Key Points
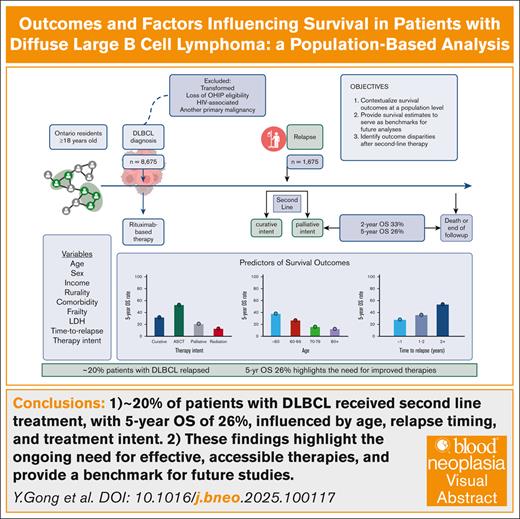
Around 20% of patients with DLBCL received 2L treatment, with a 5-year OS of 26%, influenced by age, relapse timing, and treatment intent.
These findings highlight the ongoing need for effective, accessible therapies and provide a benchmark for future studies.
Visual Abstract
Given the rapidly evolving treatment landscape for diffuse large B-cell lymphoma (DLBCL), we performed a contemporary analysis of survival outcomes in patients aged ≥18 years with DLBCL at the population level using linked administrative data sets in Ontario, Canada (ICES). Among 8675 patients (median age, 67 years; 44% female) treated with frontline rituximab-based therapy, 1675 (19%) were treated with second-line therapy (2L). The 2-year and 5-year overall survival (OS) from 2L were 33% and 26%, respectively. Univariate analysis demonstrated that curative-intent therapy (autologous stem cell transplantation [ASCT]) (58% of patients) was associated with better OS than palliative radiotherapy (hazard ratio [HR], 0.56; P < .0001). Patients aged ≥60 years showed inferior OS than those aged <60 years (age 60-69 years: HR, 1.35; P =.0002; aged 70-79 years: HR, 1.64; P < .0001; age ≥80 years: HR, 2.08; P < .0001). In addition, early relapse was associated with worse outcomes than relapses occurring after 2 years (<3 months: HR, 1.45; P =.0002; 3-6 months: HR, 1.51; P =.0001; 6-12 months: HR, 1.88; P < .0001). Multivariable analysis confirmed these associations while accounting for lactate dehydrogenase, comorbidity burden, frailty, and income. Exploratory analysis indicated that third-line chimeric antigen receptor T-cell (CAR-T) therapy was associated with improved outcomes compared to a historical cohort of patients treated with palliative therapy before 2020 (2-year OS 56% vs 21%). This population-based analysis suggests that curative-intent therapy (ASCT and CAR-T) is associated with improved OS over conventional treatment approaches. The outcomes presented here provide benchmarks for future analyses aimed at assessing the effects of novel treatments in the 2L on outcomes.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma, accounting for 30% to 40% of cases globally.1 Despite the introduction of rituximab,2-4 one-third of patients will experience relapsed or refractory (R/R) disease.5-7 The standard of care (SOC) for R/R DLBCL for eligible patients has been salvage chemotherapy (SC) followed by autologous stem cell transplantation (ASCT) for chemotherapy-sensitive disease.5 However, most patients either do not respond to SC or progress after ASCT.8,9
Recent novel therapies have emerged, improving the management of R/R disease and are now being evaluated in the frontline setting. Although R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) remains the standard frontline treatment, a polatuzumab-based regimen (polatuzumab vedotin, cyclophosphamide, doxorubicin, prednisone, and rituximab) has shown promise in improving progression-free survival.10,11 In addition, randomized trials evaluating new treatments and risk-adapted approaches for frontline DLBCL are ongoing. In the R/R setting, T-cell-directed therapies, including chimeric antigen receptor T-cell (CAR-T) therapy and bispecific engagers, have reshaped treatment.12-18 Initially approved for third-line use,14-16 CAR-T therapy is now the SOC for second-line treatment in patients with early relapse, demonstrating superior overall survival (OS) over ASCT.17,18
Although prior studies have reported on outcomes of unselected patients with DLBCL,19-22 a comprehensive population-based evaluation of outcomes following the availability of CAR-T therapy remains limited. This study aimed to contextualize treatment strategies and survival outcomes for an unselected cohort of patients with DLBCL in Ontario, from primary treatment and beyond.
Methods
Study design
This was a population-based retrospective cohort study using linked population-based administrative data in Ontario, Canada, held at ICES (formerly the Institute for Clinical Evaluative Sciences) using unique encoded identifiers. The data set includes anonymized, individual-level records for all 14 million residents of Ontario. Use of the data in this project is authorized under Section 45 of Ontario’s Personal Health Information Protection Act and does not require review by a research ethics board.
Study population and cohort creation
The study cohort consisted of patients aged ≥18 years with newly diagnosed DLBCL and received first-line curative treatment with rituximab-containing chemoimmunotherapy between 1 January 2012 and 31 December 2022. Patients with DLBCL were identified through the Ontario Cancer Registry (OCR), using International Classification of Diseases for Oncology, third revision codes, and the New Drug Funding Program (NDFP) in Ontario. The OCR is a province-wide cancer registry that captures >95% of cancer diagnoses and all cancer deaths in Ontario residents since 1964.23,24 The NDFP is a publicly funded drug program that covers the cost of many cancer drugs, including rituximab. The following exclusion criteria were applied: central nervous system, HIV-associated, and transformed lymphomas, invalid health card number, and any other cancer (ie, not DLBCL) in OCR in the 2 years before cohort entry date or anytime during follow-up.
Second-line definition
As the date of relapse is not available in administrative records, patients who received second-line treatment were considered as a surrogate for relapse. The criteria for the second-line of treatment were: curative-intent treatment was defined as receipt of platinum-based SC, or mobilization regimen, with or without ASCT; palliative-intent treatment encompassed nonplatinum based systemic chemotherapy, oral therapies (etoposide, cyclophosphamide, or procarbazine) identified through the Ontario Drug Database (ODB) in those aged ≥65 years, or palliative-intent radiotherapy administered >3 months after the completion of frontline therapy (to prevent misclassification as consolidation radiotherapy).
Systemic therapies and radiation treatments were identified through the Cancer Activity Level Reporting database, which contains patient-level data on radiation and systemic therapy services. The ODB contains all prescription medications for all Ontario residents ≥65 years.25
Covariates
Demographic information included age, sex, and socioeconomic information, including income quintile (based on residential postal code) and rurality. Age was categorized into the following groups: <60, 60-69, 70-79, and ≥80 years. Comorbidity burden was measured using the Johns Hopkins Adjusted Clinical Groups System, Version 10,16 whereby patients were assigned up to 32 aggregated diagnosis groups (ADGs) to characterize their comorbidity burden in the 2 years before the end of first-line treatment (cancer ADGs was excluded). Performance status at the time of first- or second-line treatment was available for a subset of patients and was assessed using the Patient Reported Functional Status (PRFS), categorized as 0 to 1 or ≥2.26,27
Lactate dehydrogenase (LDH) levels at first- or second-line treatment were obtained from the Ontario Laboratory Information System, categorized as <200 U/L, 200-400 U/L, >400 U/L, or unknown. Time to diagnosis to treatment (DTI) was defined as the time from diagnosis to initiation of rituximab-based chemoimmunotherapy.28-30
Treatment-related covariates included the intent of second-line treatment (curative or palliative), and time to second-line treatment (time to next treatment [TTNT]), categorized into ≤3, 3-6, 6-12, 12-24, and ≥24 months.
Outcomes
The outcomes were as follows: OS1, defined as the time from the initiation of first-line treatment to the date of last follow-up or death; OS2, defined as the duration from the initiation of second-line treatment to the date of last follow-up or death. Secondary outcomes included (1) progression of lymphoma, which could not be captured through administrative databases, thus operationalized as relapse-free survival (RFS), defined as the initiation of second-line DLBCL treatment defined earlier, censoring on last follow-up, or death; and (2) lymphoma-specific death.
Exploratory analysis
We conducted an exploratory analysis comparing outcomes of patients who received third-line CAR-T therapy at 1 of 3 cellular therapy institutions in Ontario, beginning in 2020 when public funding for CAR-T therapy became available in Ontario, to a historical cohort. The comparator group included only patients who received third-line palliative-intent therapy after second-line curative treatment, between 2012 and 2020, to exclude those who may have received CAR-T therapy. The exploratory outcome was OS3, defined as the time from third-line treatment to date of last follow-up or death.
Statistical analysis
Baseline data were analyzed using descriptive statistics. Baseline characteristics were compared between patients who received second-line treatment and those who did not using standardized differences (<10% indicates balance).31
OS probabilities were estimated using Kaplan-Meier curves. Multivariable Cox proportional hazard regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for factors associated with OS1 and RFS. Multivariable models adjusted for demographic covariates, comorbidity burden, frailty, and DTI time. Sensitivity analyses included known stage or PRFS, respectively.
Similarly, univariate and multivariable Cox models were used for OS2, adjusting for demographic factors, comorbidity, frailty, intent of treatment, TTNT, and LDH. Proportional hazards and linearity assumptions were checked. Sensitivity analyses included known stage or PRFS, respectively. Additional sensitivity analyses examined whether age (<70 or ≥70 years) modified the association between treatment intent or TTNT and OS. A significant interaction term would indicate that the prognostic factors differ between younger and older patients.
Cumulative incidence functions for lymphoma-related deaths were estimated while accounting for the competing risk of non-lymphoma-related death. Time to lymphoma-related death was modeled using a cause-specific Cox proportional hazard model.
For the exploratory analysis third-line comparison, inverse probability of treatment weighting (IPTW) was used to adjust for confounding covariates in survival analysis.32 Propensity scores for CAR-T therapy vs comparator group were calculated using logistic regression accounting for age, sex, comorbidity burden, frailty, the interval between first-line and second-line treatment, and second-line and third-line treatment, respectively. Covariate balance was assessed using standardized differences.
For all analyses, a 2-tailed P value of <.05 was deemed statistically significant. Analyses were performed using SAS software, version 9.4 (Cary, NC).
Results
Study population
We identified a cohort of 8675 patients diagnosed with DLBCL who initiated treatment with rituximab-based chemoimmunotherapy (Table 1). The median age of the entire cohort was 68 years (interquartile range [IQR], 57-76 years), with 45% of patients aged ≥70 years, 44% female, 58% presenting with advanced-stage (III-IV) disease among those with available staging information (51%), and 36% with PRFS 2-4 among those with available PRFS information (37%). Patients received a median of 6 cycles of primary therapy (IQR, 4-6).
Patient characteristics of the overall cohort, and by relapse status
| . | All patients (N = 8675) . | No second-line treatment (n = 7000) . | Second-line treatment (n = 1675) . | Standardized difference . |
|---|---|---|---|---|
| Age at cohort entry, y | ||||
| Median (Q1-Q3) | 68 (57-76) | 68 (57-77) | 67 (57-75) | 0.08 |
| 18-60, n (%) | 2585 (29.8) | 2063 (29.5) | 522 (31.2) | 0.04 |
| 60-69, n (%) | 2179 (25.1) | 1705 (24.4%) | 474 (28.3) | 0.09 |
| 70-79, n (%) | 2463 (28.4) | 2016 (28.8) | 447 (26.7) | 0.05 |
| ≥80, n (%) | 1448 (16.7) | 1216 (17.4) | 232 (13.9) | 0.10 |
| Sex, n (%) | ||||
| Female | 3851 (44.4) | 3180 (45.4) | 671 (40.1) | 0.11 |
| Income quintile, n (%) | ||||
| 1 | 1577 (18.2) | 1289 (18.4) | 285-289∗ | 0.03 |
| 2 | 1774 (20.4) | 1444 (20.6) | 330 (19.7) | 0.02 |
| 3 | 1713 (19.7) | 1350 (19.3) | 363 (21.7) | 0.06 |
| 4 | 1678 (19.3) | 1350 (19.3) | 328 (19.6) | 0.007 |
| 5 | 1914 (22.1) | 1550 (22.1) | 364 (21.7) | 0.01 |
| Rural residence, n (%) | 1124 (13.0) | 902 (12.9) | 218-222∗ | 0.01 |
| Cancer stage at diagnosis, n (%) | ||||
| I | 886 (10.2) | 800 (11.4) | 86 (5.1) | 0.23 |
| II | 971 (11.2) | 838 (12.0) | 133 (7.9) | 0.14 |
| III | 687 (7.9) | 510 (7.3) | 177 (10.6) | 0.12 |
| IV | 1891 (21.8) | 1393 (19.9) | 498 (29.7) | 0.23 |
| Missing | 4240 (48.9) | 3459 (49.4) | 781 (46.6) | 0.06 |
| Days from diagnosis to treatment, n (%) | ||||
| 0-28 | 2459 (28.3) | 1914 (27.3) | 545 (32.5) | 0.11 |
| 29-56 | 4046 (46.6) | 3324 (47.5) | 722 (43.1) | 0.09 |
| ≥57 | 2170 (25.0) | 1762 (25.2) | 408 (24.4) | 0.02 |
| No. of cycles of frontline treatment | ||||
| Median (Q1-Q3) | 6 (4-6) | 6 (4-6) | 6 (5-6) | 0.04 |
| Noncancer ADGs | ||||
| Median (Q1-Q3) | 10 (8-13) | 10 (8-13) | 10 (8-13) | 0.01 |
| Frailty (ACG-based), n (%) | 1237 (14.3) | 1008 (14.4) | 229 (13.7) | 0.02 |
| Year initiated frontline therapy, n (%) | ||||
| 2012-2015 | 2968 (34.2) | 2319 (33.1) | 649 (38.7) | 0.12 |
| 2016-2019 | 3131 (36.1) | 2487 (35.5) | 644 (38.4) | 0.06 |
| 2020-2022 | 2576 (29.7) | 2194 (31.3) | 382 (22.8) | 0.19 |
| . | All patients (N = 8675) . | No second-line treatment (n = 7000) . | Second-line treatment (n = 1675) . | Standardized difference . |
|---|---|---|---|---|
| Age at cohort entry, y | ||||
| Median (Q1-Q3) | 68 (57-76) | 68 (57-77) | 67 (57-75) | 0.08 |
| 18-60, n (%) | 2585 (29.8) | 2063 (29.5) | 522 (31.2) | 0.04 |
| 60-69, n (%) | 2179 (25.1) | 1705 (24.4%) | 474 (28.3) | 0.09 |
| 70-79, n (%) | 2463 (28.4) | 2016 (28.8) | 447 (26.7) | 0.05 |
| ≥80, n (%) | 1448 (16.7) | 1216 (17.4) | 232 (13.9) | 0.10 |
| Sex, n (%) | ||||
| Female | 3851 (44.4) | 3180 (45.4) | 671 (40.1) | 0.11 |
| Income quintile, n (%) | ||||
| 1 | 1577 (18.2) | 1289 (18.4) | 285-289∗ | 0.03 |
| 2 | 1774 (20.4) | 1444 (20.6) | 330 (19.7) | 0.02 |
| 3 | 1713 (19.7) | 1350 (19.3) | 363 (21.7) | 0.06 |
| 4 | 1678 (19.3) | 1350 (19.3) | 328 (19.6) | 0.007 |
| 5 | 1914 (22.1) | 1550 (22.1) | 364 (21.7) | 0.01 |
| Rural residence, n (%) | 1124 (13.0) | 902 (12.9) | 218-222∗ | 0.01 |
| Cancer stage at diagnosis, n (%) | ||||
| I | 886 (10.2) | 800 (11.4) | 86 (5.1) | 0.23 |
| II | 971 (11.2) | 838 (12.0) | 133 (7.9) | 0.14 |
| III | 687 (7.9) | 510 (7.3) | 177 (10.6) | 0.12 |
| IV | 1891 (21.8) | 1393 (19.9) | 498 (29.7) | 0.23 |
| Missing | 4240 (48.9) | 3459 (49.4) | 781 (46.6) | 0.06 |
| Days from diagnosis to treatment, n (%) | ||||
| 0-28 | 2459 (28.3) | 1914 (27.3) | 545 (32.5) | 0.11 |
| 29-56 | 4046 (46.6) | 3324 (47.5) | 722 (43.1) | 0.09 |
| ≥57 | 2170 (25.0) | 1762 (25.2) | 408 (24.4) | 0.02 |
| No. of cycles of frontline treatment | ||||
| Median (Q1-Q3) | 6 (4-6) | 6 (4-6) | 6 (5-6) | 0.04 |
| Noncancer ADGs | ||||
| Median (Q1-Q3) | 10 (8-13) | 10 (8-13) | 10 (8-13) | 0.01 |
| Frailty (ACG-based), n (%) | 1237 (14.3) | 1008 (14.4) | 229 (13.7) | 0.02 |
| Year initiated frontline therapy, n (%) | ||||
| 2012-2015 | 2968 (34.2) | 2319 (33.1) | 649 (38.7) | 0.12 |
| 2016-2019 | 3131 (36.1) | 2487 (35.5) | 644 (38.4) | 0.06 |
| 2020-2022 | 2576 (29.7) | 2194 (31.3) | 382 (22.8) | 0.19 |
Reported as numbers and percentages, unless otherwise specified.
ADGs, aggregated diagnosis groups; Q1, first quartile; Q3, third quartile.
Refers to the use of a range of numbers to minimize the risk of reidentification, in accordance with ICES policies.
Among this cohort, 1675 patients (19%) received second-line treatment, serving as a surrogate of R/R disease. The median age of these patients was 67 years (IQR, 57-75 years), with 996 (59%) aged <70 years, 679 (41%) aged ≥70 years, and 232 (14%) aged ≥80 years, 40% were female, and 76% had advanced-stage disease where stage was available (53%).
Outcomes following first-line treatment
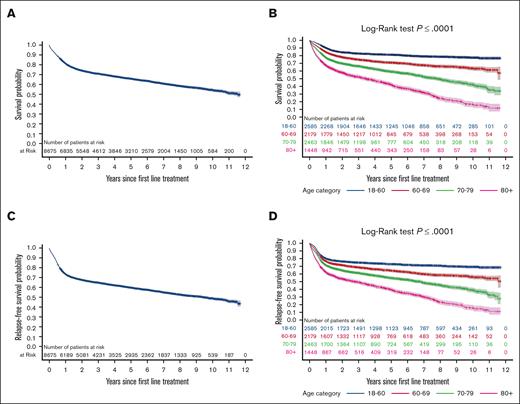
With a median follow-up of 3.4 years (IQR, 1.2-6.7 years), the 2-year and 5-year OS1 were 74% and 66%, respectively, with an OS1 of 50% overall (Figure 1A). The 2-year RFS rates were 68%, 60% at 5 years, and 46% at the end of follow-up (Figure 1C).
Kaplan-Meier survival curves illustrating survival outcomes of patients following first-line treatment. (A) OS (OS1) for the entire cohort during follow-up. (B) OS1 stratified by age. (C) RFS during the follow-up. (D) RFS stratified by age.
Kaplan-Meier survival curves illustrating survival outcomes of patients following first-line treatment. (A) OS (OS1) for the entire cohort during follow-up. (B) OS1 stratified by age. (C) RFS during the follow-up. (D) RFS stratified by age.
Both OS1 and RFS decreased with increasing age, suggesting inferior outcomes in older patients compared to those <60 years (Figure 1B,D). In the multivariable analysis (adjusting for sex, income, rurality, frailty, and comorbidity burden), independent predictors of OS1 included older age compared with those aged <60 years (aged 60-69 years: adjusted HR [aHR], 1.51; 95% CI, 1.34-1.69; P < .0001; aged 70-79 years: aHR, 2.30; 95% CI, 2.06-2.56; P < .0001; aged ≥80 years: aHR, 3.64; 95% CI, 3.25-4.09; P < .0001), elevated LDH levels (LDH, 200-399 U/L: aHR, 1.41; 95% CI, 1.25-1.58; P < .0001; ≥400 U/L: aHR, 2.22; 95% CI, 1.96-2.50; P < .0001), and a shorter DTI of 0-28 days compared with ≥57 days (aHR, 1.18; 95% CI, 1.07-1.30; P =.0009; DTI, 29-56 days: aHR, 0.97; 95% CI, 0.88-1.05; P =.43; Table 2). Similar associations were observed for the multivariable Cox regression model for the RFS outcome (supplemental Table 2). Sensitivity analyses on the multivariable model, including stage or PRFS, did not change the aforementioned associations when these additional predictors were included (supplemental Table 3).
Adjusted Cox regression analyses to identify predictors of OS from first-line therapy
| . | Adjusted∗ . | |
|---|---|---|
| HR (95% CI) . | P value . | |
| Age | ||
| 60-69† | 1.51 (1.34-1.69) | <.0001 |
| 70-79† | 2.30 (2.06-2.56) | <.0001 |
| ≥80† | 3.64 (3.25-4.09) | <.0001 |
| Sex | ||
| Male | 1.26 (1.17-1.35) | <.0001 |
| Days from diagnosis to treatment‡ | ||
| 0-28 | 1.18 (1.07-1.30) | .0009 |
| 29-56 | 0.97 (0.88-1.05) | .42 |
| LDH | ||
| 200-399 | 1.14 (1.02-1.26) | .017 |
| ≥400 | 1.59 (1.48-1.7) | <.0001 |
| Unknown | 1.06 (1.05-1.06) | <.0001 |
| . | Adjusted∗ . | |
|---|---|---|
| HR (95% CI) . | P value . | |
| Age | ||
| 60-69† | 1.51 (1.34-1.69) | <.0001 |
| 70-79† | 2.30 (2.06-2.56) | <.0001 |
| ≥80† | 3.64 (3.25-4.09) | <.0001 |
| Sex | ||
| Male | 1.26 (1.17-1.35) | <.0001 |
| Days from diagnosis to treatment‡ | ||
| 0-28 | 1.18 (1.07-1.30) | .0009 |
| 29-56 | 0.97 (0.88-1.05) | .42 |
| LDH | ||
| 200-399 | 1.14 (1.02-1.26) | .017 |
| ≥400 | 1.59 (1.48-1.7) | <.0001 |
| Unknown | 1.06 (1.05-1.06) | <.0001 |
Adjusted for frailty, comorbidity burden as measured by aggregated diagnosis groups, income quintile, and rurality.
Reference group, age 18 to 59 years.
Reference group, >56 days.
Among those undergoing second-line therapy (supplemental Tables 4 and 5), 976 patients (58%) received curative-intent SC, of whom 440 patients underwent ASCT (45% of those receiving SC, or 26% of the overall R/R cohort). In addition, 338 patients (20%) received palliative-intent systemic therapy (146 oral therapies, 17 investigational treatments, and 175 multiagent regimens), whereas 361 patients (22%) underwent palliative-intent radiotherapy.
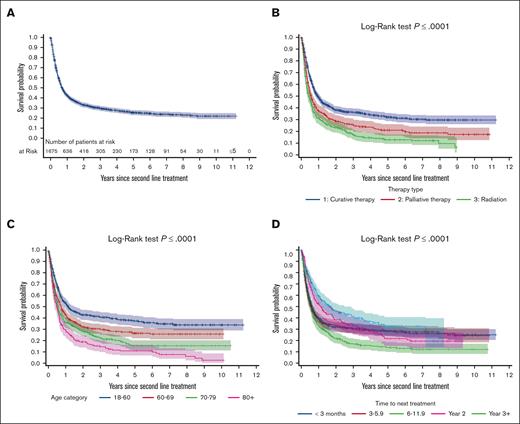
Outcomes from second-line treatment
With a median follow-up of 0.62 years (IQR, 0.25-2) from second-line treatment, the 2-year and 5-year OS2 of patients with R/R DLBCL were 33% and 26%, respectively (Figure 2A). To better characterize factors determining OS2, we evaluated OS2 stratified by age, curative vs palliative treatment, and TTNT. In univariate Cox regression analysis, curative-intent second-line treatment significantly improved OS2 compared to palliative radiation (HR, 0.56; 95% CI, 0.49-0.65; P < .0001), as did palliative-intent systemic therapy (HR, 0.79; 95% CI, 0.66-0.93; P =.006; Table 3). The 5-year OS2 for the curative-intent population was 32% (95% CI, 29-36), and 53% (95% CI, 47-58) in patients who received transplant, whereas OS2 was only 17% (95% CI, 14-20) for the palliative-intent population (Figure 2B; supplemental Table 6).
Kaplan-Meier survival curves illustrating survival outcomes of patients from the time of relapse. (A) OS (OS2) for all patients who received second-line treatment to the end of the follow-up. (B) OS2 is categorized by treatment intent, distinguishing between curative and palliative therapies. (C) OS2 stratified by age. (D) OS2 based on the time to first relapse.
Kaplan-Meier survival curves illustrating survival outcomes of patients from the time of relapse. (A) OS (OS2) for all patients who received second-line treatment to the end of the follow-up. (B) OS2 is categorized by treatment intent, distinguishing between curative and palliative therapies. (C) OS2 stratified by age. (D) OS2 based on the time to first relapse.
Unadjusted and adjusted Cox regression analyses to identify predictors of OS after second-line therapy
| . | Unadjusted . | Adjusted∗ . | ||
|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Age, y | ||||
| 60-69† | 1.35 (1.16-1.59) | .0002 | - | |
| 70-79† | 1.64 (1.40-1.92) | <.0001 | - | - |
| ≥80† | 2.08 (1.73-2.49) | <.0001 | - | - |
| Age | ||||
| 10-year increment | 1.17 (1.11-1.23) | <.0001 | ||
| TTNT, mo | ||||
| <3‡ | 1.45 (1.19-1.77) | .0003 | 1.58 (1.29-1.94) | <.0001 |
| 3-5.9‡ | 1.51 (1.23-1.85) | <.0001 | 1.34 (1.0-1.65) | .007 |
| 6-11.9‡ | 1.88 (1.53-2.41) | .23 | 1.69 (1.37-2.08) | <.0001 |
| 12-23.9‡ | 1.27 (1.00-1.61) | .20 | 1.22 (0.96-1.56) | .10 |
| Treatment intent | ||||
| Curative systemic§ | 0.56 (0.49-0.65) | <.0001 | 0.70 (0.59-0.83) | <.0001 |
| Palliative systemic§ | 0.79 (0.66-0.93) | .005 | 0.71 (0.59-0.86) | .0003 |
| LDH | ||||
| 200-399 | 1.14 (1.02-1.26) | .017 | 1.40 (1.18-1.66) | .0001 |
| ≥400 | 1.59 (1.48-1.7) | <.0001 | 2.52 (2.11-3.02) | <.0001 |
| Unknown | 1.06 (1.05-1.06) | <.0001 | 1.51 (1.25-1.82) | <.0001 |
| . | Unadjusted . | Adjusted∗ . | ||
|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Age, y | ||||
| 60-69† | 1.35 (1.16-1.59) | .0002 | - | |
| 70-79† | 1.64 (1.40-1.92) | <.0001 | - | - |
| ≥80† | 2.08 (1.73-2.49) | <.0001 | - | - |
| Age | ||||
| 10-year increment | 1.17 (1.11-1.23) | <.0001 | ||
| TTNT, mo | ||||
| <3‡ | 1.45 (1.19-1.77) | .0003 | 1.58 (1.29-1.94) | <.0001 |
| 3-5.9‡ | 1.51 (1.23-1.85) | <.0001 | 1.34 (1.0-1.65) | .007 |
| 6-11.9‡ | 1.88 (1.53-2.41) | .23 | 1.69 (1.37-2.08) | <.0001 |
| 12-23.9‡ | 1.27 (1.00-1.61) | .20 | 1.22 (0.96-1.56) | .10 |
| Treatment intent | ||||
| Curative systemic§ | 0.56 (0.49-0.65) | <.0001 | 0.70 (0.59-0.83) | <.0001 |
| Palliative systemic§ | 0.79 (0.66-0.93) | .005 | 0.71 (0.59-0.86) | .0003 |
| LDH | ||||
| 200-399 | 1.14 (1.02-1.26) | .017 | 1.40 (1.18-1.66) | .0001 |
| ≥400 | 1.59 (1.48-1.7) | <.0001 | 2.52 (2.11-3.02) | <.0001 |
| Unknown | 1.06 (1.05-1.06) | <.0001 | 1.51 (1.25-1.82) | <.0001 |
Adjusted for sex, frailty, comorbidity burden as measured by aggregated disease groups, income quintile, and rurality.
Reference group, age 18-59 years.
Reference group, ≥24 months.
Reference group, palliative radiation.
Increasing age was associated with inferior OS2 compared with patients aged <60 years (aged 60-69 years: HR, 1.35; 95% CI, 1.16-1.59; P =.0002; aged 70-79 years: HR, 1.64; 95% CI, 1.40-1.92; P < .0001; aged ≥80 years: HR, 2.08; 95% CI, 1.73-2.49; P < .0001). Patients aged 18-60 years had more favorable OS2 rates, with 43% at 2 years and 38% at 5 years, whereas those aged ≥80 years had the lowest rates, with 20% at 2 years and 12% at 5 years (Table 4). Age also correlated with the use of ASCT. Among 179 patients aged ≥70 years who received curative-intent therapy, a lower proportion underwent consolidation with ASCT than those aged <70 years. Specifically, 58% (260/450) of patients aged 18-60 years received ASCT, compared with just 12% (22/179) of patients aged ≥70 years (supplemental Table 7).
OS rates from second-line treatment to end of follow-up or death from any cause by age and time to second-line treatment
| . | 2-year survival (95% CI), % . | 5-year survival (95% CI), % . |
|---|---|---|
| Age, y | ||
| 18-60 | 43 (39-48) | 38 (33-42) |
| 60-69 | 33 (28-37) | 27 (23-32) |
| 70-79 | 29 (25-34) | 16 (12-21) |
| ≥80 | 20 (15-25) | 12 (7-17) |
| Time to second-line treatment, y | ||
| <1 | 32 (29-36) | 28 (25-32) |
| 1-2 | 52 (41-61) | 36 (25-47) |
| ≥2 | 60 (51-68) | 54 (44-63) |
| . | 2-year survival (95% CI), % . | 5-year survival (95% CI), % . |
|---|---|---|
| Age, y | ||
| 18-60 | 43 (39-48) | 38 (33-42) |
| 60-69 | 33 (28-37) | 27 (23-32) |
| 70-79 | 29 (25-34) | 16 (12-21) |
| ≥80 | 20 (15-25) | 12 (7-17) |
| Time to second-line treatment, y | ||
| <1 | 32 (29-36) | 28 (25-32) |
| 1-2 | 52 (41-61) | 36 (25-47) |
| ≥2 | 60 (51-68) | 54 (44-63) |
Early relapse, as defined here by TTNT <1 year, was observed in 1231 patients (73% of the R/R cohort). This group exhibited worse OS2 outcomes (TTNT, <3 months: HR, 1.45; 95% CI, 1.19-1.77; P =.0002; TTNT, 3-6 months: HR, 1.51; 95% CI, 1.23-1.85; P =.0001; TTNT, 6-12 months: HR, 1.88; 95% CI, 1.53-2.31; P < .0001) compared to those with late TTNT, defined as ≥2 years. Conversely, patients with TTNT of 12-24 months did not demonstrate statistically significantly inferior outcomes relative to those with TTNT ≥2 years (HR, 1.27; 95% CI, 0.99-1.61; P =.054; Table 3). OS2 survival rates stratified by TTNT are shown in Figure 2D and Table 4. Treatment details according to TTNT are presented in supplemental Table 8.
In multivariable analysis (adjusting for sex, income, rurality, frailty, and comorbidity burden), independent predictors of OS2 included older age (per 10-year increase; aHR, 1.17; 95% CI, 1.11-1.23; P < .0001), early relapse (TTNT, <3 months: aHR, 1.59; 95% CI, 1.29-1.94; P < .0001; TTNT 3-5.9 months: aHR, 1.34; 95% CI, 1.0-1.65; P =.007; TTNT, 6-11.9 months: aHR, 1.69; 95% CI, 1.37-2.08; P < .0001), and elevated LDH levels (LDH, 200-399 U/L: aHR, 1.40; 95% CI, 1.18-1.66; P =.0001; ≥400 U/L: aHR, 2.52; 95% CI, 2.11-3.02; P < .0001; Table 3; supplemental Figure 1). Furthermore, both curative-intent and palliative-intent therapies were associated with improved OS over palliative radiation (curative intent: aHR, 0.70; 95% CI, 0.59-0.83; palliative systemic therapy: aHR, 0.71; 95% CI, 0.59-0.86; Table 3).
Sensitivity analyses for the OS2 multivariable model, including stage or PRFS, did not alter the associations with key predictors of interest (age, TTNT, and intent of treatment; supplemental Tables 9 and 10).To evaluate the differential effects of age, TTNT, and treatment intent, we performed a sensitivity analysis using Cox regression incorporating interactions of these factors. Analysis of the interaction between age and TTNT revealed that for patients aged <70 years, early relapse (<1 year) was associated with significantly worse OS2, whereas for patients aged ≥70 years, early relapse was not significantly associated with worse OS2 than late relapse. The interaction analysis between age and treatment intent showed that the benefits of curative and palliative-intent systemic therapies over radiation therapy were more pronounced in patients <70 years, and inferior OS2 outcomes for older patients were observed across treatment intents (supplemental Table 11).
Cause-specific death
A total of 3050 deaths (35% of cohort) were observed. Of the 2539 (83%) deaths with recorded cause, 78.6% were attributed to lymphoma, 2.2% to other cancers, and 19.2% to noncancer causes (supplemental Figure 2). Among patients who received second-line treatment, there were 1143 deaths, of which 90.9% with known cause were lymphoma-related, 1.3% were from other cancers, and 7.8% were noncancer-related (supplemental Figure 2). The 2-year and 5-year cumulative incidences of lymphoma-related death were 19.5% (95% CI, 18.7-20.4) and 23% (95% CI, 22.5-24.3), respectively (supplemental Figure 3). Cumulative incidence functions showed that lymphoma deaths peaked early and plateaued ∼2 years after first treatment, whereas non-lymphoma-related deaths increased steadily over time. Following second-line treatment, the 2-year and 5-year incidences were 52.5% (95% CI, 50.0-55.0) and 56.6% (95% CI, 54.0-59.1; supplemental Figure 4). Cause-specific Cox regression, censoring on non-lymphoma death, confirmed the impact of age, treatment intent, and TTNT on survival outcomes (supplemental Table 12; supplemental Figure 4).
Third-line therapy
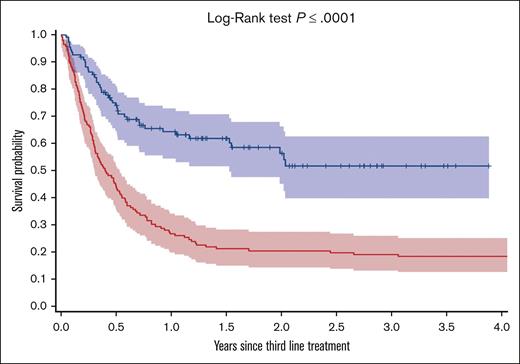
In an exploratory analysis, 110 patients who received CAR-T therapy at 3 Ontario centers from 2020 onward were compared to a historical control cohort of 147 patients who received third-line therapy without CAR-T therapy (supplemental Table 13 for treatment details). IPTW-adjusted covariates were well balanced between the groups, including age and time to treatment (supplemental Table 14). Patients in the CAR-T therapy cohort had significantly improved OS3 compared with the control group (2-year OS3, 56% [95% CI, 45-66] vs 21% [95% CI, 15-28], P < .0001; Figure 3; IPTW-weighted: HR,0.40; 95% CI, 0.28-0.58; P < .0001).
Kaplan-Meier survival curves illustrating survival outcomes for patients receiving CAR-T therapy in comparison to those undergoing palliative therapy as a third-line treatment.
Kaplan-Meier survival curves illustrating survival outcomes for patients receiving CAR-T therapy in comparison to those undergoing palliative therapy as a third-line treatment.
Discussion
In this contemporary population-based study, ∼20% of patients with DLBCL received second-line treatment. Among these, 679 patients (41%) were aged ≥70 years, with 232 (14%) aged ≥80. Although 58% of patients received curative-intent treatment (defined here as platinum-containing SC), the 5-year survival rate following second-line treatment was only 26%, underscoring the poor prognosis for these patients. Several factors were found to be strongly associated with inferior survival outcomes, including increasing age, early relapse (within 12 months of first-line treatment), and palliative-intent treatment. These worse outcomes observed in older patients with DLBCL highlight an urgent need for tailored, age-appropriate treatment strategies to address this disparity. As the treatment landscape is rapidly evolving, additional studies are needed to evaluate the outcomes of patients who receive CAR-T therapy in earlier lines of therapy and those who do not receive CAR-T therapy. The outcomes presented here provide important benchmarks for such future analyses.
The 5-year OS of 66% in the frontline setting and 26% in the second line, aligns with prior retrospective analyses from the rituximab era.20,33,34 A recent Swedish analysis of 737 patients with R/R DLBCL (median age 71 years) noted a similar relapse rate of 23%, with a 2-year OS of 27%, in a cohort of patients where 64% underwent curative-intent second-line treatment (34% completed ASCT); this is comparable to the 58% 2-year OS in this study in which 45% completed ASCT.20 Patients who received curative-intent treatment had improved outcomes compared to those who received palliative-intent treatment, with a 5-year OS2 of 32% vs 17%. This is particularly true for patients consolidated with ASCT (∼50%) who had 5-year OS2 of 53%. In contrast, the other half of patients treated with curative intent who do not undergo ASCT had very poor outcomes.35 In this context, 2 recent randomized trials have demonstrated improved survival for those with early relapse, establishing CD19-directed CAR-T therapy, axicabtagene ciloleucel, and lisocabtagene maraleucel, as the new SOC for high-risk transplant-eligible patients with early relapse.17,18 Moreover, continued development of novel therapies could further broaden access to curative treatments for a wider range of patients, including older, more frail patients and those who relapse after CAR-T therapy.36-38 Hence, the population-based survival rates presented here offer valuable benchmarks for future analyses to assess whether incorporating novel treatments into earlier lines of therapy improves survival outcomes.
Our study confirms that age is a critical prognostic factor for survival in patients with R/R DLBCL, with a significant decline in 5-year survival rates as age increases, both for first-line and second-line treatment. Patients aged <60 years had a 5-year OS2 rate of 38%, which dropped to just 12% for those aged >80 years, and a 17% relative increased risk of mortality for each additional decade of life. These findings are consistent with existing literature and contribute to the understanding of outcomes by age in the R/R setting.20,22,39-41 Several key observations can be drawn from our results. In this cohort, nearly half of the patients were aged ≥70 years, and those receiving standard chemotherapy without ASCT had poor outcomes, with a 5-year OS2 of only 15%. However, older patients who underwent ASCT had similar outcomes to younger patients, consistent with prior reports,42,43 suggesting that age alone should not be a barrier to curative-intent treatments. This is especially relevant now that second-line CAR-T therapy has become the SOC, with recent single-arm trials demonstrating efficacy in transplant-ineligible patients (often based on age cutoffs) for axicabtagene ciloleucel and lisocabtagene maraleucel.44,45 Although a study by Chihara et al found that patients aged ≥75 years were less likely to receive CAR-T therapy, yet subgroup analyses and retrospective real-world studies show comparable outcomes in both older and younger patients.44-54 As such, age-based eligibility criteria traditionally used for ASCT may not apply to CAR-T therapy, offering a curative option for patients previously ineligible for ASCT. Currently, no standardized criteria for CAR-T therapy eligibility exist. Future research expanding our understanding of CAR-T therapy–related morbidity and mortality, as well as developing strategies to predict and manage severe toxicities, may help optimize patient selection, balancing the benefits of CAR-T therapy with its associated risks. Finally, the inferior survival outcomes in older patients shown here underscore the ongoing need for age-inclusive therapies that are both tolerable and effective.
Our study reinforces the strong association between early relapse (within 12 months of the end of therapy, 73% of patients9) and inferior survival outcomes, with a 39% increased risk of mortality compared with later relapse. These are consistent with earlier studies in transplant-eligible patients.8,55 Despite the known prognostic impact of early relapse, these patients have historically been treated similarly to those with late relapse using SC and ASCT, with consistently poor outcomes across various settings.20,56,57 To address varying definitions of primary refractory DLBCL in the literature,58-61 Bock et al compared patients with stable or progressive disease at the end of frontline treatment (primary refractory) to those who relapsed within 3, 3-6, and 6-12 months.57 They found that primary refractory patients had the worst outcomes, whereas no significant differences were noted among the early relapse groups. Our results align with Bock et al, showing similar 2-year OS rates within the first year.
The cause-specific analysis showed that most known deaths were due to lymphoma, consistent with prior studies.34,62-64 The cumulative incidence of lymphoma-related death was highest in the first 2 years following frontline treatment, similar to prior observations.63,65,66 This observation varied by age; older patients continued to have a higher risk of lymphoma-related death beyond this timeframe. In contrast, the probability of death from other causes steadily increased during follow-up, likely reflecting the older patients in this cohort.67,68 These data highlight an important strength of our study is its ability to estimate the cumulative incidence of lymphoma deaths and identify the factors influencing lymphoma outcomes while accounting for the competing risk of death from other causes, particularly relevant in older populations.
Our exploratory analysis of patients receiving CAR-T therapy as third-line treatment compared with a cohort receiving palliative-intent regimens before CAR-T therapy became available suggests a potentially significant impact on outcomes, despite the lack of controlled trial data in this setting.56 Despite differences in patient characteristics across clinical trial cohorts (Canadian Clinical Trial Group [CCTG] LY.12, Collaborative Trial in Relapsed Aggressive Lymphoma [CORAL]) and observational institutional cohorts (MD Anderson, The Lymphoma Specialized Program of Research Excellence [SPORE]), which limit direct comparison, our adjusted population-based analysis is consistent with a matched analysis comparing ZUMA-1 CAR-T therapy outcomes to SC across clinical trial cohorts and observational cohorts in refractory lymphoma.69
Due to the nature of administrative databases, misclassification may have occurred. Lymphoma progression based on imaging or biopsy could not be captured. Instead, we used the initiation of the next line of treatment as a surrogate for relapse; as such, the RFS from the first treatment does not accurately reflect true progression-free survival. As a result, we could not calculate second-line DTI or model its association with OS2. The proportion of patients with R/R DLBCL is likely higher than the 20% reported, as our definition includes only those who received second-line treatment and excludes those who were unfit or died before receiving it. However, the proportion receiving second-line treatment aligns with prior reports. Treatment intent (curative vs palliative) was inferred based on regimens; though some patients may have received platinum-based SC without proceeding to ASCT, this is expected to be a small minority. Notably, 45% of those who received SC proceeded to ASCT, which aligns with the expected 50% reported in this setting.70-74 Because of limitations of the ODB, we could not capture all patients <65 receiving oral alkylator therapies (etoposide, cyclophosphamide, or procarbazine). This data set lacked detailed prognostic disease-related factors, including pathologic data (cell of origin, molecular markers), extranodal disease, and advanced imaging features. Finally, treatment availability in Ontario may differ from other jurisdictions, making extrapolation challenging.
Despite these limitations, our study has several strengths. This data set is current and relevant to the era of novel immunotherapies, encompassing patients diagnosed with DLBCL as recently as 2022. The large population-based design effectively captures the diversity of patients and outcomes typical of routine clinical practice. The heterogeneity in outcomes is in part attributed to the independent effect of age, time to relapse, and treatment intent while adjusting for other known determinants of survival. Curative-intent treatment appears to have demonstrable value with favorable OS, and it would appear CAR-T therapy is of benefit with early data captured at a population level.
Conclusion
In conclusion, our study reveals significant heterogeneity in outcomes for patients with DLBCL requiring second-line treatment. These outcomes were influenced by age, early relapse, and intent of treatment. Our data highlight the need for the development and integration of accessible therapies that are both effective and safe, particularly to address the outcome disparities observed in patients with early relapse and advanced age. Strategies to improve outcomes include enhancing frontline treatments to reduce relapse rates and expanding the range of second-line curative options to increase the curable proportion of patients with DLBCL. Collectively, the survival estimates presented here provide valuable benchmarks for future comparisons within the evolving treatment landscape of DLBCL. This is especially relevant as we assess the impact of CAR-T therapy in the second-line setting, the use of polatuzumab-based therapy in the frontline, and the recent approval of bispecific T-cell engagers in the third-line and beyond setting.
Acknowledgments
Parts of this material are based on data and/or information compiled and provided by the Ontario Ministry of Health (MOH), Ontario Health (OH), and Canadian Institute for Health Information (CIHI). This document used data adapted from the Statistics Canada Postal CodeOM Conversion File, which is based on data licensed from Canada Post Corporation, and/or data adapted from the MOH Postal Code Conversion File, which contains data copied under license from Canada Post Corporation and Statistics Canada. The authors thank IQVIA Solutions Canada Inc for the use of their Drug Information File.
This study was supported by ICES, which is funded by an annual grant from the MOH and the Ministry of Long-Term Care (Ontario, Canada), and by the Princess Margaret Cancer Foundation (Ontario, Canada).
The opinions, results, and conclusions reported in this paper are those of the authors and are independent of the funding and data sources. No endorsement by ICES, MOH, CIHI, OH, its partners, or the Province of Ontario is intended or should be inferred. ICES and Ontario Health Data Plaform (OHDP) had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Authorship
Contribution: I.Y.G., J.K., M.C., A.P., M.C.C., and D.H. conceived of and designed the study; I.Y.G., J.K., M.C., and A.P. acquired, analyzed, or interpreted data; I.Y.G., J.K., and M.C. drafted the manuscript; A.C. performed the statistical analysis; and all the authors performed critical revision of the manuscript for important intellectual content.
Conflict-of-interest disclosure: M. Crump received honoraria from Canada’s drug agency Canadian Agency for Drugs and Technologies in Health (CADTH) and from Kite/Gilead; and research funding from Epizyme/Ipsen and Roche. A.P. received honoraria from AbbVie, Kite/Gilead, and AstraZeneca. D.R. reports consultancy with, and a current holding of stock options in, Need Inc. L.M. received honoraria from AbbVie. J.K. reports data safety and monitoring board membership at Karyopharm; research funding from F. Hoffmann-La Roche Ltd, AstraZeneca, Merck, and Novartis; honoraria from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Genmab, Gilead, Incyte, Janssen, Merck, Novartis, Pfizer, F. Hoffmann-La Roche Ltd, and Seattle Genetics; and consultancy with AbbVie, Bristol Myers Squibb, Gilead, Merck, F. Hoffmann-La Roche Ltd, and Seattle Genetics.
Correspondence: John Kuruvilla, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, 6-424, 700 University Ave, Toronto, ON M5G 1Z5, Canada; email: john.kuruvilla@uhn.ca.
References
Author notes
The data set from this study is held securely in coded form at ICES (formerly the Institute for Clinical Evaluative Sciences). Although legal data sharing agreements between ICES and data providers (eg, health care organizations and government) prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access (http://www.ices.on.ca/DAS; email: das@ices.on.ca).
The full-text version of this article contains a data supplement.