leukemia cells but not for the N+L leukemia cells (supplemental Figure 8), underlining the differences in sensitivity of T-ALL–like leukemias to NOTCH1 dimers. Furthermore, we performed RNA-seq of leukemia cells that were transduced with wt ICN1, ICN1-R1984A dimer mutant, or the EV and treated with GSI in vitro for 4 days. Using the top variable genes in the different cell conditions and unsupervised hierarchical clustering, we identified HES4 in the gene set modulated by the absence of ICN1 dimerization (Figure 4B; supplemental Figure 9).
The HES4 gene is required to maintain growth of established T-cell leukemias. (A) Flow cytometric analysis of GFP+ NGFR+ cell abundance in NOTCH1-ΔE–derived (N-only) established CB leukemias, transduced with lentivectors encoding the wt intracellular Notch1 domain (ICN1), the ICN1-R1984A dimer mutant, or the EV as control, together with a truncated nerve growth factor receptor (tNGFR) as a marker. Two days after the transduction, the NGFR+ cells were sorted using FACS and treated with GSI Compound E (1 μM) or dimethyl sulfoxide (DMSO) as mock control for 4 days. The absolute cell numbers were calculated using an internal beads-based control. The mean values of duplicate experiments are plotted. ∗∗P < .01 (2-way ANOVA). (B) Heat map and hierarchical clustering based on the normalized values of gene expression RNA-seq data derived from human ICN1 (WT), ICN1-R1984A (R1984A), and EV N-only established CB leukemia cells as reported in panel A. Differentially expressed genes, scaled to mean = 0 and SD = 1, are represented (adjusted P value ≤.1). (C) Flow cytometric analysis of GFP+ NGFR+mTagBFP2+ cell abundance of NOTCH1-ΔE-derived (N-only) established CB leukemias, doubly transduced with ICN1-R1984A/tNGFR lentiviruses and HES4/mTagBFP2 or EVs as control. The transduced cells were sorted using FACS and treated with GSI Compound E (1 μM) or DMSO as a mock control for 4 days. The absolute cell numbers were calculated with an internal beads-based control. The mean values of duplicate experiments are plotted. ∗P < .05; ∗∗∗P < .001 (2-way ANOVA). (D) Flow cytometric analysis of the NGFR+mTagBFP2+ cell abundance of PDX transduced with ICN1-R1984A/tNGFR lentiviruses alone or in combination with the HES4/mTagBFP2 vector. Cells transduced with ICN1/tNGFR or EV alone were also included as control. The transduced cells were sorted using FACS and treated with GSI Compound E (1 μM) or DMSO as mock control for 3 days. The absolute cell numbers were calculated using an internal beads-based control. The mean values of duplicate experiments are plotted. ∗∗P < .01; ∗∗∗P < .001 (2-way ANOVA). (E) Abundance of the shRNA-transduced Cherry+ cell fraction, tracked over time in culture by flow cytometry. The CUTLL-1, TALL-1, and RPMI-8402 cell lines were independently transduced with 2 different clones of shRNA/mCherry lentiviral constructs against HES4 gene or scramble control as indicated, sorted using FACS, and cultured in vitro. The graphs report the result of 3 independent experiments performed in triplicate. ∗∗∗P < .001 (2-way ANOVA). (F) Abundance of the HES4-transduced mTagBFP2+ cell fraction, tracked over time in culture by flow cytometry. The DND41 and ALL-SILL cell lines were independently transduced with the HES4/mTagBFP2 lentiviral construct or EVs as control. The graphs report the result of 3 independent experiments performed in triplicate. ∗∗∗P < .001 (2-way ANOVA).
The HES4 gene is required to maintain growth of established T-cell leukemias. (A) Flow cytometric analysis of GFP+ NGFR+ cell abundance in NOTCH1-ΔE–derived (N-only) established CB leukemias, transduced with lentivectors encoding the wt intracellular Notch1 domain (ICN1), the ICN1-R1984A dimer mutant, or the EV as control, together with a truncated nerve growth factor receptor (tNGFR) as a marker. Two days after the transduction, the NGFR+ cells were sorted using FACS and treated with GSI Compound E (1 μM) or dimethyl sulfoxide (DMSO) as mock control for 4 days. The absolute cell numbers were calculated using an internal beads-based control. The mean values of duplicate experiments are plotted. ∗∗P < .01 (2-way ANOVA). (B) Heat map and hierarchical clustering based on the normalized values of gene expression RNA-seq data derived from human ICN1 (WT), ICN1-R1984A (R1984A), and EV N-only established CB leukemia cells as reported in panel A. Differentially expressed genes, scaled to mean = 0 and SD = 1, are represented (adjusted P value ≤.1). (C) Flow cytometric analysis of GFP+ NGFR+mTagBFP2+ cell abundance of NOTCH1-ΔE-derived (N-only) established CB leukemias, doubly transduced with ICN1-R1984A/tNGFR lentiviruses and HES4/mTagBFP2 or EVs as control. The transduced cells were sorted using FACS and treated with GSI Compound E (1 μM) or DMSO as a mock control for 4 days. The absolute cell numbers were calculated with an internal beads-based control. The mean values of duplicate experiments are plotted. ∗P < .05; ∗∗∗P < .001 (2-way ANOVA). (D) Flow cytometric analysis of the NGFR+mTagBFP2+ cell abundance of PDX transduced with ICN1-R1984A/tNGFR lentiviruses alone or in combination with the HES4/mTagBFP2 vector. Cells transduced with ICN1/tNGFR or EV alone were also included as control. The transduced cells were sorted using FACS and treated with GSI Compound E (1 μM) or DMSO as mock control for 3 days. The absolute cell numbers were calculated using an internal beads-based control. The mean values of duplicate experiments are plotted. ∗∗P < .01; ∗∗∗P < .001 (2-way ANOVA). (E) Abundance of the shRNA-transduced Cherry+ cell fraction, tracked over time in culture by flow cytometry. The CUTLL-1, TALL-1, and RPMI-8402 cell lines were independently transduced with 2 different clones of shRNA/mCherry lentiviral constructs against HES4 gene or scramble control as indicated, sorted using FACS, and cultured in vitro. The graphs report the result of 3 independent experiments performed in triplicate. ∗∗∗P < .001 (2-way ANOVA). (F) Abundance of the HES4-transduced mTagBFP2+ cell fraction, tracked over time in culture by flow cytometry. The DND41 and ALL-SILL cell lines were independently transduced with the HES4/mTagBFP2 lentiviral construct or EVs as control. The graphs report the result of 3 independent experiments performed in triplicate. ∗∗∗P < .001 (2-way ANOVA).
N-only leukemia cells were also doubly transduced with ICN1-R1984A lentiviruses and HES4 or the EV as control. Transduced cells were then purified using FACS and treated with GSI in vitro for 4 days in bare plastic without feeder cells. The expression of HES4 enforced the expansion of N-only leukemia cells and rescued the growth inhibition induced by GSI treatment (Figure 4C). Similar results were also obtained for 5 independent clones of GSI-sensitive PDX (Figure 4D). In addition, the CUTLL-1, TALL-1, and RPMI-8402 cell lines with high HES4 expression levels (supplemental Figure 10) were transduced with a scramble control or shHES4 constructs. Interestingly, the shRNA-mediated decrease in HES4 expression in both cell lines inhibited the in vitro expansion observed in the control cells (Figure 4E). Furthermore, the DND-41 and ALL-SILL cell lines with low endogenous HES4 levels were transduced with lentiviruses that carried the HES4 gene and showed a greater expansion when compared with the control cells (Figure 4F).
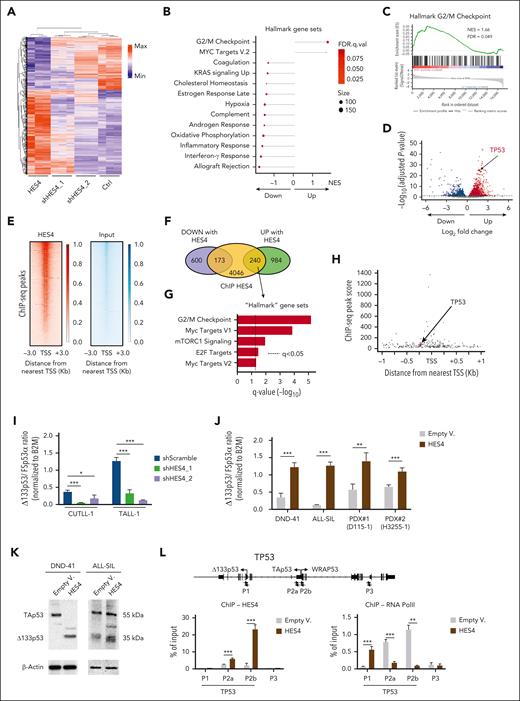
Subsequently, we performed RNA-seq on the CUTLL-1 cell line that was transduced with a lentivector encoding HES4, 2 different clones of shRNA lentiviral constructs against HES4 genes, or the scramble control (Figure 5A; supplemental Figure 11). Using GSEA, we found that genes involved in the G2/M checkpoint were among the most enriched gene sets when HES4 was expressed (Figure 5B-D; supplemental Table 12). We validated these data in HES4-transduced CUTLL-1, DND-41, and ALL-SIL cells and observed a significant increase in the cycling activity and a reduction in the number of apoptotic cells (supplemental Figure 12). Chromatin immunoprecipitation sequencing (ChIP-seq) data from unmanipulated CUTLL-1 cells, combined with RNA-seq analysis, identified 240 genes with HES4 binding regions and high expression when HES4 was overexpressed (Figure 5E-F; supplemental Figures 13 and 14; supplemental Tables 13-15). Within this refined data set, the G2/M checkpoint gene set exhibited the highest level of significance in terms of enrichment (Figure 5G). Moreover, the TP53 gene was one of the most upregulated genes when HES4 was expressed (Figure 5D) with a predicted HES4 binding site in the promoter region (Figure 5H).
HES4 enforces the expression of the Δ133p53 isoform in CUTLL-1 human T-ALL cell line. (A) Heat map and hierarchical clustering of the gene expression RNA-seq data from the human CUTLL-1 cell line after transduction with the HES4/mTag2BFP, 2 shHES4/Cherry, or empty/Cherry (Ctrl) lentivectors as indicated. After 10 days from the transduction, mTag2BFP+ or Cherry+ cells were sorted using FACS for RNA isolation and sequencing. The top 2000 differentially expressed genes, scaled to mean = 0 and SD = 1, are represented (adjusted P value ≤.1). (B) Lollipop plots of the Hallmark GeneSets significantly enriched in CUTLL-1 cells by GSEA of genes identified in the analysis of the RNA-seq data. (C) GSEA showing the enrichment score for the Hallmark G2/M checkpoint gene signature obtained by analysis of the RNA-seq data in CUTLL-1 cells. (D) Volcano plot of up- and downregulated genes by HES4 expression in CUTLL-1 cells as determined by RNA-seq assay (adjusted P value <.05). (E) Heat map of the ChIP-seq reads for HES4 and input around the transcriptional starting site (TSS) in the CUTLL-1 cell line. Scales indicate the normalized counts in the ChIP-Seq signal. (F) Venn diagram showing the up- and downregulated genes following HES4 expression in CUTLL-1 cells, determined by RNA-seq, and the genes with HES4 peaks within 1 Kb around the TSS as determined by the ChIP-seq assay. (G) Plots of the Hallmark GeneSets significantly enriched in GSEA of upregulated genes (n.240) following HES4 expression in CUTLL-1 cells as identified by the integrated analysis of the RNA-seq and ChIP-seq data sets. (H) Location of predicted HES4 sites relative to the TSS, and the ChIP-seq peak score of up- and downregulated genes following HES4 expression in CUTLL-1 cells as determined by the RNA-Seq assay. (I-J) The Δ133p53 messenger RNA expression level in the CUTLL-1 and TALL-1 cell lines after transduction with the sh-scramble or shHES4 lentivectors as indicated (I) and in the DND-41 and ALL-SIL cell lines and 2 independent clones of PDXs after transduction with HES4 lentiviruses or the EV as control (J). Four days after the transduction, the cells were sorted using FACS for RNA isolation to perform the TaqMan reverse transcriptase-digital droplet polymerase chain reaction assay. The values in the plot indicate the ratio of Δ133p53 messenger RNA expression level over the full length FSp53α isoform, both normalized to B2M gene expression as control. In each data set, 3 biologic replicates for each condition are indicated. (K) The western blot analysis of the P53 isoforms in the DND-41 and ALL-SIL cell lines following transduction with the HES4 lentiviruses or EV as negative control. (L) ChIP-quantitative polymerase chain reaction (qPCR) analysis. Local ChIP was performed using antibodies against HES4 and RNA polymerase II in the DND-41 cell line after transduction with the HES4 lentiviruses or EV as negative control. In the schematic map of the TP53 human region, the primer pairs used for ChIP-qPCR assays are indicated by the small arrows. Values are expressed as a fraction of input DNA controls. Mean values are plotted for assays performed in triplicate. The error bars indicate the SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 (Student t test). FDR, false discovery rate q-value; Min, minimum; Max, maximum; NES, normalized enrichment score; PolII, polymerase II.
HES4 enforces the expression of the Δ133p53 isoform in CUTLL-1 human T-ALL cell line. (A) Heat map and hierarchical clustering of the gene expression RNA-seq data from the human CUTLL-1 cell line after transduction with the HES4/mTag2BFP, 2 shHES4/Cherry, or empty/Cherry (Ctrl) lentivectors as indicated. After 10 days from the transduction, mTag2BFP+ or Cherry+ cells were sorted using FACS for RNA isolation and sequencing. The top 2000 differentially expressed genes, scaled to mean = 0 and SD = 1, are represented (adjusted P value ≤.1). (B) Lollipop plots of the Hallmark GeneSets significantly enriched in CUTLL-1 cells by GSEA of genes identified in the analysis of the RNA-seq data. (C) GSEA showing the enrichment score for the Hallmark G2/M checkpoint gene signature obtained by analysis of the RNA-seq data in CUTLL-1 cells. (D) Volcano plot of up- and downregulated genes by HES4 expression in CUTLL-1 cells as determined by RNA-seq assay (adjusted P value <.05). (E) Heat map of the ChIP-seq reads for HES4 and input around the transcriptional starting site (TSS) in the CUTLL-1 cell line. Scales indicate the normalized counts in the ChIP-Seq signal. (F) Venn diagram showing the up- and downregulated genes following HES4 expression in CUTLL-1 cells, determined by RNA-seq, and the genes with HES4 peaks within 1 Kb around the TSS as determined by the ChIP-seq assay. (G) Plots of the Hallmark GeneSets significantly enriched in GSEA of upregulated genes (n.240) following HES4 expression in CUTLL-1 cells as identified by the integrated analysis of the RNA-seq and ChIP-seq data sets. (H) Location of predicted HES4 sites relative to the TSS, and the ChIP-seq peak score of up- and downregulated genes following HES4 expression in CUTLL-1 cells as determined by the RNA-Seq assay. (I-J) The Δ133p53 messenger RNA expression level in the CUTLL-1 and TALL-1 cell lines after transduction with the sh-scramble or shHES4 lentivectors as indicated (I) and in the DND-41 and ALL-SIL cell lines and 2 independent clones of PDXs after transduction with HES4 lentiviruses or the EV as control (J). Four days after the transduction, the cells were sorted using FACS for RNA isolation to perform the TaqMan reverse transcriptase-digital droplet polymerase chain reaction assay. The values in the plot indicate the ratio of Δ133p53 messenger RNA expression level over the full length FSp53α isoform, both normalized to B2M gene expression as control. In each data set, 3 biologic replicates for each condition are indicated. (K) The western blot analysis of the P53 isoforms in the DND-41 and ALL-SIL cell lines following transduction with the HES4 lentiviruses or EV as negative control. (L) ChIP-quantitative polymerase chain reaction (qPCR) analysis. Local ChIP was performed using antibodies against HES4 and RNA polymerase II in the DND-41 cell line after transduction with the HES4 lentiviruses or EV as negative control. In the schematic map of the TP53 human region, the primer pairs used for ChIP-qPCR assays are indicated by the small arrows. Values are expressed as a fraction of input DNA controls. Mean values are plotted for assays performed in triplicate. The error bars indicate the SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 (Student t test). FDR, false discovery rate q-value; Min, minimum; Max, maximum; NES, normalized enrichment score; PolII, polymerase II.
To explore potential reasons why the HES4-mediated increase in TP53 gene expression reduced the level of apoptotic cells, we assessed the transcriptional variants of the TP53 gene in the DND41 and T-ALL1 cell lines after transduction with HES4 or empty constructs and the sh-scramble or shHES4 lentivectors, respectively, using PacBio long-read isoform sequencing. It has been reported that p53 transcriptional variants encode dominant-negative p53 protein isoforms, which have the opposite effects of the full length p53 in controlling cell proliferation and apoptosis.25-27 These variants are mainly generated by the activation of a different promoter, such as the Δ133p53 isoform27-29 (supplemental Figure 15A). Through isoform sequencing profiling it was found that HES4 expression promoted an increase in the p53I4α short isoforms, thereby encoding the Δ133p53 protein variant and reducing the full-length FSp53α transcript (supplemental Figure 15B). We also found that the transcriptional level of Δ133p53 directly correlated with HES4 expression in 15 human T-ALL cell lines (supplemental Figure 16A). We validated these observations at the transcriptional level in 6 different T-ALL cell lines with high HES4 transcriptional levels, including CUTLL-1 and TALL-1, after transduction with the sh-scramble or shHES4 lentivectors (Figure 5I; supplemental Figure 16B) and in the DND-41 and ALL-SIL cell lines and in 2 independent clones of PDXs after transduction with HES4 lentiviruses or EV as control (Figure 5J). Using western blot, we also confirmed the increase in Δ133p53 protein expression in HES4-transduced DND-41 and ALL-SIL cell lines when compared with the control cells (Figure 5K). In addition, we performed a local ChIP assay using anti-HES4 and anti-RNA polymerase II antibodies and a series of 4 primer sets that spanned the 2 TP53 promoters, P1 and P2, involved in the expression of the Δ133p53 and full-length FSp53α isoforms, respectively29 (supplemental Figure 17). In the DND-41, ALL-SIL, and CUTLL-1 cell lines transduced with HES4 virus or the EV as control, we also assessed the level of the H3K27Ac and H3K27me3 histone markers, which are associated with active and repressed gene promoters, respectively. We found that the P1 promoter was significantly more enriched in RNA polymerase II and the H3K27Ac histone marker in the HES4-transduced cells when compared with the EV controls, whereas the P2 promoter was significantly more enriched in HES4 (Figure 5L; supplemental Figures 17-19), suggesting that the binding of HES4 primes an isoform switch in the TP53 gene in favor of the antiapoptotic, dominant-negative Δ133p53 isoform.
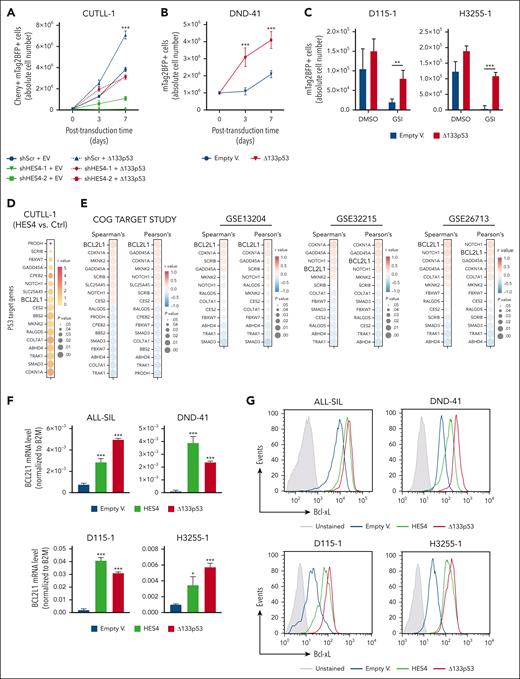
Furthermore, the CUTLL-1 and TALL-1 cell lines were independently transduced with 2 different clones of the shHES4/mCherry lentivectors or scramble control, in addition to the Δ133p53/mTagBFP2 construct or the EV. Interestingly, the expression of Δ133p53 rescued the growth inhibition induced by the shRNA-mediated decrease in HES4 gene expression (Figure 6A; supplemental Figure 20). Furthermore, the Δ133p53-overexpressing DND-41 cell line showed greater cell expansion when compared with the control cells (Figure 6B). Similar findings were also obtained with 2 independent PDX clones (Figure 6C). To identify potential effectors of the Δ133p53 protein in human T-ALL, the RNA-seq data generated from the CUTLL-1 cell line (Figure 5) and 4 different publicly available gene expression data sets (Children's Oncology Group (COG) TARGET study [n = 264],7 GSE13204 [n = 174],30,31 GSE32215 [n = 228],32 and GSE26713 n = 117]33) were used, and we correlated the HES4 expression with a list of 343 p53-target genes.34 We found a statistically significant direct correlation between the HES4 and BCL2L1 expression levels in the CUTLL1 cells (Figure 6D), in 15 human T-ALL cell lines (supplemental Figure 16A), and in other large cohorts of patients with T-ALL, totaling 783 patient samples (Figure 6E; supplemental Figures 21 and 22). Interestingly, the shRNA-mediated decrease in HES4 gene expression in the CUTLL-1, TALL-1, RPMI-8402, PEER, KARPAS-45, and PF382 cell lines reduced the expression of BCL2L1 at the transcriptional and protein levels, thereby supporting the idea that the effects of HES4 and the Δ133p53 axis on BCL2L1 gene expression was not dependent on HES4 overexpression (supplemental Figure 23). Moreover, we found a statistically significant direct correlation between the Δ133p53 and BCL2L1 expression levels in 15 human T-ALL cell lines (supplemental Figure 24A-B). We also assessed the expression level of BCL2L1 in the ALL-SIL, DND-41, and CUTLL-1 cell lines and in the D115-1 and H3255-1 PDXs after transduction with lentivectors that encoded HES4, Δ133p53, or the EVs. Both HES4 and Δ133p53 induced the expression of the BCL2L1 transcript (Figure 6F; supplemental Figure 24C) and increased the level of the B-cell lymphoma extra-large (Bcl-xL) antiapoptotic protein as determined by flow cytometry (Figure 6G; supplemental Figure 24D-E). In addition, we found that the monoallelic 994A mutant form of Δ133p53, which has been described as being present in T-ALL cells and leading to the substitution of arginine 248 with glutamine in the DNA-binding domain,17 also induced the expression of the BCL2L1 transcript and Bcl-xL protein (supplemental Figure 25), suggesting that the dominant negative activity of the Δ133p53 isoform is also maintained in the 994A mutant allele. We also performed a local ChIP assay with a p53 antibody cocktail (ThermoFisher) that recognized the main p53 isoforms, including Δ133p53, and with antibodies against RNA polymerase II and the H3K27Ac and H3K27me3 histone markers using 4 primer sets that spanned the human BCL2L1 locus (supplemental Figure 26A). We examined the DND-41, ALL-SIL, and CUTLL-1 cell lines that were transduced by HES4 virus or the EV and found that the BCL2L1 promoter was significantly more enriched in p53, RNA polymerase II, and the H3K27Ac histone marker in the HES4-transduced cells when compared with the controls (supplemental Figure 26B).
The BCL2L1 gene encodes Bcl-xL protein, which is a member of the Bcl-2 family and a well-known antiapoptotic protein that regulates mitochondrial function and prevents caspase activation.35 Therefore, using a standard flow cytometric annexin V binding assay, we assessed the apoptotic level in the ALL-SIL and DND-41 cell lines and in 2 independent PDX clones transduced with HES4, Δ133p53, or the empty lentivectors and after GSI treatment for 3 days. Interestingly, both HES4 and Δ133p53 expression reduced the number of apoptotic cells (Figure 6H). Similar findings were also generated in the same cell subsets of 2 PDX clones after transduction with the ICN1-R1984A dimer mutant, either alone or in combination with the HES4 or Δ133p53 constructs, and followed by treatment with GSI for 3 days before flow cytometric analysis (Figure 6I; supplemental Figure 27). Taken together, these results highlight the antiapoptotic role of the Notch-dimer target HES4 through a functional axis that involves the Δ133p53 and Bcl-xL proteins.
NOTCH1 dimer target genes in patients with high-risk T-ALL
To assess the relevance of the reported findings in human T-ALL, we investigated the relationship between the transcriptional level of NOTCH1 dimer target genes and a proapoptosis gene signature. Specifically, we performed single-cell RNA-seq profiling of leukemia cell subsets from 41 primary human T-ALLs without any expansion in immunocompromised mice and included 23 in-house sequenced samples and 18 cases from 3 independent, publicly available data sets, namely GSE227122,36 GSE161901,37 and GSE13250938 (supplemental Figure 28). Using the Leiden clustering algorithm, we identified 20 distinct populations across the 4 sample sets analyzed by the single-cell RNA-seq assay (Figure 7A; supplemental Figure 28). We also calculated the enrichment scores of the NOTCH1 dimer target genes (NOTCH1 dimer [NDIM] signature), as defined by the reported RNA-seq data (Figure 2), and of those genes associated with a proapoptosis signature from the Kyoto Enciclopedia of Genes and Genomes (KEGG) pathway database. Subsequently, we compared the score intensity of 2 signatures across the different cell clusters (Figure 7B) and found that, in the #1, #6, #14, #15, and #17 clusters, the scores for the NDIM signature were inversely correlated with the values of the proapoptosis signature (Figure 7C), suggesting an augmented NOTCH1 dimeric signaling and reduced apoptosis rate within these specific cell subsets. We also used a panel of 42 oligo-conjugated antibody-seq to evaluate the protein level of cell surface markers associated with T-cell leukemias and normal development (supplemental Table 7) in the 23 in-house sequenced samples. Through this approach, we determined the immunophenotypic differences of each identified cell subset by calculating the fold change expression of surface cell markers recognized by the oligo-conjugated antibodies in the antibody-seq assay. Interestingly, we observed that the CD5 and CD2 cell markers were highly expressed in the #6 and #15 clusters (Figure 7D).
To recapitulate our findings in patients with T-ALL, we employed machine learning approaches to cluster, in 2-dimensions, the transcriptomes of 262 patients with T-ALL from the COG TARGET study.7 We identified 5 different groups of patients with T-ALL using the k-means algorithm and selecting a number of clusters that would allow minimal overlap between them (Figure 7E; supplemental Figures 29 and 30). We also calculated the enrichment score of the NDIM signature using Gene Set Variation Analysis, along with the transcriptional expression levels of the HES4 and BCL2L1 genes. Notably, the patients with T-ALL who were grouped in cluster #1 were characterized by the highest median score for the NDIM signature and for the HES4 and BCL2L1 genes (Figure 7F). A significant direct correlation in cluster #1 was also observed between NOTCH1 and HES4 messenger RNA levels (Pearson correlation coefficient, R = 0.27) (Figure 7G). Furthermore, after calculating the gene set variation analysis scores for the NDIM and proapoptosis (KEGG) signatures, we found that only the values in clusters #1 and #3 showed a strong significant difference (Figure 7H), suggesting that specific T-ALL subtypes are more sensitive to NOTCH1 dimeric signaling.
We also investigated the score level of the NDIM signature by grouping the patients based on the transcriptional expression of the CD2 and CD5 markers. Notably, patients with a high expression level of both CD2 and CD5 showed the highest median score for the NDIM signature when compared with the other groups of patients (CD2highCD5low, CD2lowCD5high, and CD2lowCD5low) (Figure 7I; supplemental Figure 31). Furthermore, to focus on the importance of the NDIM signature levels, we subdivided the patients with T-ALL into NDIMhigh or NDIMlow cases based on unsupervised hierarchical clustering (supplemental Figures 32 and 33) and observed a poorer event-free survival rate for NDIMhigh patients (Figure 7J; supplemental Table 9). Interestingly, cases with higher transcriptional levels of HES4 or of the CD2 and CD5 markers also exhibited significantly poorer event-free survival (Figure 7K-L; supplemental Figure 34; supplemental Table 9). Overall, these results identified a specific subtype of patients with high-risk T-ALL who are characterized by a high expression level of NOTCH1 dimer target genes that are inversely correlated with a proapoptosis gene signature and by persistently high levels of CD2 and CD5 surface markers.
Discussion
NOTCH1 signaling is crucial for tumor development and maintenance.9-12,39-46 In mice, Notch1 dimers are required for T-cell maturation and leukemic transformation.16 Despite these results, the role of NOTCH1 dimers in human leukemogenesis and primary T-cell leukemia was still unclear. In this study, using 3 different synthetic models of human T-ALL, we reported that NOTCH1 dimeric signaling is required for the malignant transformation of human T-cell precursors. Interestingly, we identified HES4 as a direct target of NOTCH1 dimerization. It was already shown that HES4 is a direct target of Notch1 dimers22,47,48 and that its knockdown significantly reduced human T-cell development.49 However, in this study, we identified a novel functional axis that involves NOTCH1 dimers and HES4, which primes an isoform switch in the TP53 gene in favor of the antiapoptotic, dominant-negative Δ133p53 isoform.25-27 DNA alterations in the TP53 gene are indeed present in patients with T-ALL and are associated with disease relapse.7,50 Our findings suggest that HES4 enforces the expression of the Δ133p53 isoform, which contributes to disease progression through the concomitant block of proapoptotic and typical p53 target genes. Furthermore, its dominant-negative activity is preserved in T-ALL cell lines with mutated p53, such as CUTLL1.17 In line with these observations, previous reports have shown previously that the expression of the Δ133p53 isoform is associated with disease progression and therapy resistance.27,51-53
By relying on different machine learning approaches and using publicly available data sets,7,30-33 we also reported that a high expression level of the NDIM signature inversely correlated with a proapoptosis gene signature in a specific T-ALL subtype. By integrating the clinical information available, we observed that the NDIM signature was enriched in patients with particular clinical traits and a significantly poorer event-free survival when compared with other cases. Interestingly, it was also reported that, in patients with breast cancer, HES4 is a potential important biomarker for NOTCH1 related therapies,54 supporting the idea that the antiapoptotic effect induced by NOTCH1 dimers through the activation of HES4 expression might be extended to other Notch-related cancers as well. Nonetheless, more data are needed to corroborate this hypothesis.
This study provides substantial evidence to support the proposed model. Nevertheless, several mechanistic aspects remain unresolved. For instance, the selective binding of HES4 to Δ133p53 regulatory elements and its role in upregulating Δ133p53 expression require further investigation to clarify the underlying molecular mechanisms. In addition, this study does not comprehensively assess the genome-wide overlap between HES4 binding and other canonical Hes factors. Although E-box motifs were identified as significant across all data sets, they were not among the top-ranked motifs, suggesting that HES4 binding in human cells may involve additional DNA-binding regions. Furthermore, the association of HES4 with DNA motifs beyond the canonical Hes factor binding sites raises intriguing questions about the involvement of potential cofactors or alternative binding preferences, which warrant further characterization. Although the specificity of the HES4 ChIP-seq antibodies was addressed through additional controls, further validation is required to fully elucidate HES4’s genome-wide binding capacity. Lastly, extending these findings to contexts beyond leukemic T-cells could provide broader biologic insights. These limitations emphasize the need for further research to deepen our understanding of HES4’s regulatory roles and to refine the proposed model.
In conclusion, this work defined the crucial role of NOTCH1 dimerization in human T-cell leukemogenesis and in established leukemia by identifying a novel functional axis that involves the HES4 transcription factor and the dominant-negative Δ133p53 isoform, which leads to a reduction in apoptosis. Furthermore, we identified a distinct subset of patients with T-ALL who were characterized by a specific NDIM signature that was also present in patients with breast cancer. Taken together, these data highlight the role of Notch dimerization in T-ALL and other Notch-related cancers, suggesting the possibility that disrupting NOTCH1 dimerization or inactivating its effectors may be therapeutically relevant.
Acknowledgments
The authors are grateful to Rossella Di Paola and Tommaso Mazza for their help with the Illumina next-generation DNA sequencing. The authors also thank Chiara Di Giorgio at the Fondazione IRCCS Casa Sollievo della Sofferenza in San Giovanni Rotondo, Italy, for proofreading the text and Alessia Reboa for her assistance in the realization of the visual abstract.
This study was funded by the Foundation with the South, Brain2South, grant 2015-0251 (V.G.); the Italian Ministry of Health, Ricerca Finalizzata Young Researcher, GR-2016-02361287 (V.G.); the Italian Association for Cancer Research (AIRC), My First AIRC Grant, MFAG18487 and IG 2019-ID. 23070 project (V.G.); Worldwide Cancer Research, 20-0318 (V.G.), and the AIRC, Fellowship for Italy, #21010, 3-year AIRC Pietro Finocchiaro fellowship (F.T.).
Authorship
Contribution: F.T., C.P., A.P.W., and V.G. conceptualized the study; A.T., M.S., A.P.W., and V.G. contributed resources; F.T., C.P., E.D.S., and V.G. were responsible for data curation; F.T., C.P., K.D., and V.G. performed formal analysis; F.T., C.P., E.D.S., D.F.S., V.C., S.D.I., C.D.N., M.C., G.B., E.G., and V.G. were responsible for methodology; F.T., C.P., E.G., and V.G. did investigation; F.T., C.P., and V.G. were responsible for validation and visualization, and wrote the original draft; V.G. was responsible for funding acquisition; E.D.S., C.D.N., and D.F.S. contributed to project administration; M.S., A.P.W., and V.G. performed supervision; and F.T., C.P., M.S., A.P.W., and V.G. reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vincenzo Giambra, Hematopathology Laboratory, Institute of Regenerative Medicine, Fondazione IRCCS Casa Sollievo della Sofferenza, Viale Padre Pio 7, 71013 San Giovanni Rotondo, Italy; email: v.giambra@operapadrepio.it.
References
Author notes
The RNA sequencing data are accessible at the National Center for Biotechnology Information-Sequence Read Archive (NCBI-SRA) PRJNA1010174, PRJNA1010175, and PRJNA1011511. The chromatin immunoprecipitation sequencing data are accessible at NCBI-SRA PRJNA980610. The long-read isoform sequencing data are accessible at NCBI-SRA PRJNA1161830. The single-cell RNA-seq data are accessible at NCBI-SRA PRJNA784728. Other original data are available on request from the corresponding author, Vincenzo Giambra (v.giambra@operapadrepio.it).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



![HES4 transcriptional expression confers growth advantage in the transformation of human T-cell progenitors. (A) Flow cytometric analysis of GFP+ and mCherry+ cell abundance in the CB cells doubly transduced, respectively, with NOTCH1-ΔE/GFP or shRNA/mCherry lentiviral constructs. Transduced cells were cultured in vitro on OP9-DL1 feeders and serially passaged every 5 days. GFP+ mCherry+ alive cells were measured at the indicated time points using flow cytometry for DRAQ7 exclusion. The graphs report the result of 2 independent experiments performed in triplicate. ∗∗∗P < .001 (2-way analysis of variance [ANOVA] with Dunnett test, comparing the sh-scramble (shScr) control mean with the other values). (B) Flow cytometric tracking of doubly transduced (GFP+mTagBFP2+) CB cells at each serial passage onto OP9-DL1 feeders every 5 days. The CB cells were transduced with lentiviral particles with NOTCH1-ΔE-R1984A/GFP dimer mutant lentiviruses, in addition to the HES4/mTagBFP2 construct or EV as control on days 0 and 5. The mean values ± range for duplicate experiments are plotted. ∗∗P < .01; ∗∗∗P < .001 (Student t test). (C) Flow cytometric tracking of mTagBFP2+ CB cells, transduced with HES4/mTagBFP2 lentivector or EV as control on days 0 and 5. The transduced cells were serially passaged onto OP9-DL1 feeders every 5 days. The mean values ± range for duplicate experiments are plotted. ∗∗∗P < .001 (Student t test). (D) Limiting dilution growth assays. CB cells were transduced with HES4/mTagBFP2 lentivector or EV as control on day 0, cultured until day 3, and then sorted using FACS into individual wells of a 96-well plate on precoated plastic in T-cell expansion media (StemCell Technologies) and maintained in vitro for 3 weeks. The entire content of each well was assayed by flow cytometry and the frequency of well-initiating cells was calculated using the ELDA software. In the ELDA plot, the cell dose vs the log fraction of negative cultures is represented. Dotted lines represent the confidence interval of 95%. The estimated frequencies are indicated in the box on the right side. P values were calculated using the ELDA tool. (E) t-distributed stochastic neighbour embedding (tSNE) plots based on the flow cytometric tracking of CB cells, singly transduced with NOTCH1ΔE/GFP or HES4/mTagBFP2 lentivectors at day 10 of in vitro coculture on OP9-DL1 feeders. (F) The Kaplan-Meier survival curves of primary NSG mice injected with CB cells transduced with NOTCH1-ΔE, NOTCH1-ΔE-R1984A, or HES4 lentiviruses after 10 days of in vitro growth. The survival curve of secondary recipient mice transplanted with primary HES4 CB leukemia is indicated with a dashed line. The number of recipient animals for each cell type is indicated in parentheses. Data are pooled from 2 separate cohorts of animals.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/145/24/10.1182_blood.2024027020/2/m_blood_bld-2024-027020-gr3.jpeg?Expires=1765960333&Signature=s~ZoWbOfOizjP3PbRhQsvEMFb4FRzXi-~fQT-scXV0yPdcVtjxdVKbbyVBTtprnqm7FIGIMn6QQkQoTJ0HEK1EM3TZfDR2OPYBdZChVPIaMdkXG7C-Eoufmcuc2ZLp6P9Vij5m05uX6puGkbdBOn2lRRKx1bYTH-l20EAVpdjFVmR7M5iS8GJJAdK9t2vSSmG78Vw4mbgJ2JzJ3bC~nDDD753eXOQ9ubxisGK3QRp6tSR5RTR03PDe0m4tKtp1TZRyH4o0RHnW3LI00yQcmgwf9VPmhBZCCjPejqP~pJiIjikS4L0fAE6iVM-ZOkv~d2~0Lbh9WxJlO9bF56S9KO9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![The Δ133p53 isoform is an antiapoptotic protein that promotes the survival of human T-ALL cells through induction of Bcl-xL expression. (A) The abundance of the Cherry+ mTagBFP2+ cell fraction, tracked over time in culture by flow cytometry. The CUTLL-1 cell line was independently transduced with 2 different clones of shRNA/mCherry lentiviral constructs against the HES4 gene or with the scramble control, in combination with the Δ133p53/ mTagBFP2 construct or the EV as indicated. Cherry+ mTagBFP2+ cells were sorted using FACS, cultured in vitro, and measured at the indicated time points by flow cytometry. The graphs report the result of 3 independent experiments performed in triplicate. ∗∗∗P < .001 (2-way ANOVA). (B) Abundance of the Δ133p53-transduced mTagBFP2+ cell fraction, tracked over time in culture by flow cytometry. The DND-41 cell line was independently transduced with the Δ133p53/mTagBFP2 lentiviral construct or EVs as control. mTagBFP2+ alive cells were measured at the indicated time points by flow cytometry for propidium iodide (PI) exclusion. The graphs report the result of 3 independent experiments performed in triplicate. ∗∗∗P < .001 (2-way ANOVA). (C) Abundance of the Δ133p53-transduced mTagBFP2+ cell fraction in 2 independent clones of PDXs. Two days after transduction the cells were sorted using FACS and treated with the GSI Compound E (1 μM) for 3 days and measured at the indicated time points by flow cytometry for PI exclusion. The graphs report the result of 3 independent experiments performed in triplicate. ∗∗P < .01; ∗∗∗P < .001 (Student t test). (D) Correlation analysis of messenger RNA (mRNA) expression among HES4 and selected p53 target genes in the human CUTLL-1 cell line after transduction with HES4 or the EV as reported in Figure 5. (E) Correlation analysis of mRNA expression between HES4 and selected p53 target genes across 4 different gene expression data sets of human T-ALLs. The mRNA expression levels of different genes among 262 patients with T-ALL from the COG TARGET study were normalized using rLog. The Affymetrix microarray (HG-U133 Plus 2.0) data were downloaded from the Gene Expression Omnibus (GEO) database (accession numbers: GSE13204 [n = 174], GSE32215 [n = 228], and GSE26713 [n = 117]) and normalized using RMA (Robust Multiarray Averaging). (F) The BCL2L1 mRNA expression level in the ALL-SIL and DND-41 cell lines and in the D115-1 and H3255-1 PDXs after transduction with the lentivectors that encoded HES4, Δ133p53, or EVs. Cells were collected 4 days after the transduction and sorted using FACS before RNA isolation and performing the TaqMan reverse transcriptase-digital droplet polymerase chain reaction assay. The values in the plot indicate the ratio of the BCL2L1 mRNA expression level normalized to the B2M gene expression as control. In each data set, 3 biologic replicates for each condition are indicated. Error bars indicate the SD. ∗P < .05; ∗∗∗P < .001 (Student t test). (G) Protein expression level of Bcl-xL determined by flow cytometry in the ALL-SIL and DND-41 cell lines and in the D115-1 and H3255-1 PDXs transduced as reported in panel F. (H) Flow cytometric analysis of the early apoptotic level using annexin V binding and 7-aminoactinomycin D (7AAD) exclusion in the ALL-SIL and DND-41 cell lines and the D115-1 and H3255-1 PDXs transduced as reported in panel F. Two days after the transduction, the cells were sorted using FACS and treated with GSI for 3 days before flow cytometry analysis. The graphs report the results of 2 independent experiments performed in triplicate. Each reported statistical value was compared with EV as the control. ∗∗P < .01; ∗∗∗P < .001 (Student t test). (I) Flow cytometric analysis of early apoptotic level by annexin V binding and 7AAD exclusion in the D115-1 and H3255-1 PDXs after transduction with lentivectors encoding wt intracellular NOTCH1 domain (ICN1) or ICN1-R1984A dimer mutant alone and in combination with the HES4 or Δ133p53 constructs. Cells transduced with EV were also included as control. Two days after the transduction, the cells were sorted using FACS and treated with GSI for 3 days before flow cytometry analysis. The graphs report the result of 2 independent experiments performed in triplicate. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 (Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/145/24/10.1182_blood.2024027020/2/m_blood_bld-2024-027020-gr6hi.jpeg?Expires=1765960333&Signature=okBJMux6xGf4Af7zL-2mIULQ6UKTJhmGbrJQrrxGjGrBCncDhshCcqTKm9w2IenTCdM29IhE9pa2XwSx8dGHPGtsupVSp03vfz7YSTjVN4Q4YrM1LGOIcSJ3QZDZGr8WkBZYwbH6jHE2V~8wcOeNj~-LB3mBPl4Y4PQ29adaV~-fG7wfmzGrshPEo9SRnIohmquQun58ZA44ruI1UTJ0p-BWam-VkYX-4XMBST-auAfy5Z3DCWLKIcW5Ui29oYUeihAjvGgVDw90mq4DbxWzB9DxG0xsHPup5uUPtBhKsMu8M272BNrrUezV8aKzvd~lmYVssPiRkxk1anVcTbzDYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

