Key Points
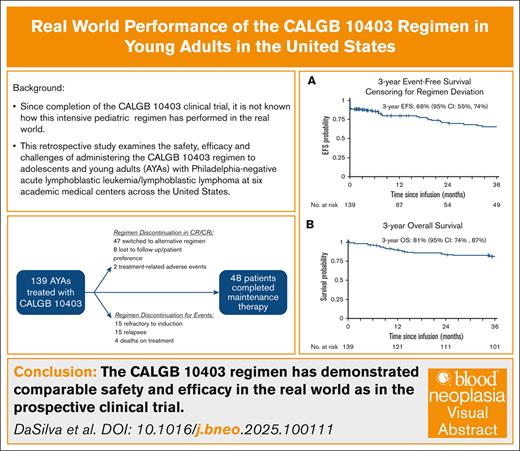
Real-world efficacy and safety of the C10403 regimen in AYA patients with newly diagnosed ALL match prospective trial results.
Completing the regimen through maintenance therapy remains a challenge for patients and clinicians in adult oncology clinics.
Visual Abstract
The Cancer and Leukemia Group B 10403 (C10403) trial prospectively demonstrated the safety and efficacy of administering an asparaginase-containing pediatric regimen for the treatment of adolescents and young adults (AYAs) with acute lymphoblastic leukemia. Since its implementation as standard of care, it is unknown how the C10403 regimen performs beyond the clinical trial setting. To bridge this knowledge gap, we designed a multicenter retrospective cohort study to examine the safety, efficacy, and challenges of completing C10403 in the “real world.” From October 2012 through June 2020, a total of 139 patients began induction as per the C10403 regimen across 6 US academic cancer centers. The median age was 26 years (range, 17-39), 69% were male, 55% were non-Hispanic White, and 27% were Hispanic. Among them, 122 patients (88%) achieved complete remission or complete remission with incomplete count recovery (CR/CRi) with C10403, 48 (35%) completed maintenance therapy, and 47 (34%) changed postremission regimens while in CR/CRi. The 3-year event-free survival (EFS) was 66% (95% confidence interval [CI], 55-74), and the 3-year overall survival (OS) was 81% (95% CI, 74-87). Four deaths occurred while on C10403 treatment: 1 during induction; and 3 later in the treatment course. The most common grade 3 or 4 adverse events during induction included alanine aminotransferase elevation (22%) and sepsis (14%). B-cell immunophenotype (hazard ratio [HR], 2.45; 95% CI, 1.09-5.48), Philadelphia chromosome–like genetics (HR, 3.05; 95% CI, 1.25-7.44), and Hispanic ethnicity (HR, 2.00; 95% CI, 1.06-3.78) were associated with worse EFS in univariate analyses. Overall, these real-world results are comparable to those of the C10403 trial. Further improvements are needed to enhance outcomes and regimen tolerability in the AYA population.
Introduction
From 2008 to 2012, the US Intergroup conducted Cancer and Leukemia Group B (CALGB) 10403 (C10403), a prospective phase 2 clinical trial investigating whether a pediatric regimen for acute lymphoblastic leukemia (ALL) was feasible, safe, and effective when administered to adolescents and young adults (AYAs; aged 17-39 years) with newly diagnosed Philadelphia chromosome–negative (Ph–) ALL.1 The results of C10403 demonstrated that adult oncologists are able to safely administer a pediatric ALL regimen to AYAs with excellent outcomes. The 3-year event-free survival (EFS) and overall survival (OS) of 59% and 73%, respectively, were far superior to historic survival data in this population.2,3 The C10403 results led to a significant change in clinical practice, with the C10403 regimen and other pediatric regimens emerging as the standard of care for AYAs with newly diagnosed Ph– ALL at many US centers and being recommended by the National Comprehensive Cancer Network.4
Although C10403 was a practice-changing trial, only one-third of patients completed protocol treatment. The high rate of protocol noncompletion in C10403 highlighted the challenges in administering an intensive pediatric ALL regimen to AYAs. Furthermore, although toxicities were expected and manageable, more grade 3 to 4 asparaginase-related adverse events (AEs) were noted in AYAs than children, including liver test abnormalities and pancreatitis.1,5 Finally, Hispanic patients were underrepresented in C10403 compared to the epidemiology of AYA patients with ALL in the United States.6
For these reasons, we were interested in understanding the performance of the C10403 regimen when administered to patients outside of the context of a clinical trial. Therefore, we created a multi-institutional US cohort of AYA patients with newly diagnosed Ph– ALL who were treated with C10403 after 2012 to assess adherence, efficacy, and toxicity of the regimen when administered as standard of care.
Methods
Patients
AYA patients (aged 17-40 years) with newly diagnosed B-cell or T-cell Ph– ALL or lymphoblastic lymphoma (LBL) who received initial treatment with the C10403 regimen at 1 of 6 US centers (supplemental Table 1) between October 2012 and June 2020 were included. Deviations from the trial regimen with respect to corticosteroid selection (prednisone vs dexamethasone) and dosing of pegylated (PEG)-asparaginase were permitted. Patients with Ph+ ALL or Burkitt-type ALL were excluded, as were patients who received prior treatment for ALL (except hydroxyurea, steroids, or a single dose of chemotherapy) or were enrolled in an investigational protocol. Data lock occurred on 30 June 2023.
Institutional review boards of each participating center approved this study. Retrospective chart review, data collection, and data sharing were conducted in accordance with the protocol.
Study end points and definitions
Primary end points were induction response rate, 3-year EFS, and 3-year OS. Induction response was defined as achieving <5% blasts on bone marrow by morphology or achieving metabolic response on positron emission tomography–computed tomography by the end of induction or extended induction. Similar to the C10403 trial,1 EFS was defined as the time from regimen initiation (day 1) to any of the following events: (1) failure to achieve bone marrow response by the end of induction or extended induction; (2) failure to achieve metabolic response of lymphoma on positron emission tomography–computed tomography, as determined by the clinician, by the end of induction or extended induction; (3) relapse at any site; (4) development of a second malignancy; or (5) death.1 OS was defined as the time from regimen initiation to death from any cause.
Measurable residual disease (MRD) was defined as the detection of disease in the bone marrow by flow cytometry or by next-generation sequencing (NGS) using ClonoSEQ. For flow cytometry, MRD positivity was defined as the detection of any blast cell with a sensitivity of 10–4. For NGS, MRD positivity was defined as the detection of an immunoglobulin H clonal sequence in B-cell ALL (B-ALL) or T-cell receptor clonal sequence in T-cell ALL (T-ALL) at or above the level of detection for the assay with a sensitivity of 10–6.7
Ph-like ALL was assessed and defined per individual institutional standards. Overweight and obese body mass index (BMI) were defined as 25 to <30 kg/m2 and ≥30 kg/m2, respectively. High-risk cytogenetics included hypodiploid, complex karyotype (≥3 chromosomal abnormalities), or KMT2A rearrangement.
AEs were defined using the Common Terminology Criteria for Adverse Events version 5.0.8 Specific grade ≥3 AEs of interest including liver enzyme abnormalities, pancreatitis, and venous thromboembolism (central nervous system [CNS] and non-CNS) were collected per treatment phase (induction or after remission) by manual chart review.
Statistical analysis
Descriptive statistics were used to summarize baseline patient demographic and disease characteristics, as well as patient outcomes after treatment. Kaplan-Meier curves were used to evaluate OS (event defined as death) and EFS (event defined as induction refractory, second malignancy, relapse, or death). OS was censored for loss to follow-up, whereas EFS was censored for the addition of a non–protocol-defined agent (eg, blinatumomab or switching to an alternative regimen), hematopoietic cell transplant (HCT), or loss to follow-up. Cumulative incidence curves were used to evaluate relapse rates, with death as a competing risk. Associations with OS and EFS were assessed using Cox regression, and associations with MRD-negative remission were assessed with logistic regression. This study was approved by the institutional review boards of each participating center. All tests were conducted at a significance level of .05. SAS version 9.4 (SAS Institute, Cary, NC) was used to conduct the analyses.
Results
Patient and disease characteristics
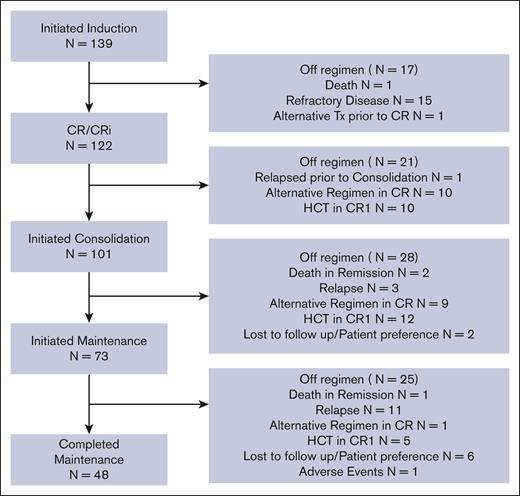
The study cohort consisted of 139 newly diagnosed AYA patients with ALL treated with the C10403 regimen as standard of care (Table 1; Figure 1). The median age at treatment initiation was 26 years (interquartile range, 21-32); 47 patients (34%) were aged 30 to 40 years. Most patients were male (n = 96 [69%]) and non-Hispanic White (n = 76 [55%]); 38 (27%) were Hispanic, 18 (13%) were Asian, and 4 (3%) were Black. Seventy-nine patients (57%) had an overweight (25-29.9 kg/m2) or obese BMI (≥30 kg/m2) at diagnosis. More patients had B-ALL (n = 103 [74%]) than T-ALL (n = 36 [26%]). High-risk cytogenetics were common (n = 38 [27%]), and 12 of 43 tested patients (28%) harbored Ph-like genetics, as locally defined. Corticosteroid use during induction favored prednisone (n = 120) over dexamethasone (n = 17; supplemental Table 2). There were 16 patients who received at least 1 dose of Erwinia asparaginase and 17 patients who had at least 1 dose of PEG-asparaginase capped (supplemental Table 2).
CONSORT diagram. AYA patients with ALL treated with C10403 from 2012 to 2020.
The study cohort was slightly older than the C10403 clinical trial cohort (median age, 26 vs 24 years; patients aged 30-40 years, 34% vs 25%) and had a greater proportion of patients who were Hispanic (27% vs 15%) or Asian (13% vs 3%).1 Patients were otherwise similar in terms of baseline demographics and leukemia characteristics (supplemental Table 3).
Response and per-protocol regimen completion
Figure 1 summarizes patient responses and patient disposition (regimen completion and reasons for discontinuation). Of the 139 patients who initiated induction treatment, 122 (88%) achieved CR/CRi by the end of induction/extended induction; 15 (11%) were refractory, and 2 (1%) were unevaluable (1 due to early treatment-related death and 1 due to early deviation from the C10403 regimen). Of the 122 patients with CR/CRi, 108 had MRD data available, with 63 (58%) achieving an MRD-negative remission by flow cytometry and/or NGS (supplemental Figure 1). Forty-seven patients (39%) in CR/CRi were switched to alternative treatment regimens before the completion of the C10403 regimen, including HCT (n = 27), hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) (n = 9), and blinatumomab (n = 8; supplemental Table 4). In total, only 48 of the patients (35%) who started C10403 induction completed maintenance chemotherapy (course V). Of the 27 patients who proceeded to HCT in CR1 directly after treatment with C10403, 24 patients (89%) had known high-risk genetics (cytogenetics or Ph-like) and/or were MRD positive after induction therapy (supplemental Table 5). Comparing patterns of regimen deviation by immunophenotype, of the 36 patients with T-ALL, 47% completed the C10403 regimen through maintenance, whereas only 30% of patients with B-ALL completed maintenance (supplemental Table 6).
Relapse, EFS, and OS
The median follow-up for surviving patients was 72 months (range, 5-127). Fifteen patients (11%) were deemed refractory to either induction and/or extended induction. Among the 122 patients who achieved CR/CRi after induction or extended induction, the 3-year cumulative incidence of relapse was 22% (95% confidence interval [CI], 15-30).
Over the available follow-up period for all patients, 36 patients (26%) died. Of those who died, 25 patients had at least 1 disease relapse, with the primary cause of death attributed to relapsed ALL in 16 of 25 patients and treatment toxicity in 7 of 25 patients (supplemental Table 7). Four died while receiving C10403 treatment (1 died during induction and 3 died after induction while in CR), and 5 patients died after transplant without a documented disease relapse (supplemental Table 7).
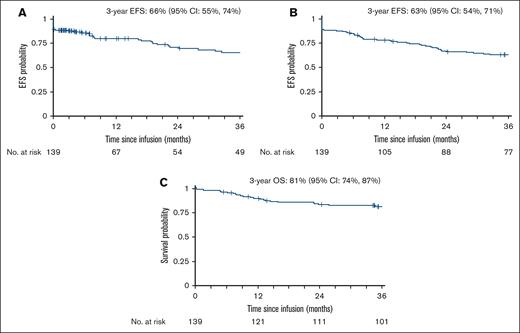
The 3-year EFS was 63% (95% CI, 54-71). The 3-year EFS after censoring for alternative therapies and HCT while in first complete remission (CR1) was 66% (95% CI, 55-74; Figure 2A-B). The 3-year OS was 81% (95% CI, 74-87; Figure 2C). Supplemental Table 8 describes the 3-year EFS and OS of the study cohort relative to the C10403 clinical trial cohort.1
EFS and OS among AYAs with newly diagnosed ALL treated with C10403. (A) EFS censored for discontinuation of C10403 regimen while in CR/CRi or at last follow-up. (B) EFS censored at last follow-up only. (C) OS of the entire cohort.
EFS and OS among AYAs with newly diagnosed ALL treated with C10403. (A) EFS censored for discontinuation of C10403 regimen while in CR/CRi or at last follow-up. (B) EFS censored at last follow-up only. (C) OS of the entire cohort.
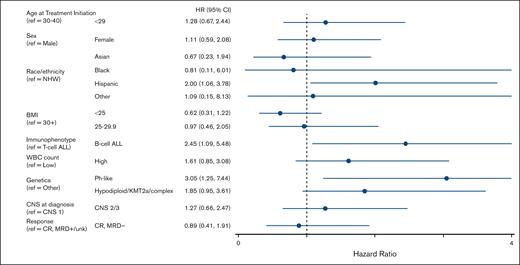
Univariate Cox regression analyses revealed the following variables to be associated with significantly inferior EFS for patients treated with the C10403 regimen (Figure 3; supplemental Table 9): Hispanic ethnicity relative to non-Hispanic White (hazard ratio [HR], 2.00; 95% CI, 1.06-3.78; P = .03); B-ALL relative to T-ALL (HR, 2.45; 95% CI, 1.09-5.48; P = .03); and Ph-like genetics relative to Ph– non–high-risk genetics (HR, 3.05; 95% CI, 1.25-7.44; P = .014). Compared to patients with a BMI ≥30 kg/m2, those with a BMI <25 kg/m2 trended toward better EFS (HR, 0.62; 95% CI, 0.31-1.22; P = .16) but did not meet statistical significance.
Forest plot depicting univariable analyses of variable associated with EFS. Analyses included 91 patients. NHW, Non-Hispanic White; ref, reference; unk, unknown.
Forest plot depicting univariable analyses of variable associated with EFS. Analyses included 91 patients. NHW, Non-Hispanic White; ref, reference; unk, unknown.
Toxicity
Table 2 highlights grade 3 to 4 nonhematologic AEs that occurred during induction or after remission. The most common grade 3 to 4 AEs during induction were alanine aminotransferase (ALT) elevation (n = 31 [22%]), sepsis (n = 20 [14%]), and bilirubin elevation (n = 17 [12%]). Similarly, ALT elevation (n = 36 [36%]), aspartate aminotransferase elevation (n = 20 [20%]), and sepsis (n = 17 [17%]) were the most common grade 3 to 4 AEs after remission. Compared with the C10403 clinical trial cohort,5 during induction, there were fewer occurrences of grade 3 to 4 sepsis (14% vs 25%) and similar instances of ALT elevation (22% vs 29%), grade 3 to 4 pancreatitis (4% vs 3%), and allergic reactions (2% vs 1%; supplemental Table 10). After remission, compared to the C10403 clinical trial cohort, the frequency of grade 3 to 4 pancreatitis (9% vs 8%) and allergic reactions (12% vs 13%) was similar, although the frequency of sepsis (17% vs 39%) and elevated ALT (36% vs 56%) was lower (supplemental Table 10).5
Discussion
In the early 2000s, it was recognized that older adolescents (aged 16-20 years) treated with a pediatric ALL regimen in pediatric treatment settings had significantly better outcomes than the same-aged patients treated with adult regimens in adult treatment settings. This prompted interest in treating young adults with pediatric chemotherapy in an effort to reduce treatment failure and obviate the need for allogeneic HCT. The concern with this approach was that applying pediatric ALL chemotherapy to older patients, along with the intensified use of corticosteroids, vincristine, CNS prophylaxis, and asparaginase, would be unacceptably toxic.
The US Intergroup C10403 trial was launched to establish whether a pediatric regimen, adopted from the Children’s Oncology Group (COG) AALL0232 trial, could be administered in adults aged <40 years and improve outcomes.9 The study met its goal and demonstrated improved measures of disease response compared to young adult patients (aged 16-29 years) treated on historical CALGB protocols: median EFS (78.1 vs 30 months), median disease-free survival (DFS; 81.7 vs 34 months), and 3-year OS (73% vs 58%).1 The study also established that the toxicity profile was manageable, albeit with more toxicity than observed in younger children.5
Although the C10403 trial established the benefit of a pediatric regimen in AYAs, it was notable that most patients (61%) did not finish the entire protocol.1,5 Although better, only 58% of patients aged ≥18 years completed treatment per COG AALL0232.5,9 Taken together, these data highlight ongoing concerns regarding feasibility of administering an intensive pediatric regimen in this patient population, a concern that may be amplified outside the supportive structure of a clinical trial. Additionally, whether outcomes in patient populations more representative of the United States would be similar was unknown.
Therefore, we conducted this study to understand performance of the C10403 pediatric ALL regimen in the real-world setting, among a more diverse patient population. To our knowledge, our study of 139 AYA patients with Ph– ALL and LBL treated at 6 US centers represents the largest study describing the performance of the C10403 regimen when administered as a standard of care in the United States. Compared to the C10403 cohort, our cohort was more representative of the AYA ALL demographics in the United States (median age, 26 years; 27% Hispanic).1,6 We found a 3-year OS of 81% and a 3-year EFS of 66%, which are comparable to the results of the C10403 publication. Our results demonstrate definitively, to our knowledge, for the first time that the positive outcomes of C10403 are being regularly achieved outside the context of a clinical trial.
In our cohort, Ph-like genetics, B-ALL immunophenotype, and Hispanic ethnicity were associated with inferior EFS. Ph-like genetics have been previously associated with inferior outcomes, including in the C10403 cohort; therefore, these results are not surprising.1,10 The association of Hispanic ethnicity with inferior outcomes demonstrated in our cohort may be mediated by the known link between Hispanic ethnicity and Ph-like genetics.11 Because our study included patients diagnosed between 2012 and 2020, before the universal examination of Ph-like genetics in B-ALL, missing data precluded our ability to perform adjusted analyses. Notably, in contrast to the C10403 cohort, B-ALL was associated with worse EFS than T-ALL.1 This may be due to the fact that our cohort had more Hispanic patients with Ph-like B-ALL or that there was an increased frequency of patients with T-ALL who completed the C10403 regimen compared with patients with B-ALL. A similar association has been reported in other cohorts.12 Finally, although obesity (BMI ≥30 kg/m2) was associated with inferior DFS in the C10403 clinical trial, we were unable to show a statistically significant association between BMI and EFS in our cohort, which may be related to inadequate sample size or other differences between cohorts.
Furthermore, we demonstrated the safety of C10403 in the real-world setting. There was only 1 death during induction and 3 subsequent deaths while on treatment with C10403, which compares favorably to the 9 deaths during induction and 2 deaths in remission in the trial.1,5 We also did not observe an increased frequency of grade 3 to 4 thrombosis, anaphylaxis, hepatotoxicity, or pancreatitis in the real-world setting compared to the trial cohort. Thus, the C10403 regimen was associated with a toxicity profile in the real-world setting as favorable as that seen on clinical trial.
Despite encouraging results, only 35% of our cohort completed the C10403 regimen, which is similar to the 39% of patients in the clinical trial who completed treatment per protocol.5 The reasons for protocol noncompletion varied in our cohort, with more real-world patients deviating from the regimen in favor of alternative therapies, including HCT in CR1. The tendency for treatment discontinuation for alternative therapy and/or HCT may be related to the availability of novel agents (such as blinatumomab, administered in 8 patients who were directly coming off the C10403 regimen) and better tools for risk stratification (identification of Ph-like biology and MRD assessment), which prompted treatment adjustments, including allocation to HCT in CR1 to prevent overt relapse. Still, some patients did discontinue AYA chemotherapy due to toxicity and/or inability to handle treatment complexity, highlighting areas of unmet need. Reports of alternative pediatric ALL regimens have been associated with higher rates of regimen completion in AYAs; for example, AYA patients aged 15 to 50 years who received treatment with a Dana-Farber Cancer Institute (DFCI) pediatric ALL protocol had a regimen completion rate of 60.8%, with 8.4% of patients going for HCT in CR1.12 Comparatively, patients aged 18 to 39 years who received this regimen had a completion rate of 57.3%.12 This higher rate of completion may have been due to differences in the patient population and treatment circumstances, including more concise geography and relatively high rate of safety net resources in states in the US Northeast.
Taken together, our study adds to the knowledge regarding the feasibility and efficacy of applying pediatric style chemotherapy outside of a clinical trial. Our results are remarkably similar to the results reported by the trial, which is an important finding and validates the wide adoption of this regimen by practitioners who treat ALL in AYAs. A limitation of our study is the lack of generalizability: although our cohort was treated in the “real world,” all patients received treatment at National Cancer Institute–designated cancer centers/specialized treatment centers, which has been associated with improved OS.13,14 Considering this study is retrospective, assessment of prespecified AEs may contain inaccuracy or omission, although AE reporting in the context of prospective trials is also imperfect. We also acknowledge that many advances in ALL treatment occurred during the time frame in which our cohort was treated, including the use of blinatumomab for MRD-positive patients and the increasing use of NGS-MRD testing, which may have favorably biased our outcomes. Finally, our modest sample size and heterogeneity of genetic interrogation at diagnosis limit the ability to make conclusions regarding outcomes of specific genetic subsets, including any possible benefit of transplant in CR1.
Recently, the ECOG 1910 study resulted in the approval of blinatumomab during consolidation for adults with B-ALL.15 In this study, the addition of 4 cycles of blinatumomab to consolidation chemotherapy in MRD-negative patients resulted in an improved 3-year relapse-free survival (80% vs 64%) and OS (85% vs 68%) compared with the chemotherapy-only arm.15 Similarly, data presented from the COG AALL1731 study showed that the addition of 2 cycles of blinatumomab to chemotherapy resulted in improved 3-year DFS (96% vs 87.9%) compared with chemotherapy alone for pediatric patients with newly diagnosed standard-risk B-ALL at an average or high risk of relapse.16 How best to integrate frontline blinatumomab into C10403 is not yet formally established but is being routinely integrated into therapy based on the results of ECOG 1910 and AALL 1731. Outcomes in AYA patients treated as per the C10403 regimen with blinatumomab and whether chemotherapy could be safely de-escalated in the context of blinatumomab require further study. Of note, the successor study to C10403, the Alliance A041501 (ClinicalTrials.gov identifier: NCT03150693) study, is testing the addition of inotuzumab after induction but was suspended due to increased toxicities (primarily infection) late during treatment.17 The study is planned to continue with dose adjustments to inotuzumab, addition of blinatumomab, and recommendations to mitigate risk of severe infection.
In conclusion, among newly diagnosed AYA patients with Ph– ALL and LBL, the C10403 regimen has comparable safety and efficacy in the real world, as was demonstrated in the prospective phase 2 clinical trial. Completion of this regimen through maintenance remains low due to treatment failure, regimen toxicity, and regimen complexity. Thus, further studies are warranted to decrease toxicity and improve outcomes in this regimen, potentially through the addition of novel agents in place of conventional therapies.
Authorship
Contribution: B.D., L.S.M., and M.R.L. designed the study and wrote the manuscript; B.D., M.R.L., R.D.V., A.D., K.R., J.S.G., V.T., S.F., and H.J. were responsible for data collection and validation; E.C.L. contributed to study design and data collection; A.Z. analyzed the data and wrote and edited the manuscript; and A.S.D., M.L., W.S., R.D.C., M.S., and J.T.L. wrote and edited the manuscript.
Conflict-of-interest disclosure: L.S.M. reports advisory/consulting fees from CTI Biopharma, Bristol Myers Squibb (BMS), Autolus, Kite, Pfizer, Cargo, Astellas, Vor Bio, Medexus, and Incyte; research funding from Jasper, Kite, BMS, Adaptive Biotechnologies, Wugen, and Vor Bio. J.T.L. has received consulting fees from Adaptive Biotechnologies, Autolus, Amgen, Kite, Pfizer, and Takeda; research funding from AbbVie; and honoraria from Adaptive Biotechnologies. R.D.C. has received research funding from Amgen, Kite/Gilead, Incyte, Merck, Pfizer, Servier, and Vanda Pharmaceuticals; consultancy fees/honoraria from Autolus, Amgen, Jazz, Kite/Gilead, and Pfizer; membership on an advisory committee for Autolus and PeproMene Bio; and spouse was employed by and owned stock in Seagen. M.R.L. reports research funding to institution from Novartis and AbbVie; consulting fees from Novartis, Jazz, Kite, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Marlise R. Luskin, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Dana 2056, Boston, MA 02215; email: marlise_luskin@dfci.harvard.edu.
References
Author notes
Data are available on request from the corresponding author, Marlise R. Luskin (marlise_luskin@dfci.harvard.edu).
The full-text version of this article contains a data supplement.
Lori S. Muffly and Marlise R. Luskin are joint senior authors.