Key Points
Spacer-CUB interactions maintain the ADAMTS13 global latency to structurally constrain its proteolytic function.
An opening anti-ADAMTS13 CUB1 mAb uncouples the Spacer-CUB domain interaction indirectly to disrupt the ADAMTS13 global latency.
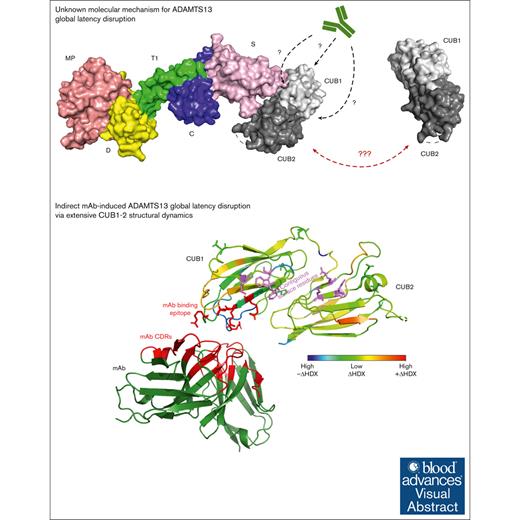
Visual Abstract
Allosteric regulation of ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type-1 motif, member 13) activity involves an interaction between its spacer (S) and 2 complement C1r/C1s, Uegf and BMP1 (CUB; CUB1-2) domains to keep the enzyme in a closed, latent conformation. Monoclonal antibodies (mAbs) uncouple the S-CUB interaction to open the ADAMTS13 conformation and thereby disrupt the global enzyme latency. The molecular mechanism behind this mAb-induced allostery remains poorly understood. To gain insights in the mAb-induced S-CUB uncoupling and global latency disruption, we combined hydrogen/deuterium exchange mass spectrometry (HDX-MS) with structural analysis of ADAMTS13 CUB1-2 mutants. Thereby, the CUB1 L3 and L9 loops were fine-mapped as the 17G2 mAb binding epitope. Indirect S-CUB uncoupling was observed as mAb binding–induced extensive structural dynamics within both CUB1-2 domains without directly targeting the contiguous CUB1 surface that engages with the ADAMTS13 S domain. HDX-MS analysis revealed the short interdomain linker to structurally cover the central CUB1-2 domain interface, which also showed some protein regions that became more exposed upon mAb binding. Therefore, repositioning of the central CUB1-2 interface appears crucial to transfer structural dynamics between both domains. Nevertheless, mutagenesis of the short linker did not disrupt the ADAMTS13 global latency because its closed conformation was preserved. Presumably, allosteric disruption of the global latency requires a structural impact extending beyond the central interface repositioning. Because anti-ADAMTS13 autoantibodies from patients with immune-mediated thrombotic thrombocytopenic purpura (iTTP) also induce an open ADAMTS13 conformation, our novel insights in the antibody-mediated global latency disruption boost our understanding of the iTTP disease pathology.
Introduction
In normal hemostasis, the function of von Willebrand factor (VWF) is regulated by the enzyme ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type-1 repeats, member 13).1 By cleaving VWF, ADAMTS13 reduces the size and consequently the activity of this multimeric glycoprotein. When ADAMTS13 is deficient, for example because of anti-ADAMTS13 autoantibodies as seen in patients with immune-mediated thrombotic thrombocytopenic purpura (iTTP), ultralarge VWF multimers accumulate, leading to the formation of pathological microthrombi.1-3
ADAMTS13 circulates as a 190-kDa enzyme with multiple domains. The N-terminal part of the enzyme, called MDTCS, consists of a metalloprotease (MP) domain with the active site for VWF proteolysis, a disintegrin-like (D) domain, a first thrombospondin type-1 repeat (TSP1), a cysteine-rich (C), and a spacer (S) domain. The C-terminal tail, known as T2C2, contains 7 more thrombospondin type-1 repeats (TSP2-8) and 2 CUB (CUB1-2) domains that are essential for proteolysis restriction and substrate recognition.4-7 Intriguingly, the ADAMTS13 function is conformation-dependent, in which the interaction between the S and CUB1-2 domains plays a central role.8-12 ADAMTS13 circulates in a globally latent state, characterized by a closed, hairpin-like conformation in which the T2C2 tail folds back, allowing the CUB1-2 domains to noncovalently interact with the central S domain.9-13 Molecular docking simulations using the crystal structures of both MDTCS and CUB1-2 domains revealed the individual amino acids that maintain this S-CUB interaction.8 These include Arg568, Phe592, Arg660, Tyr661, and Tyr665 in the S domain and 2 clusters that form a contiguous surface in the CUB1-2 domains (Trp1245, Trp1250, and Lys1252; and Arg1326, Glu1387, and Glu1389 in CUB1 and CUB2; respectively).8
Disruption of the S-CUB interaction leads to opening of the ADAMTS13 conformation, which, in turn, increases the enzymatic activity by twofold to fivefold.9-12,14 Physiologically, the S-CUB disruption is regulated via allosteric interaction with the VWF substrate, after binding of the VWF D4-CK domains to the ADAMTS13 CUB1-2 domains.4,15 Using our monoclonal antibody (mAb) 1C4, this open ADAMTS13 conformation is specifically recognized and revealed this open conformation to be a novel biomarker for iTTP.16,17 From the polyclonal immune response of patients with iTTP, we previously identified that pathological immunoglobulin G autoantibodies against the S and CUB1-2 domains cause the conformational opening of ADAMTS13.17,18
We previously also described the anti-CUB1 mAb 17G2, which opens the ADAMTS13 conformation and thereby mimics the disruption of the enzyme’s global latency.14,16 Although being broadly conserved across different species and essential for the ADAMTS13 activity regulation,12,14 the molecular details of the conformational mechanism that allosterically disrupt the S-CUB interactions, remain elusive. In this study, we aimed to elucidate the molecular mechanism by which our mAb 17G2 can disrupt the global latency of ADAMTS13. To this end, we fine-mapped the 17G2 epitope, used hydrogen/deuterium exchange mass spectrometry (HDX-MS) to study 17G2-induced structural changes within the CUB1-2 domains and examined novel ADAMTS13 CUB1-2 mutants to assess the role of individual CUB1-2 domain regions in the conformational regulation of ADAMTS13.
Methods
Protein generation
For use in various enzyme-linked immunosorbent assay (ELISA) setups in this study, we produced and purified the in-house–developed murine anti-human ADAMTS13 mAbs 3H9, 1C4, 15D1, and 17G2, as previously described.13,14,16,19 The mAb 3H9 recognizes the active site within the MP domain of ADAMTS13 and was used here as a coating and detection antibody in ELISAs. The anti-S mAb 1C4 specifically recognizes a cryptic epitope that is only available in open ADAMTS13 and was used here as an ELISA coating antibody to specifically capture open ADAMTS13. The anti-S mAb 15D1 and anti-CUB1 mAb 17G2 were used as detection antibodies in ELISAs. The anti-CUB1 mAb 17G2 is an activating mAb that induces an open ADAMTS13 conformation. The truncated C-terminal ADAMTS13 variant T2C2 (ie, all ADAMTS13 domains from TSP2 up to CUB2) was expressed by a stable Chinese hamster ovary–derived cell line and was purified using immobilized metal affinity chromatography as described before.20,21
HDX-MS
Triplicate HDX-MS experiments were similarly performed as previously described.22,23 In brief, after T2C2 protein incubation with or without our anti-CUB1 mAb 17G2, an autosampler (LEAP Technologies, Carborro, NC) was used to prepare protein samples (50 pmol) in 1:7 (volume-to-volume ratio) exchange buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid], 10 mM sodium phosphate, pD 7.0) in >99.9% pure deuterium solvent (Cambridge Isotope Laboratories, Tewksbury, MA) at 20°C. As an undeuterated reference, protein samples were incubated in equivalent hydrogenated exchange buffer (pH 7.0) at 20°C. After incubation for 10, 100, 500, and 1000 seconds, exchange reactions were quenched in precooled quench buffer (2 M guanidinium chloride, 250 mM TCEP [Tris(2-carboxyEthyl)phosphine]), pH 2.5) at 1°C. Protein samples were injected into a nano Acquity (Waters, Milford, MA) with a temperature-controlled HDX manager and digested on a 2.1 × 30 mm 1:1 (weight-to-weight ratio) pepsin-to-protease XIII dual digestion column (NovaBioAssays, Woburn, MA). Peptides were trapped and desalted for 3 minutes using a 2.1 × 5 mm2 bridged ethyl hybrid C18 column (Waters, Milford, MA). Peptides were separated and eluted using a 95% to 5% water-to-acetonitrile flow gradient (40 μL/min) with 0.1% formic acid. After protein injections, blank water samples containing 0.1% formic acid were injected to reduce peptide carryover between protein injections to <10% of the peak area. Mass spectra were acquired using a Synapt G2-Si high definition mass spectrometer (Waters, Milford, MA) in positive resolution configuration with ion mobility separation. According to manufacturer’s instructions, mass accuracy was determined through simultaneous infusion of 1 μg/mL leucine-enkephalin as a reference lock-mass compound.
ProteinLynx Global Server 3.0.2 software (Waters, Milford, MA) identified peptides based on their mass-to-charge ratio and traced them back to their originating protein sequence. The sequences of pepsin and protease XIII were included to remove autodigested peptides from the data set. ProteinLynx Global Server data was imported in DynamX 3.0 software (Waters, Milford, MA) to manually inspect individual peptides. Relative deuterium uptake was analyzed as a function of incubation time, and low signal-to-noise intensity peptides were excluded. By applying a rainbow color scale (blue, low HDX; red, high HDX), HDX heat maps were generated to visualize the relative fractional deuterium uptake levels. Comparative analysis was performed by side-by-side evaluation of HDX heat maps for protein samples in presence and absence of our mAb. Differential HDX heat maps were obtained by subtracting HDX in presence of our mAb from HDX in absence of our mAb (blue, high –ΔHDX; green, low ΔHDX; and red, high +ΔHDX). PyMOL 2.5.3 software was used to map (differential) HDX heat maps onto the crystal structure of the ADAMTS13 CUB1-2 domains (protein data bank [PDB] ID: 7B01). More details on the working principle of HDX-MS are provided in supplemental Figure 1.
Mutagenesis and expression of ADAMTS13 CUB1-2 mutants
Fourteen nonexpression pUC57 vectors containing the ADAMTS13 CUB1-2 sequence were synthesized, each containing a single-residue mutation in CUB1 (H1196Y, D1204H, Q1210R, R1219Q, D1259G, R1272K, R1274H, C1275R, R1277L, E1293G, and Y1296P) or CUB2 (N1345D, E1392P, and Q1398H; GenScript, Rijswijk, The Netherlands). Using MreI and XbaI restriction enzymes, the CUB1-2 sequence was isolated from the 14 nonexpression vectors and used to replace the original CUB1-2 sequence of a mammalian pcDNA6.1 expression vector expressing full-length, wild-type human ADAMTS13 with C-terminal V5 and 6× His tags.8,24 After cloning, Sanger sequencing (LGC Genomics, Berlin, Germany) confirmed the correct CUB1-2 sequence of each mutant. Jetprime reagent (PolyPlus, Milmort, Belgium) was used according to the manufacturer’s guidelines to transiently transfect empty human embryonic kidney 293 cells with wild-type or 1 of the 14 mutant full-length ADAMTS13 expression vectors. A pMax green fluorescent protein expression vector (Lonza, Basel, Switzerland) was cotransfected to verify successful expression at 48 hours after transfection using fluorescence microscopy (Carl Zeiss Microscopy GmbH, Jena, Germany). Conditioned expression medium containing either wild-type or CUB1-2 mutant full-length ADAMTS13 proteins was collected 72 hours after transfection and further characterized using various ELISA setups.
ADAMTS13 ELISA
To quantify antigen levels of wild-type and CUB1-2 mutant full-length ADAMTS13 in conditioned medium, our previously described in-house ELISA was used.25 Minor modifications were applied because the CUB1-2 mutations could possibly affect the antigen recognition by our biotinylated anti-CUB1 17G2 detection antibody. Therefore, the biotinylated anti-S 15D1 detection antibody was used as an alternative. To confirm the 17G2 mAb binding epitope and assess the general CUB1-2 domain folding upon ADAMTS13 CUB1-2 mutagenesis, we evaluated the binding capacity of our 17G2 mAb to wild-type and mutant ADAMTS13 captured on our anti-MP mAb 3H9. In addition, we also studied the conformation (open or closed) of wild-type and mutant ADAMTS13 by capturing these variants on our in-house–developed anti-S mAb 1C4, which can only capture open ADAMTS13 (open-closed ELISA assay).16 More detailed information on all 3 ELISA assays is provided in the supplemental Data.
Statistics
Statistical analysis was performed using GraphPad Prism 8.0.1 (GraphPad Software, San Diego, CA) software. When evaluating multiple groups for difference between means, Shapiro-Wilk normality tests, ordinary 1-way or 2-way analysis of variance tests with Bonferroni multiple comparison were performed. Statistical significance was achieved when P values were below .05 (∗), .01 (∗∗), .001 (∗∗∗), and .0001 (∗∗∗∗). All experiments were repeated in triplicate and data are presented as mean with error bars reflecting 1 standard deviation.
Results
Antibody binding induces extensive structural dynamics within both ADAMTS13 CUB1-2 domains
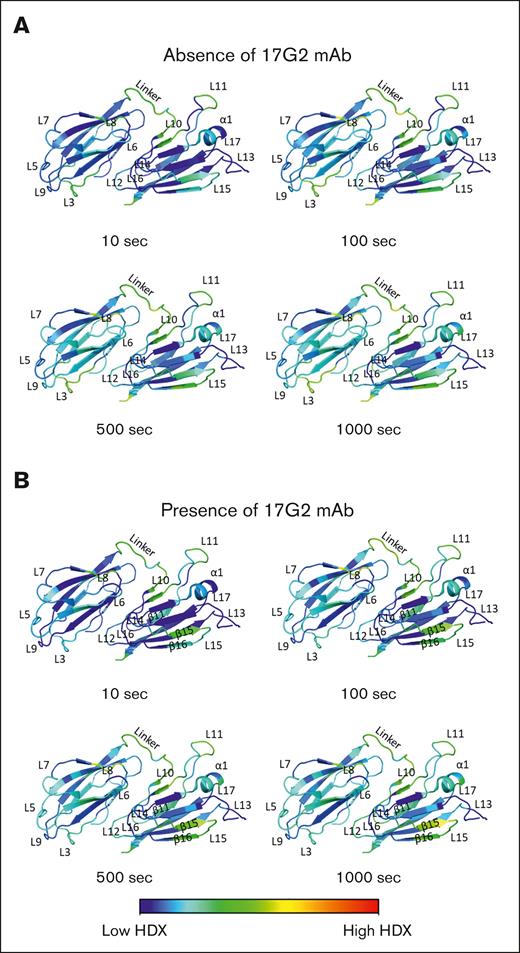
In a first set of experiments, the HDX pattern for the CUB1-2 domains was defined in absence of the 17G2 mAb. Here, a total of 202 unique, overlapping peptides fully covered the CUB1-2 protein sequence with an average residue redundancy of 9.52 (supplemental Figure 2). The HDX heat map in rainbow color scale (blue, low HDX; red, high HDX), reflecting the relative fractional deuterium uptake for the ADAMTS13 CUB1-2 domains in absence of the anti-CUB1 mAb 17G2 at 10, 100, 500, and 1000 seconds of exchange is given in supplemental Figure 3. Next, we the HDX heat map was visualized in the CUB1-2 domain crystal structure (PDB ID: 7B01; Figure 1A).8 In this structure, the antiparallel β-strands are intertwined by loops or an α-helix to form a jelly roll fold.8 As illustrated by the blue section of the color scaling, most of the β-strands and the α-helix revealed low HDX for increasing timepoints in accordance with the expected stability of these secondary structures (Figure 1A; supplemental Figure 3). As expected, increased HDX (green to yellow color) was observed over time for the less rigid intertwining loops and the short linker sequence that connects both CUB domains (Figure 1A; supplemental Figure 3). Moreover, increased HDX was observed for the C-terminal CUB2 residues (Trp1411-Thr1427) that previously remained uncrystallized (supplemental Figure 3).8 Interestingly, the CUB1 L5 and CUB2 L13 loops homing the radially positioned N-linked glycans, showed low HDX, suggesting a structurally stable conformation.8,26 Taken together, our HDX heat map supports the overall stable protein structure of the ADAMTS13 CUB1-2 domains and further highlights the more exposed or covered regions within these domains.
HDX patterns for the ADAMTS13 CUB1-2 domains. (A-B) HDX heat maps in the rainbow color scale (blue, low HDX; red, high HDX) plotted onto the ADAMTS13 CUB1-2 crystal structure (PDB ID: 7B01) reflecting the relative fractional deuterium uptake at the 10, 100, 500, and 1000 seconds incubation time points in absence (A) and presence (B) of the 17G2 mAb. In these annotated structures, the antiparallel β-strands are intertwined by loops or an α-helix to form a jelly roll fold.8
HDX patterns for the ADAMTS13 CUB1-2 domains. (A-B) HDX heat maps in the rainbow color scale (blue, low HDX; red, high HDX) plotted onto the ADAMTS13 CUB1-2 crystal structure (PDB ID: 7B01) reflecting the relative fractional deuterium uptake at the 10, 100, 500, and 1000 seconds incubation time points in absence (A) and presence (B) of the 17G2 mAb. In these annotated structures, the antiparallel β-strands are intertwined by loops or an α-helix to form a jelly roll fold.8
Next, the HDX pattern of the CUB1-2 domains in presence of the 17G2 mAb were determined (blue, low HDX; red, high HDX; supplemental Figure 3; Figure 1B). Binding of our mAb 17G2 did not significantly change the HDX heat map for most of the β-strands and the CUB2 α-helix, confirming their intramolecular stability even after mAb binding (Figure 1B). Interestingly, potent HDX protection was noticed for the peptides covering the CUB1 L3 and L9 loops (Figure 1B; supplemental Figure 5), which could hint toward the anti-CUB1 17G2 mAb binding epitope. In addition, weaker HDX protection was also observed for the incompletely resolved CUB2 L13 loop8 as well as for some amino acid residues within the CUB1 L7 loop, the short linker, and the CUB2 L14 and L16 loops (Figure 1B). In contrast, elevated HDX was noticed for the CUB1 L6 and L8 loops; the short linker region; the CUB2 L10, L11, L12, L15, and L17 loops; but also for the CUB2 β11, β15, and β16 strands (Figure 1B; supplemental Figure 4).
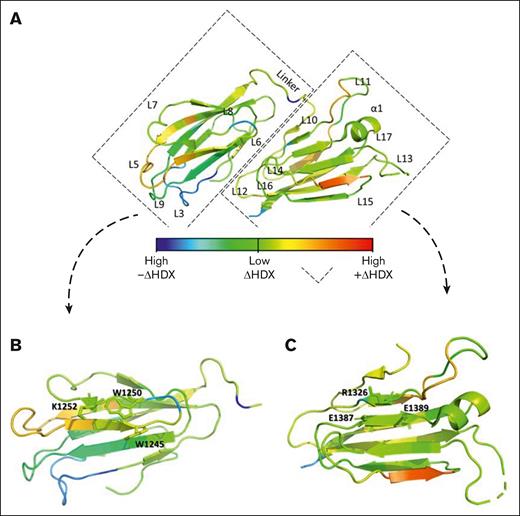
A differential HDX heat map (blue, high –ΔHDX; green, low ΔHDX; red, high +ΔHDX) for the CUB1-2 domains in presence vs absence of 17G2 was obtained as shown in Figure 2A and supplemental Figure 5. Blue and dark green colors indicate negative relative fractional deuterium uptake levels and thus represent regions that become less exposed upon mAb binding. Yellow and orange colors indicate positive relative fractional deuterium uptake levels, signifying regions with mAb-induced increased surface exposure and thus flexibility. Notably, HDX differences upon mAb binding were not only observed in the CUB1 domain, but also within the CUB2 domain as most clearly indicated by several yellow and orange colored protein regions (Figure 2A). Interestingly, minimal HDX difference (light green colors) was observed for the CUB1 (Trp1245, Trp1250, and Lys1252) and CUB2 (Arg1326, Glu1387, and Glu1389) residues that form the contiguous CUB1-2 domain surface that interacts with the spacer domain (Figure 2B-C).8 These results suggest that 17G2 binding may not interfere with the S-CUB interface and may instead disrupt the global latency through indirect, extensive structural rearrangements in both CUB1-2 domains.
Differential HDX pattern of the ADAMTS13 CUB1-2 domains. (A) Differential HDX heat map in the rainbow color scale (blue, high –ΔHDX; green, low ΔHDX; red, high +ΔHDX) plotted onto the ADAMTS13 CUB1-2 crystal structure reflecting the difference in relative fractional deuterium uptake in 17G2 mAb presence and absence at the 1000 seconds incubation time point. (B) Inset of the differential HDX heat map for the ADAMTS13 CUB1 domain with the Trp1245, Trp1250, and Lys1252 residues of the contiguous surface that interact with the spacer domain presented as green–colored sticks reflecting minimal HDX difference in presence and absence of the 17G2 mAb. (C) Inset of the differential HDX heat map of the ADAMTS13 CUB2 domain with the Arg1326, Glu1387, and Glu1389 residues of the contiguous surface presented as light-green–colored sticks reflecting minimal HDX difference in presence and absence of the 17G2 mAb.
Differential HDX pattern of the ADAMTS13 CUB1-2 domains. (A) Differential HDX heat map in the rainbow color scale (blue, high –ΔHDX; green, low ΔHDX; red, high +ΔHDX) plotted onto the ADAMTS13 CUB1-2 crystal structure reflecting the difference in relative fractional deuterium uptake in 17G2 mAb presence and absence at the 1000 seconds incubation time point. (B) Inset of the differential HDX heat map for the ADAMTS13 CUB1 domain with the Trp1245, Trp1250, and Lys1252 residues of the contiguous surface that interact with the spacer domain presented as green–colored sticks reflecting minimal HDX difference in presence and absence of the 17G2 mAb. (C) Inset of the differential HDX heat map of the ADAMTS13 CUB2 domain with the Arg1326, Glu1387, and Glu1389 residues of the contiguous surface presented as light-green–colored sticks reflecting minimal HDX difference in presence and absence of the 17G2 mAb.
Fine mapping of the 17G2 mAb reveals an epitope outside of the CUB1 contiguous surface
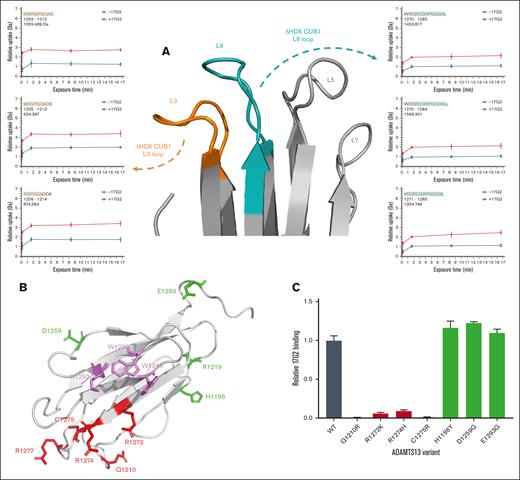
Based on our observation that 17G2 mAb binding–induced potent HDX protection of the CUB1 L3 and L9 loops (Figures 1B and 2A), we next performed fine mapping experiments to validate this 17G2 mAb binding epitope. Figure 3A shows in detail the HDX difference of the relevant loops in absence or presence of our 17G2 mAb. To verify if these loops could shape a nonlinear 17G2 mAb binding epitope, 6 full-length ADAMTS13 mutants with single CUB1 residue substitutions were generated based on nonconserved residues between human and mouse ADAMTS13 that are outward-facing in the CUB1-2 crystal structure. These included the D1204H, Q1210R, R1272K, R1274H, C1275R, and R1277L substitutions (Figure 3B).8 As a control, 4 additional full-length ADAMTS13 CUB1 mutants (H1196Y, R1219Q, D1259G, and E1293G) were generated outside the L3 and L9 loops at positions at which no potent HDX protection was observed (Figure 3B). Notably, no detectable expression was obtained for mutants D1204H, R1277L, and R1219Q (n = 3), evidenced by undetectable ADAMTS13 antigen levels (data not shown), most likely because of folding deficits that hamper protein secretion. The remaining ADAMTS13 CUB1 mutants were tested for their capacity to bind our 17G2 mAb. As shown in Figure 3C, all ADAMTS13 CUB1 L3 and L9 mutants (Q1210R, R1272K, R1274H, and C1275R) revealed strongly reduced or even abolished 17G2 binding. When compared to wild-type ADAMTS13, the 17G2 binding capacity was maintained for our control CUB1 substitutions (H1196Y, D1259G, and E1293G). These data indicate that the epitope of our mAb 17G2 is not located within the CUB1 contiguous surface (Trp1245, Trp1250, and Lys1252) responsible for the S-CUB interaction (Figure 3B, violet residues),8 again suggesting that 17G2 binding indirectly disrupts the S-CUB interaction, most likely through extensive structural rearrangements within the CUB1-2 domains, rather than by directly competing for S-CUB binding residues.
Binding epitope of the anti-ADAMTS13 CUB1 mAb 17G2. (A) Section of the CUB1-2 domains presenting the ADAMTS13 CUB1 L3 (in orange), L5, L7, and L9 (in cyan) loops, differences in HDX (ΔHDX) are presented by the deuterium uptake plots for each binding epitope loop. (B) Cartoon representation of the CUB1 domain (light gray) with mutated, single residues presented as sticks. The L3 and L9 loop mutations are colored in red, whereas the control mutations are indicated in green. The CUB1 residues of the contiguous surface that docks on the S domain are colored in violet.8 Of note, the D1204H substitution is located at the backside of the CUB1 domain and is visually covered by the front β-strands of CUB1. (C) Abolished binding of the 17G2 mAb to single residue, full-length ADAMTS13 mutants (Q1210R, R1272K, R1274H, and C1275R) captured on our anti-MP mAb 3H9, confirmed the CUB1 L3 and L9 loops as the HDX-identified mAb epitope, whereas the mAb 17G2 could bind the 3H9-captured ADAMTS13 mutants with control mutations outside the L3 or L9 loops (H1196Y, D1259G, and E1293G) equally well compared to wild-type (WT) ADAMTS13.
Binding epitope of the anti-ADAMTS13 CUB1 mAb 17G2. (A) Section of the CUB1-2 domains presenting the ADAMTS13 CUB1 L3 (in orange), L5, L7, and L9 (in cyan) loops, differences in HDX (ΔHDX) are presented by the deuterium uptake plots for each binding epitope loop. (B) Cartoon representation of the CUB1 domain (light gray) with mutated, single residues presented as sticks. The L3 and L9 loop mutations are colored in red, whereas the control mutations are indicated in green. The CUB1 residues of the contiguous surface that docks on the S domain are colored in violet.8 Of note, the D1204H substitution is located at the backside of the CUB1 domain and is visually covered by the front β-strands of CUB1. (C) Abolished binding of the 17G2 mAb to single residue, full-length ADAMTS13 mutants (Q1210R, R1272K, R1274H, and C1275R) captured on our anti-MP mAb 3H9, confirmed the CUB1 L3 and L9 loops as the HDX-identified mAb epitope, whereas the mAb 17G2 could bind the 3H9-captured ADAMTS13 mutants with control mutations outside the L3 or L9 loops (H1196Y, D1259G, and E1293G) equally well compared to wild-type (WT) ADAMTS13.
Effect of CUB1-2 mutations on the S-CUB interaction
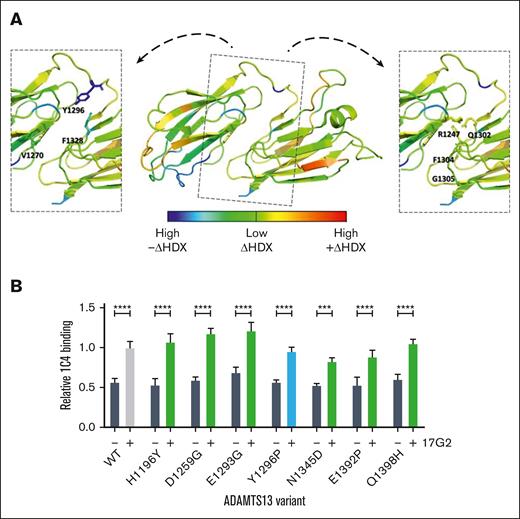
Because the results of both the HDX and CUB1 mutants point to indirect, extensive structural rearrangements within CUB1-2 as the cause of 17G2-induced disruption of the S-CUB interaction, we focused on identifying the most likely region involved in these structural changes. Because both CUB domains are connected by a short interdomain linker (Leu1290-Glu1298) that positions these domains in an opposing orientation with a large central interface shaped by hydrogen and hydrophobic interactions,8 we hypothesized that changes in this linker and interface could reorient the CUB1-2 domains leading to S-CUB uncoupling. Among the known residues involved in the CUB1-2 interface,8 HDX-MS revealed a decreased surface exposure for the Val1270, Tyr1296, and Phe1328 residues (blue and dark green), whereas the Arg1247, Gln1302, Phe1304, and Gly1305 residues (light green and yellow) became more exposed upon 17G2 mAb binding (Figure 4A). Given the pronounced HDX-identified covering of the Tyr1296 residue (Figures 2A and 4A; supplemental Figure 5), we hypothesized that a Y1296P substitution in the linker would disrupt the hydrophobic nature of the interface and adjust the orientation of the short linker,27 leading to the destabilization of both CUB1-2 domains and the disruption of the S-CUB interaction. Therefore, we generated the full-length ADAMTS13 Y1296P substitution mutant to assess its open or closed conformation. As controls, we generated full-length ADAMTS13 mutants with single-residue mutations at locations with minimal HDX difference in presence or absence of mAb 17G2, because these mutations are expected not to be involved in the 17G2-induced structural rearrangements. These included the 3 described CUB1 mutants in Figure 3 (H1196Y, D1259G, and E1293G) as well as 3 additional mutants with single-residue CUB2 substitutions (N1345D, E1392P, and Q1398H; supplemental Figure 5). Because these mutants present the allosteric regulation of full-length ADAMTS13, the impact of specific CUB1-2 domains regions on the allosteric ADAMTS13 regulation could be studied. All full-length mutants could be expressed and binding of the 17G2 mAb was unaltered (Figure 3C; supplemental Figure 6). When testing the open or closed conformation via ELISA, all 6 full-length ADAMTS13 CUB1-2 control mutants displayed a closed conformation similar to wild-type ADAMTS13, because they could be successfully opened upon 17G2 mAb incubation (Figure 4B). However, also the Y1296P mutant showed a closed conformation, which could be opened by 17G2 (Figure 4B). Thus, changing the Tyr1296 in the short linker did not induce spontaneous uncoupling of the S and CUB domains, as hypothesized. Most likely, more extensive rearrangements are needed to replicate 17G2-induced uncoupling of the S-CUB interaction.
Structural effects of the 17G2 mAb binding on the CUB1-2 interface. (A) Differential HDX heat map in the rainbow color scale (blue, high –ΔHDX; green, low ΔHDX; red, high +ΔHDX) plotted onto the ADAMTS13 CUB1-2 crystal structure with insets highlighting CUB1-2 interface residues that became less (Val1270, Tyr1296, and Phe1328) or more (Arg1247, Gln1302, Phe1304, and Gly1305) mobile upon 17G2 mAb binding. Our mAb 1C4 recognizes a conformationally sensitive epitope in the spacer domain of open ADAMTS13. All control CUB1-2 mutants (green) showed a similarly closed conformation as WT ADAMTS13. (B) Despite its lowered mobility upon 17G2 binding, the conformation of the Y1296P interface mutant (blue) remained similarly closed as for WT ADAMTS13, whereas its conformation could still be opened by preincubation of the 17G2 mAb.
Structural effects of the 17G2 mAb binding on the CUB1-2 interface. (A) Differential HDX heat map in the rainbow color scale (blue, high –ΔHDX; green, low ΔHDX; red, high +ΔHDX) plotted onto the ADAMTS13 CUB1-2 crystal structure with insets highlighting CUB1-2 interface residues that became less (Val1270, Tyr1296, and Phe1328) or more (Arg1247, Gln1302, Phe1304, and Gly1305) mobile upon 17G2 mAb binding. Our mAb 1C4 recognizes a conformationally sensitive epitope in the spacer domain of open ADAMTS13. All control CUB1-2 mutants (green) showed a similarly closed conformation as WT ADAMTS13. (B) Despite its lowered mobility upon 17G2 binding, the conformation of the Y1296P interface mutant (blue) remained similarly closed as for WT ADAMTS13, whereas its conformation could still be opened by preincubation of the 17G2 mAb.
Discussion
In this study, we used HDX-MS and ADAMTS13 mutagenesis to characterize the structural dynamics within the ADAMTS13 CUB1-2 domains, aiming to elucidate the molecular mechanism by which the anti-CUB1 mAb 17G2 induces the opening of ADAMTS13. The mAb binding epitope was fine-mapped at near-residual resolution and was located in the CUB1 L3 and L9 loops. Within these loops, residues Gln1210, Arg1272, Arg1274, and Cys1275 are involved in mAb binding because substitution of these residues abolished 17G2 binding. These residues differ from those of the contiguous CUB1 surface (Trp1245, Trp1250, and Lys1252) responsible for the S domain docking,8 suggesting that 17G2 does not directly interfere with the CUB1-2 docking interface. Instead, 17G2 binding likely induces extensive structural rearrangements that indirectly disrupt the S-CUB interaction, as corroborated by the increased surface exposure of protein regions in CUB1 as well as in CUB2. Intriguingly, such indirect structural effects have previously been proposed to induce the open ADAMTS13 conformation, leading to its activation at subphysiological pH.8
Previous hypotheses suggested that mAb binding–induced structural changes regulate ADAMTS13’s global latency by altering the orientation of key amino acid residues at the S-CUB interface.8 In our study, potent HDX-MS protection revealed the short linker Tyr1296 residue as a likely candidate to adjust this CUB1-2 domain interface. At this position, we substituted the tyrosine residue into a proline residue to destabilize the central CUB1-2 interface and possibly reorient the crucial CUB1-2 residues that shape the contiguous surface.27 However, the Y1296P substitution in full-length ADAMTS13 did not disrupt the global ADAMTS13 latency, because the closed ADAMTS13 conformation was preserved. Possibly, the structural impact of this single-residue substitution is minor compared with the broader structural dynamics upon 17G2 binding.
The effects of posttranslational modifications on the open or closed ADAMTS13 conformation remain poorly understood. Notably, the ADAMTS13 CUB1 L5 and CUB2 L13 loops house homologous N-glycans, a characteristic not observed in other tandem CUB domains.8,26 Although the N-glycan–bearing region of the CUB2 L13 loop was previously unresolved, it was presumed to be flexible and the N-glycan was thought to sterically hinder ligand docking.8 However, our observation of low HDX in the absence of our mAb 17G2 suggests that these loops adopt a structurally stable conformation. Even after the addition of 17G2, the CUB2 L13 loop continued to exhibit low HDX, indicating that these N-glycans might only play a limited role in the mAb-induced opening of ADAMTS13.
Mutagenesis frequently causes protein folding or secretion issues, which presumably also caused the undetectable expression of 3 of our full-length ADAMTS13 CUB1-2 mutants. Notably, VWF96 proteolysis by the R1219Q ADAMTS13 mutant was previously reported to show no additional functional activation in presence of our mAb 17G2, which likely occurred because of folding issues of the conserved CUB1 L4 loop that thereby abolished the 17G2 mAb binding.8 In this study, reevaluation of this R1219Q substitution showed complete loss of ADAMTS13 protein secretion in repeated transfections. This aligns with an earlier report of a patients with congenital TTP with this arginine being substituted into a tryptophan residue to cause not only a reduced ADAMTS13 activity but also an impaired secretion in vitro and lower ADAMTS13 antigen levels in vivo.28
In >55% of patients with acute phase iTTP, autoantibodies targeting the CUB1-2 domains are present,20 and isolated patient-derived anti-CUB autoantibody fractions have previously been shown to induce an open ADAMTS13 conformation in healthy individuals.17,18 Thereby, such antibodies largely contribute to the iTTP pathophysiology. Notably, we previously demonstrated that our mAb 17G2 mimics the allosteric ADAMTS13 opening in vitro, similarly to the effect of patient-derived autoantibodies.14 Therefore, the herein reported indirect, extensive structural rearrangements may represent an important feature for the early onset of the open ADAMTS13 conformation in the iTTP pathophysiology. Subsequently, cryptic S domain regions that become exposed, could possibly act as an immunogenic hot spot to potentially trigger the further recruitment of inhibitory and/or clearance autoantibodies.14,29-31
In conclusion, we have characterized the HDX pattern at near-residual resolution, revealing the structural dynamics within the ADAMTS13 CUB1-2 domains upon binding of an opening antibody. We identified and mapped both exposed and covered CUB1-2 regions onto the previously resolved static crystal structure. The extensive structural rearrangements in both CUB1-2 domains suggest that our mAb 17G2 indirectly uncouples the S-CUB interaction, leading to the disruption of the global ADAMTS13 latency. However, the role of the short linker and central interface in maintaining or adjusting the orientation of the contiguous surface of the CUB1-2 domains requires further clarification.
Acknowledgments
This study was supported by research funding from the Fonds Wetenschappelijk Onderzoek (FWO; grant G009923N [K.V.]), the National Institutes of Health (grants HL143794 and HL166654 [R.L.]), and FWO “Krediet voor een lang verblijf in het buitenland” (V403222N [Q.B.]).
Authorship
Contribution: Q.B. conceptualized the study, designed experimental strategies, performed experiments, analyzed data, prepared figures, and wrote the manuscript; E.R.L. helped with designing and performing hydrogen/deuterium exchange mass spectrometry experiment; I.P., F.B., and J.A. performed experiments; C.T. and S.F.D.M. discussed data and edited the manuscript; R.L. and K.V. performed study conceptualization, experimental design, data analysis, data discussion, manuscript writing, and provided funding; and all authors discussed results, supplied critical feedback, and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karen Vanhoorelbeke, Laboratory for Thrombosis Research, IRF Life Sciences, KU Leuven Campus Kulak Kortrijk, Etienne Sabbelaan 53, 8500 Kortrijk, Belgium; email: karen.vanhoorelbeke@kuleuven.be; and Renhao Li, Department of Pediatrics, Emory University School of Medicine, 2015 Uppergate Dr NE, Atlanta, GA 30322; email: renhaoli@zju.edu.cn.
References
Author notes
Original data are available on request from the corresponding author, Karen Vanhoorelbeke (karen.vanhoorelbeke@kuleuven.be).
The full-text version of this article contains a data supplement.