Key Points
SAMD1 promotes GATA binding protein 2 expression and suppresses the megakaryopoietic and erythropoietic differentiation programs.
SAMD1 facilitates di-/trimethylation of histone H3K4 status at a subset of erythroid gene promoters/enhancers.
Visual Abstract
Cell progenitor to progeny transitions depend on precise transcriptional mechanisms to adjust gene expression. The sterile α-motif–containing 1 protein (SAMD1) regulates a shift in transcriptional activity during embryonic stem cell exit from pluripotency. SAMD1 interacts with, and facilitates the activity of, the histone H3 lysine-specific demethylase 1 (LSD1; a lysine demethylating enzyme). SAMD1 is expressed throughout many biological systems, but its role in hematopoiesis is unknown. In human and mouse hematopoietic stem/progenitor cells, we tested the role of SAMD1 in hematopoiesis and erythropoiesis using loss-of-function approaches. SAMD1 promoted expression of critical drivers of hematopoiesis, including the GATA binding protein 2 transcription factor, while opposing erythroid programs. Loss of SAMD1 in ex vivo differentiating cells increased erythroid and megakaryocyte differentiation and altered the landscape of histone H3 lysine 4 (H3K4) methylation genome wide. Cohorts of SAMD1-repressed genes are linked to erythropoietic activities. SAMD1 expression promoted extracellular signal-regulated kinase signaling via stem cell factor/Kit stimulation in progenitor populations. In erythroid precursor cells, SAMD1 cooccupies chromatin with LSD1 and GATA factors. Whereas SAMD1 downregulates levels of H3K4 dimethylation genome wide, contributing to gene repression, SAMD1 also elevates transcription at select sites. To test Samd1 function in hematopoiesis, we performed competitive transplant experiments in mice. Samd1-knockdown hematopoietic stem cells (HSCs) contributed more to peripheral blood mononuclear cells vs control HSCs. Our results establish SAMD1 as a coordinator of H3K4 methylation and stem/progenitor activity in hematopoiesis and erythropoiesis.
Introduction
Hematopoiesis balances a precisely maintained pool of hematopoietic stem/progenitor cells (HSPCs) with the production of differentiated progeny.1,2 A prominent molecular feature of cell differentiation involves enzymatic modifications to histones (often termed histone “marks”).3 Correlative evidence using genome-wide mapping studies suggests that histone modifications may instruct transcriptional activity and are fundamental to mechanisms involved in self-renewal and differentiation.4 Histone modifying enzymes are frequently deregulated in hematologic malignancies,5-9 highlighting the importance of understanding their role in hematopoiesis. A variety of factors are involved in both the recruitment and activity of histone modifying enzymes, including DNA cis elements, protein-protein interactions, and context-dependent cell and chromatin states,10,11 each contributing to mechanisms of gene regulation during differentiation.
Sterile α-motif (SAM)–containing protein 1 (SAMD1) was recently described to facilitate histone 3 lysine 4 (H3K4) demethylation in embryonic stem (ES)/progenitor cells and in pathologic contexts.12,13 SAMD1 binds preferentially to nonmethylated GC-rich motifs on DNA to promote the histone demethylase function of lysine demethylase-1 (LSD1; also known as KDM1A).12 Regulation of H3K4 monomethylation or dimethylation (H3K4me1/2) levels by LSD1 is required for hematopoiesis, and loss of LSD1 impairs hematopoietic maturation and blocks progenitor commitment to the erythroid lineage.6,7 SAMD1 belongs to a family of SAM proteins capable of self-interacting as dimers/polymers or interacting with heterologous SAM-containing proteins.14 SAMs have many important functions related to cellular differentiation.15 In hematopoiesis, Samd14 is required for the survival and differentiation of erythroid progenitors in acute anemia via the tyrosine Kit receptor and erythropoietin (EPO) signaling.16,17 The SAM proteins SAMD9 and SAMD9L promote Kit signaling in HSPCs, and gain-of-function mutations in these SAM proteins cause myelodysplastic syndrome and leukemia.18,19 As the function of many SAMs and SAM-containing proteins are unknown, outstanding questions remain regarding their role in genome organization, hematopoiesis, and hematologic disease.
SAMD1 messenger RNA (mRNA) levels are elevated in the erythroid lineage.20,21 A global Samd1 knockout is embryonic lethal in mice. In SAMD1 knockout mouse ES cells, the ability of SAMD1 to deregulate gene expression is accentuated upon differentiation,12,22 suggesting SAMD1 mechanisms are involved in lineage specification. SAMD1 is elevated in acute myeloid leukemia and associated with poor prognosis.12 However, SAMD1 has not been studied in hematopoiesis or erythropoiesis. Using human and mouse SAMD1 loss-of-function systems, our studies revealed that loss of SAMD1 increases hematopoietic differentiation and alters H3K4 methylation of erythroid precursors.
Materials and methods
HUDEP-2 cell culture
Human umbilical cord–derived erythroid progenitor 2 (HUDEP-2) cells were maintained in serum-free expansion media (STEMCELL Technologies) containing 2% penicillin/streptomycin (Pen-Strep), 0.4 μg/mL dexamethasone, 50 ng/mL recombinant human (rh) stem cell factor (SCF), 3 U/mL EPO, and 1 μg/mL doxycycline. For lentiviral infections, spinoculations were performed with 500 μL of viral supernatant containing Polybrene (8 μg/mL) and 100× HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; 5 μL per infection). Forty-eight hours after infection, infected cells were either fluorescence-activated cell sorter (FACS)–purified for green fluorescent protein–positive (GFP+) cells or puromycin selected by treatment with 1 μg/mL of puromycin for 96 hours. LSD1 inhibition was conducted in control or single-guide SAMD1 (sgSAMD1) HUDEP-2 cells treated with either dimethyl sulfoxide alone or 10 nM or 100 nM of GSK-LSD1 (Cayman Chemical) for 24 hours before the collection of RNA.
Erythroid and megakaryocyte differentiation
Bone marrow from the tibia and femur of 8- to 16-week-old wild-type mice was flushed, and lineage depleted with biotin-conjugated antibodies and MojoSort streptavidin-conjugated magnetic nanobeads (BioLegend): anti-mouse CD3e (clone 145-2 C11), anti-mouse CD11b (clone M1/70), anti-mouse CD19 (clone 6D5), anti-mouse CD45R (B220; clone RA3-6B2), anti-mouse Gr-1 (clone RB6-C5), anti-mouse CD71 (clone RI7217), and anti-mouse Ter119. Lineage-depleted progenitors (1 × 106 cells) were retrovirally infected with 100 μL of viral supernatant containing Polybrene (8 μg/mL) and 100× HEPES (5 μL per infection) and spinoculated at 2600 rpm for 90 minutes at 30°C. For erythroid differentiation, cells were cultured in StemPro-34 media (Invitrogen) containing 2 mM L-glutamine, 1% Pen-Strep, 0.1 mM monothioglycerol, 1 μM dexamethasone, 0.5 U/mL EPO, and 1% murine SCF Chinese hamster ovary cell–conditioned medium. Cultures were maintained at 0.5 × 106 to 1 × 106/mL for 4 days before downstream analyses. Colony assays were performed 48 hours after infection using 7.5 × 103 FACS-purified GFP+ cells per well in 500 μL of MethoCult M3434 (StemCell Technologies). For megakaryocyte differentiation, cells were cultured in Dulbecco modified Eagle medium (Corning) containing 2 mM L-glutamine, 1% Pen-Strep, 10% fetal bovine serum (FBS), 50 ng/mL thrombopoietin, and 100 U/mL hirudin. Cultures were maintained at 5 × 106/mL for 4 days before downstream analyses.
CD34+ cell differentiation
CD34+ cells from the peripheral blood were obtained from Fred Hutchinson Cancer Center. CD34+ cells were differentiated as previously described by Lee et al.23 Briefly, cells were cultured in serum-free expansion media for 4 days containing a cocktail of rh fms-like tyrosine kinase 3 (Flt-3), SCF, interleukin-3 (IL-3), and IL-6 (StemCell Technologies), followed by 3 sequential differentiation phases for 8, 5, and 3 days. Differentiation media was composed of Iscove modified Dulbecco medium, 15% FBS, 500 μg/mL holotransferrin (Sigma-Aldrich), 2 mM glutamine, 1.5% Pen-Strep, 1% bovine serum albumin, 10 μg/mL rh insulin (Sigma-Aldrich). Differentiation media 1 also contained 1 μm dexamethasone, 1 μm β-estradiol, 5 ng/mL rhIL-3, 100 ng/mL rhSCF, and 6 U/mL EPO. Differentiation media 2 had 50 ng/mL rhSCF and 6 U/mL EPO.
Competitive transplant
Bone marrow was flushed from 8- to 12-week-old congenic CD45.1 and CD45.2 wild-type mice, and the biotin-conjugated beads and streptavidin-conjugated Allophycocyanin (APC) lineage markers, Phycoerythrin (PE) Sca-1, and Phycoerythrin-Cyanine7 (Pe-Cy7) Kit, and FACS-sorted on a Bigfoot spectral cell sorter (ThermoFisher Scientific) for lineage-negative, Sca-1+ Kit+ (LSK) cells. LSKs cells were cultured for 4 days in OptiMEM media containing 10% FBS, 1% Pen-Strep, 50 μM β-mercaptoethanol, 50 ng/mL recombinant murine (rm) SCF, 50 ng/mL rm flt-3, 10 ng/mL rmIL-6, and 10 ng/mL rmIL-3, and maintained at 0.04 × 106/mL. At 24 and 48 hours after sorting, LSKs were retrovirally infected with 50 μL of viral supernatant containing Polybrene (8 μg/mL) and 100× HEPES (5 μL per infection) and spinoculated at 2000 rpm for 120 minutes at 30°C. GFP+ LSKs were FAC sorted on day 4. CD45.1 and CD45.2 retrovirally infected LSKs (0.2 × 106 cells) along with 1 × 106 uninfected CD45.1 total bone marrow cells were transplanted into congenic CD45.1+ wild-type mice that had been lethally irradiated with 2 rounds of 500 cGy. At weeks 4, 8, 12, and 16, peripheral blood was collected and analyzed for percentages of T cells, B cells, monocytes, and granulocytes by flow cytometry. Competitive transplant was performed twice on separate cohorts of mice.
All mouse experiments were approved by the institutional animal care and use committee of the University of Nebraska Medical Center in accordance with National Institutes of Health guidelines.
CUT&RUN
A total of 1 × 106 HUDEP-2 cells were used for each condition and per replicate. Antibodies: 1:100 guinea pig anti-rabbit immunoglobulin G (Sigma SAB3700889), 1:200 LSD1, 3 μg of Samd1 (provided by R.L.),12 1:50 H3K4me2, and 1:50 H3K4me3. Primary antibody incubations were performed overnight at 4°C and secondary antibody incubation was performed with 1:100 immunoglobulin G for 1 hour. Samples were incubated with pA/G-MNase (generated in the Brahma laboratory) for 1 hour, and targeted digestion was performed with 2 mM CaCl2 at 0°C 30 minutes, followed by the release of chromatin fragments by the addition of 2× stop buffer (150 mM NaCl, 20 mM EDTA, 4 mM EGTA, 50 mg/mL RNase, and 2 pg spike-in Saccharomyces cerevisiae DNA; from the Brahma laboratory) and 30-minute incubation at 37°C. DNA was extracted using phenol-chloroform. Libraries were prepared for Illumina sequencing with unique dual-indexed (UDI) adapters (supplemental Table 1) without size selection and following the KAPA DNA polymerase library preparation kit protocol (From Kapa Biosystems) optimized to favor exponential amplification of <1000–base pair fragments over linear amplification of large DNA fragments as previously described.24 For additional details including our bioinformatics pipeline, see supplemental methods.
Statistical analysis
Statistics were performed as stated in each figure and were performed in GraphPad Prism version 8.3. Significance is defined as ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001.
Results
The SAMD1 transcription factor represses an erythroid gene expression signature
The roles of SAM-containing proteins in hematopoiesis vary widely. Some SAM proteins are involved in signal-dependent HSPC activities, lineage specification, transcription, and hematologic disease.15 SAMD1 is among 13 SAM-containing proteins that are expressed higher in megakaryocyte-erythrocyte progenitors (MEPs) than in other hematopoietic cells (Figure 1A). After Samd14, which promotes signaling in regenerative erythropoiesis, Samd1 is the second-highest expressed SAM protein in MEPs (Figure 1B). In mouse development, the highest levels of Samd1 mRNA were detected in primitive and definitive erythroid cells.25 SAMD1 interacts with the lysine demethylase LSD1, an epigenetic eraser protein that coordinates erythropoiesis.12,26 Along with the known physical and functional interactions, SAMD1 and LSD1 have similar mRNA (Figure 1C) and protein (Figure 1D) expression in hematopoiesis.
SAMD1 alters erythroid progenitor gene transcription. (A) Heat map of SAM-containing protein mRNA transcript levels in FAC-sorted mouse bone marrow–derived hematopoietic cells. Transcript levels per gene normalized to maximum expression level in all hematopoietic cell types: LT-HSC, HSC, MPP, CLP, CMP, GMP, MF, Gn, mono, NK cells, MEP, and EryA and EryB. RNA-seq data from Lara-Astiaso et al.20 (B) Quantitation of SAM-containing protein mRNA transcript levels in FAC-sorted mouse bone marrow–derived MEPs. RNA-seq data from Lara-Astiaso et al.20 (C) Quantitation of Samd1 and Lsd1 mRNA transcript levels in FAC-sorted mouse bone marrow–derived hematopoietic cells. RNA-seq data from Lara-Astiaso et al.20 (D) Quantitation of SAMD1 and LSD1 protein copy number per cell. Relative protein levels measured by mass spectrometry from Gautier et al for human erythroid progenitors.27 Prog1: Band3−CD71medGPA−, Prog2: Band3−CD71highGPA−, ProE: Band3−CD71highGPAlow, Baso1: Band3lowCD71highGPAmed, Baso2: Band3medCD71highGPAhighCD49dhigh, Poly: Band3medCD71highGPAhighCD49dmed, and Ortho: Band3highCD71medGPAhigh. (E) Experimental layout of 17-day erythroid differentiation of CD34+ HSPCs. (F) T7 endonuclease assay of day 12 CD34+ progenitors Cas9 infected with scramble (sgControl) or SAMD1 knockout (sgSAMD1) sgRNAs. (G) Western blot of SAMD1 and β-actin in day-12 CD34+ progenitors. (H) Volcano plot of RNA-seq from sgSAMD1 vs sgControl erythroid progenitors. Cutoff: P < .01 and P < .5 log2 fold change (n = 4). Enrichr analysis of 154 genes downregulated after SAMD1 knockout (“SAMD1 activated”), using data from the Reactome and MSigDB catalogs (left). Enrichr analysis of 162 genes upregulated after SAMD1 knockout (“SAMD1 repressed”), using data from the Reactome and MSigDB catalogs (right). Baso1/2, basophilic erythroblast; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; EryA/B, erythroblast A/B; GMP, granulocyte-monocyte progenitor; Gn, granulocyte; HSC, hematopoietic stem cell; LT-HSC, long-term HSC; MF, macrophage; Mono, monocyte; MPP, multipotent progenitor; NK, natural killer; Ortho, orthochromatic erythroblast; Poly, polychromatic erythroblast; ProE, proerythroblast; Prog1/2, progenitor; Puro, puromycin; RNA-seq, RNA sequencing; T7E1, endonuclease 1.
SAMD1 alters erythroid progenitor gene transcription. (A) Heat map of SAM-containing protein mRNA transcript levels in FAC-sorted mouse bone marrow–derived hematopoietic cells. Transcript levels per gene normalized to maximum expression level in all hematopoietic cell types: LT-HSC, HSC, MPP, CLP, CMP, GMP, MF, Gn, mono, NK cells, MEP, and EryA and EryB. RNA-seq data from Lara-Astiaso et al.20 (B) Quantitation of SAM-containing protein mRNA transcript levels in FAC-sorted mouse bone marrow–derived MEPs. RNA-seq data from Lara-Astiaso et al.20 (C) Quantitation of Samd1 and Lsd1 mRNA transcript levels in FAC-sorted mouse bone marrow–derived hematopoietic cells. RNA-seq data from Lara-Astiaso et al.20 (D) Quantitation of SAMD1 and LSD1 protein copy number per cell. Relative protein levels measured by mass spectrometry from Gautier et al for human erythroid progenitors.27 Prog1: Band3−CD71medGPA−, Prog2: Band3−CD71highGPA−, ProE: Band3−CD71highGPAlow, Baso1: Band3lowCD71highGPAmed, Baso2: Band3medCD71highGPAhighCD49dhigh, Poly: Band3medCD71highGPAhighCD49dmed, and Ortho: Band3highCD71medGPAhigh. (E) Experimental layout of 17-day erythroid differentiation of CD34+ HSPCs. (F) T7 endonuclease assay of day 12 CD34+ progenitors Cas9 infected with scramble (sgControl) or SAMD1 knockout (sgSAMD1) sgRNAs. (G) Western blot of SAMD1 and β-actin in day-12 CD34+ progenitors. (H) Volcano plot of RNA-seq from sgSAMD1 vs sgControl erythroid progenitors. Cutoff: P < .01 and P < .5 log2 fold change (n = 4). Enrichr analysis of 154 genes downregulated after SAMD1 knockout (“SAMD1 activated”), using data from the Reactome and MSigDB catalogs (left). Enrichr analysis of 162 genes upregulated after SAMD1 knockout (“SAMD1 repressed”), using data from the Reactome and MSigDB catalogs (right). Baso1/2, basophilic erythroblast; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; EryA/B, erythroblast A/B; GMP, granulocyte-monocyte progenitor; Gn, granulocyte; HSC, hematopoietic stem cell; LT-HSC, long-term HSC; MF, macrophage; Mono, monocyte; MPP, multipotent progenitor; NK, natural killer; Ortho, orthochromatic erythroblast; Poly, polychromatic erythroblast; ProE, proerythroblast; Prog1/2, progenitor; Puro, puromycin; RNA-seq, RNA sequencing; T7E1, endonuclease 1.
SAMD1 and LSD1 interact and colocalize on DNA to coordinate chromatin remodeling in ES cells.12 We tested the involvement of SAMD1 in erythropoiesis by differentiating human CD34+ to the erythroid lineage (Figure 1E) and deleting SAMD1 using CRISPR/CRISPR-associated protein 9 (Cas9) (Figure 1F-G).23 We performed differential expression analysis 6 days after SAMD1 knockout in Kit+ erythroid progenitors (Figure 1H; supplemental Table 2). Among the 162 SAMD1-repressed genes (fold change of >0.5, log2 false discovery rate corrected P < .01), several are central to heme metabolism (TRIM10 and TRAK2) and reactive oxygen species pathways (GLRX2 and GLCM; Figure 1H). An elevated erythroid signature after knockout suggested that SAMD1 suppressed erythropoiesis-specific gene expression. Among the 154 SAMD1-activated genes, a gene signature consistent with hemostasis and platelet activation was prominent (eg, F2RL3 [F2R-like thrombin receptor 3] and FLNA [filamin A] were downregulated in SAMD1 knockout cells; Figure 1H). Other SAMD1-activated genes are established mediators of signal transduction pathways (MAPK3 and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma [PIK3CG]).13 Interestingly, when comparing differential gene expression between control vs SAMD1 knockout RNA sequencing data sets of HepG2 (hepatocellular carcinoma cells), and differentiated and undifferentiated ES cells, only 1 gene, histone methyl-lysine binding protein 3 (L3MBTL3), was significantly upregulated in all 4 data sets (fold change of > 0.5, log2 false discovery rate corrected P < .01), highlighting cell type–specific transcriptional roles of SAMD1.12,13
SAMD1 chromatin occupancy is linked to SAMD1-regulated transcription in erythroid progenitors
SAMD1 is a transcriptional repressor in other contexts.12,13 To establish SAMD1 targets in erythroid-committed cells, we performed Cleavage Under Targets & Release Using Nuclease (CUT&RUN) using an anti-SAMD1 antibody (supplemental Figure 1). SAMD1 occupied 11 798 sites in HUDEP-2 cells (Figure 2A), 89% of which are annotated gene promoters (Figure 2B). SAMD1 occupancy was detected at 68 of 162 (42%) SAMD1-repressed and 77 of 154 (50%) SAMD1-activated genes (Figure 2C). Motif analysis of all SAMD1 occupancy sites in HUDEP-2 revealed SAMD1’s preference for binding at GC-rich DNA, consistent with previous data in embryonic cells (Figure 2D).12,13 Despite the low congruence in SAMD1-regulated transcripts between erythroid progenitors and embryonic cells, there was 42% overlap between HUDEP-2 and mouse ES cell SAMD1 peaks annotated to open reading frames, and only 51 of these sites resided nearby SAMD1-regulated genes (Figure 2E). Thus, the cohort of SAMD1 occupancy sites shared with ES cells may not be required for direct transcriptional regulation.
SAMD1 directs the transcription of erythroid progenitors. (A) Heat map of CUT&RUN signal showing SAMD1 enrichment at SAMD1 peaks compared with IgG in HUDEP-2 cells (n = 11 798). (B) Annotation of SAMD1 peaks at genomic features in HUDEP-2 cells. (C) Annotation of SAMD1 peaks at significantly changing genes from the RNA-seq of sgSAMD1 vs sgControl erythroid progenitors. (D) Enriched motif at SAMD1-bound peaks. (E) Venn diagram of SAMD1 occupancy sites in HUDEP-2 and ES cells. (F) Table of co-occupied transcription factors with SAMD1 occupancy sites from publicly available chromatin immunoprecipitation (ChIP) data in HUDEP-2, K562, and primary erythroid progenitors. The signal for each peak is derived from 2 replicates. (G) CUT&RUN chromatin occupancy of SAMD1 at the L3MBTL3, GATA2, and BCL11A loci in HUDEP-2 cells. (H) Quantitative ChIP quantitative polymerase chain reaction (qPCR) at the L3MBTL3, GATA2, and BCL11A promoters in HUDEP-2 cells infected with scramble guides in IgG and SAMD1 ChIP ( = 4). (I) Normalized RNA-seq of sgControl and sgSAMD1 primary erythroid progenitors at day 12 of erythroid differentiation ( = 4). RNA-seq counts were normalized based on reads per kilobase per million mapped reads (transcripts per million [TPM]). (J) Western blot of GATA2 and actin in day-12 CD34+ progenitors. Error bars represent standard deviation (SD). ∗P < .05; ∗∗P < .01 (2-tailed unpaired Student t test). IgG, immunoglobulin G; UTR, untranslated region.
SAMD1 directs the transcription of erythroid progenitors. (A) Heat map of CUT&RUN signal showing SAMD1 enrichment at SAMD1 peaks compared with IgG in HUDEP-2 cells (n = 11 798). (B) Annotation of SAMD1 peaks at genomic features in HUDEP-2 cells. (C) Annotation of SAMD1 peaks at significantly changing genes from the RNA-seq of sgSAMD1 vs sgControl erythroid progenitors. (D) Enriched motif at SAMD1-bound peaks. (E) Venn diagram of SAMD1 occupancy sites in HUDEP-2 and ES cells. (F) Table of co-occupied transcription factors with SAMD1 occupancy sites from publicly available chromatin immunoprecipitation (ChIP) data in HUDEP-2, K562, and primary erythroid progenitors. The signal for each peak is derived from 2 replicates. (G) CUT&RUN chromatin occupancy of SAMD1 at the L3MBTL3, GATA2, and BCL11A loci in HUDEP-2 cells. (H) Quantitative ChIP quantitative polymerase chain reaction (qPCR) at the L3MBTL3, GATA2, and BCL11A promoters in HUDEP-2 cells infected with scramble guides in IgG and SAMD1 ChIP ( = 4). (I) Normalized RNA-seq of sgControl and sgSAMD1 primary erythroid progenitors at day 12 of erythroid differentiation ( = 4). RNA-seq counts were normalized based on reads per kilobase per million mapped reads (transcripts per million [TPM]). (J) Western blot of GATA2 and actin in day-12 CD34+ progenitors. Error bars represent standard deviation (SD). ∗P < .05; ∗∗P < .01 (2-tailed unpaired Student t test). IgG, immunoglobulin G; UTR, untranslated region.
To identify whether SAMD1 co-occupied chromatin with other erythroid transcription factors, we compared SAMD1-occupied sites with 294 transcription factors using publicly available chromatin immunoprecipitation sequencing data sets from erythroid cell types (HUDEP-2, K562, and primary erythroid progenitors).28 The most frequent co-occupancy factor was MYC associated factor X (MAX) (Figure 2F; supplemental Table 3). MAX and MYC coordinate MEP differentiation, and MYC is known to be required for erythropoiesis.29,30 Another top co-occupied factor is the erythroid transcription factor GATA binding protein 1 (GATA1). GATA1 and SAMD1 are co-occupied at 9356 chromatin sites (79%) whereas GATA2 is co-occupied at 1385 sites (11%). Among predicted direct target genes of SAMD1, the L3MBTL3 gene is SAMD1-regulated and contains a SAMD1-occupied cis element (Figure 2G). SAMD1 is also at promoters and distal sites upstream of the hematopoietic transcription factor GATA2, and at the promoter and intron 1 region of the B-cell leukemia transcription factor 11A (BCL11A) locus (Figure 2G). BCL11A represses fetal hemoglobin during adult erythropoiesis.31 SAMD1 occupancy at the L3MBTL3, GATA2, and BCL11A promoters was confirmed by chromatin immunoprecipitation using an anti-SAMD1 antibody (Figure 2H). SAMD1 knockout decreased GATA2 expression by 1.7-fold and increased L3MBTL3 and BCL11A expression by twofold and 1.4-fold, respectively (Figure 2I). At day 12 of our CD34+ HSPC erythroid differentiation, GATA2 protein decreased after SAMD1 knockout (Figure 2J). These data identify SAMD1 occupancy at upregulated and downregulated gene loci and a preferred GC-rich DNA binding motif for SAMD1 in erythroid progenitors.
SAMD1 suppresses erythropoiesis and promotes Kit signaling in human CD34+ HSPCs
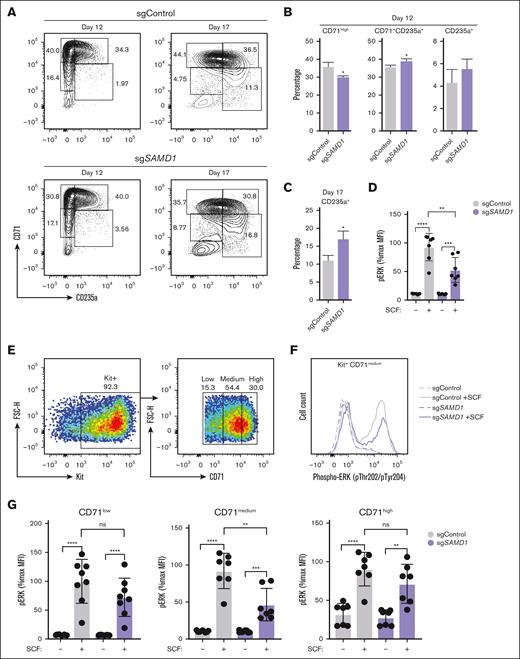
Decreased GATA2 and an increased erythroid transcriptional signature after SAMD1 knockout may reflect increased erythropoiesis. To examine whether SAMD1 knockout altered erythroid differentiation, we differentiated SAMD1 knockout human CD34+ cells using established protocols (Figure 1E).23 In cells infected with a Cas9– and SAMD1-targeting sgRNA-containing lentivirus (sgSAMD1) or a nontargeting scrambled sgRNA-expressing lentivirus (sgControl), we quantified the percentages of cells at progressive stages of erythroid differentiation by flow cytometry using cell surface markers CD71 and CD235. SAMD1 knockout increased the frequency of erythroid cells in differentiated CD34+ cultures (Figure 3A).23 SAMD1 knockout decreased the percentage of early erythroid (CD71med/high, CD235a−) cells by 1.2-fold, and increased the percentage of late erythroid (CD71−, CD235a+) cells by 1.3-fold on day 12, relative to sgControl (Figure 3B). On day 17, the percentage of late erythroid cells increased 1.5-fold in SAMD1 knockout cells vs controls (Figure 3C). Overall, erythroid-differentiating CD34+ cultures that express SAMD1 contain fewer late-stage erythroblasts compared with SAMD1-deleted cells. In conjunction with increased mRNA levels of erythroid gene expression after knockout, SAMD1 may normally reduce or limit erythroid differentiation by repressing erythroid transcriptional programs.
SAMD1 inhibits erythropoiesis and promotes Kit signaling in human CD34+ HSPCs. (A) Representative flow cytometry plot of anti-CD71 PE and anti-CD235a APC at days 12 and 17 of erythroid differentiation. (B) Quantitation of early erythroid (CD71high) and late erythroid (CD71+ CD235a+ and CD71− CD235a+) cells at day 12 of differentiation as determined by flow cytometry (n = 12). (C) Flow cytometric quantitation of mature erythrocytes (CD235a+) at day 17 of differentiation (n = 12). (D) Flow cytometric quantitation of the %max MFI in SCF-treated samples vs vehicle-treated controls at 5 minutes after SCF stimulation in the Kit+ CD71+ population at day 12 of erythroid differentiation (n = 8). (E) Representative flow cytometry gating strategy of Kit+ CD71low, CD71medium, and CD71high day-12 CD34+ HSPCs stimulated with SCF. (F) Representative plot of pERK (pThr202/pTyr204) MFI in the absence and presence of SCF stimulation in the Kit+ CD71medium population at day 12 of erythroid differentiation. (G) Quantitation of the %max MFI in SCF-treated samples vs vehicle-treated controls at 5 minutes after SCF stimulation in the Kit+ CD71low, CD71medium, and CD71high populations at day 12 of erythroid differentiation as determined by flow cytometry (n = 8). Error bars represent SD or standard error of the mean (SEM). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 (2-tailed unpaired Student t test). %max, percentage of maximum; FSC-H, forward scatter-height; MFI, median fluorescence intensity; ns, not significant.
SAMD1 inhibits erythropoiesis and promotes Kit signaling in human CD34+ HSPCs. (A) Representative flow cytometry plot of anti-CD71 PE and anti-CD235a APC at days 12 and 17 of erythroid differentiation. (B) Quantitation of early erythroid (CD71high) and late erythroid (CD71+ CD235a+ and CD71− CD235a+) cells at day 12 of differentiation as determined by flow cytometry (n = 12). (C) Flow cytometric quantitation of mature erythrocytes (CD235a+) at day 17 of differentiation (n = 12). (D) Flow cytometric quantitation of the %max MFI in SCF-treated samples vs vehicle-treated controls at 5 minutes after SCF stimulation in the Kit+ CD71+ population at day 12 of erythroid differentiation (n = 8). (E) Representative flow cytometry gating strategy of Kit+ CD71low, CD71medium, and CD71high day-12 CD34+ HSPCs stimulated with SCF. (F) Representative plot of pERK (pThr202/pTyr204) MFI in the absence and presence of SCF stimulation in the Kit+ CD71medium population at day 12 of erythroid differentiation. (G) Quantitation of the %max MFI in SCF-treated samples vs vehicle-treated controls at 5 minutes after SCF stimulation in the Kit+ CD71low, CD71medium, and CD71high populations at day 12 of erythroid differentiation as determined by flow cytometry (n = 8). Error bars represent SD or standard error of the mean (SEM). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 (2-tailed unpaired Student t test). %max, percentage of maximum; FSC-H, forward scatter-height; MFI, median fluorescence intensity; ns, not significant.
SAMD1 knockout decreased the expression of signal transduction genes in Kit+ cells. Kit signaling promotes early erythroid progenitor activity, whereas late erythroid progenitors shift to EPO dependence.32 The percentage of Kit+ erythroid cells did not change at day 12 between sgControl and sgSAMD1-expressing cells (supplemental Figure 2A). Cell surface Kit (measured by median fluorescence intensity [MFI]) was 1.2-fold higher in sgSAMD1-expressing cells (supplemental Figure 2B). To test the involvement of SAMD1 in signaling, we collected CD34+ cells at day 12 of erythroid differentiation, serum starved these cells, and stimulated the cells with the Kit ligand SCF (10 ng/mL). In Kit+ cells, phosphorylated extracellular signal-regulated kinase (pERK) levels (pThr202/pTyr204) were 1.8-fold lower in SAMD1 knockout cells vs controls, indicating SAMD1 promotes Kit signaling (Figure 3D). CD71 expression is a useful cell surface marker for drawing functional distinctions in Kit+ erythroid progenitor activity (low, medium, and high; Figure 3E).16,27 In Kit+ CD71med cells, containing colony-forming unit (CFU) erythroids, we observed 2.0-fold lower pERK levels than controls (P = .0029; Figure 3F-G). No significant differences were observed in SCF-induced pERK levels in the sgControl and sgSAMD1 cells within CD71 low and high populations (Figure 3G). SAMD1 inhibits erythroid differentiation and promotes Kit signaling.
Samd1 downregulation increases mouse erythropoiesis
Next, we tested Samd1 function in lineage-depleted mouse bone marrow (Figure 4A). Samd1-targeted short hairpin RNA (shRNA) reduced Samd1 expression 2.9-fold vs nontargeting control (Figure 4B). Samd1 protein was downregulated to different degrees using 2 distinct shRNAs (Figure 4C). We examined erythroid differentiation after 4 days of culture using preconjugated antibodies that recognize erythroid cell surface markers CD71 and Ter119 (Figure 4D). CD71low and CD71med/high contain early erythroid progenitors with progressively differentiating erythroblasts in CD71high Ter119+ and CD71− Ter119+.33,Samd1 knockdown decreased CD71low Ter119− and CD71high Ter119− 1.7-fold and 1.5-fold, respectively (Figure 4E). Conversely, Samd1 knockdown increased CD71high Ter119+ and CD71low Ter119+ by 1.8-fold and 11-fold, respectively (Figure 4E). Samd1 knockout cells appeared smaller and more mature in cytospun cultures stained with Wright-Giemsa than controls (Figure 4F). Percentages of Ter119high CD71high FSClow (late basophilic and polychromatic erythroblasts) and Ter119high CD71low FSClow (orthochromatic erythroblasts and reticulocytes) cells were 2.1-fold and 22-fold higher in Samd1 knockdown vs control cultures, indicating an increase in mature erythroid cells (supplemental Figure 3A-B).34 To evaluate Samd1 function in erythroid progenitors, we performed colony assays. Samd1 knockdown using 2 different shRNAs decreased burst-forming unit erythroid (BFU-E) colony formation by 1.5- or 1.2-fold and increased CFUs 1.3- or 1.5-fold (Figure 4G). Evaluation of the BFU-E–containing population by flow cytometry35 revealed a progressive decrease in Kit+ Ter119− CD71low (representing BFU-E–containing cells) after Samd1 knockdown compared with control cells over 4 days of differentiation (supplemental Figure 3C). Consistent with decreased CFU activity and data in human cells, Samd1 knockdown decreased Gata2 and Kit mRNA by 1.3-fold and 2.0-fold, respectively (Figure 4H). Erythroid genes hemoglobin alpha (Hba), tripartite motif–containing protein 10 (Trim10), and lysine-specific demethylase 7A (Kdm7a) increased 3.0-fold, 2.0-fold, and 1.5-fold, respectively (Figure 4H). Gata2, Trim10, and Kdm7a were SAMD1-regulated in mouse and human erythroid cells.
Samd1 inhibits murine erythropoiesis and megakaryopoiesis. (A) Schematic outlining 4-day erythroid and megakaryocyte (Mk) differentiation. (B) Samd1 mRNA in shRNA-expressing (GFP+) bone marrow at differentiation day 4 in shControl– and shSamd1_exon2–infected cells (n = 9). Normalized to 18s ribosomal RNA (rRNA). (C) Western blotting of SAMD1 in control and shSamd1-1– and shSamd1-2–infected primary mouse bone marrow (day 4 culture). (D) Representative flow cytometry plot of anti-CD71 PE and anti-Ter119 APC at day 4 of erythroid differentiation. (E) Quantitation of erythroid differentiation stages as determined by flow cytometry. Early erythroid progenitors (CD71+) and late erythroid progenitors (CD71+ Ter119+)/mature red blood cells (Ter119+; n = 12). (F) Representative images of Wright-Giemsa–stained day-4 GFP+ erythrocytes. (G) Quantitation of CFU-E (day 3) and BFU-E colonies (day 7) from GFP+ bone marrow (n = 27). (H) mRNA levels of erythroid and signaling genes Gata2, Kit, Hba (hemoglobin α), Trim10, Kdm7a, and Dusp2 in day-4 GFP+ erythroid cells (n = 9). Normalized to 18s rRNA. (I) Quantitation of mature Mks (CD41+ CD42d+) at day 4 of differentiation as determined by flow cytometry (n = 9). Error bars represent SEM and SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 (2-tailed unpaired Student t test). CFU-E, CFU-erythroid.
Samd1 inhibits murine erythropoiesis and megakaryopoiesis. (A) Schematic outlining 4-day erythroid and megakaryocyte (Mk) differentiation. (B) Samd1 mRNA in shRNA-expressing (GFP+) bone marrow at differentiation day 4 in shControl– and shSamd1_exon2–infected cells (n = 9). Normalized to 18s ribosomal RNA (rRNA). (C) Western blotting of SAMD1 in control and shSamd1-1– and shSamd1-2–infected primary mouse bone marrow (day 4 culture). (D) Representative flow cytometry plot of anti-CD71 PE and anti-Ter119 APC at day 4 of erythroid differentiation. (E) Quantitation of erythroid differentiation stages as determined by flow cytometry. Early erythroid progenitors (CD71+) and late erythroid progenitors (CD71+ Ter119+)/mature red blood cells (Ter119+; n = 12). (F) Representative images of Wright-Giemsa–stained day-4 GFP+ erythrocytes. (G) Quantitation of CFU-E (day 3) and BFU-E colonies (day 7) from GFP+ bone marrow (n = 27). (H) mRNA levels of erythroid and signaling genes Gata2, Kit, Hba (hemoglobin α), Trim10, Kdm7a, and Dusp2 in day-4 GFP+ erythroid cells (n = 9). Normalized to 18s rRNA. (I) Quantitation of mature Mks (CD41+ CD42d+) at day 4 of differentiation as determined by flow cytometry (n = 9). Error bars represent SEM and SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 (2-tailed unpaired Student t test). CFU-E, CFU-erythroid.
To explain the role of Samd1 in MEPs, consistent with expression data, we hypothesized that SAMD1 was inhibiting erythropoiesis in favor of megakaryopoiesis (Figure 1C). Using thrombopoietin to support megakaryopoiesis, we evaluated differentiation by flow cytometry after 4 days using the CD41 and CD42a surface markers of mature megakaryocytes.36 Surprisingly, the percentage of mature megakaryocytes increased 1.7-fold after Samd1 knockdown (Figure 4I). Thus, Samd1 knockdown increased erythropoiesis and megakaryopoiesis.
Samd1 alters the chromatin landscape of erythroid progenitors
SAMD1 and the histone demethylase LSD1 directly interact on chromatin.12 H3K4me2/3 marks a set of genes involved in erythroid differentiation.37 Western blotting of H3K4me2 and H3K4me3 in erythroid differentiated CD34+ cells at day 12 revealed an increase in the H3K4me2 mark and a trending decrease in H3K4me3 marks by western blotting (Figure 5A-B). To test a role for SAMD1 in H3K4 methylation status genome wide, we generated stable SAMD1 knockout HUDEP-2 cells, which did not change LSD1 expression (Figure 5C). The stage of erythroid maturation (assessed by CD71 and CD235A surface staining) was similar in sgControl- and sgSAMD1-infected HUDEP-2 expansion cultures (Figure 5D). However, in erythroid differentiation media (supplemental Figure 4A), early CD71+ erythroid progenitors decreased 1.5-fold in sgSAMD1 cultures at day 7 compared to control cells (supplemental Figure 4B-C). We observed a 3.2-fold and 1.5-fold increase in CD235a+ late erythroid progenitors/mature erythrocytes at day 7 and 10 of differentiation, respectively, indicating increased erythroid differentiation with loss of SAMD1, as was observed in our CD34+ erythroid differentiation (supplemental Figure 4B-D). Next, we performed CUT&RUN for SAMD1, LSD1, and H3K4me2/3 in sgControl and sgSAMD1 HUDEP-2 cells. SAMD1 knockout increased the average H3K4me2 peak signal genome wide, whereas H3K4me3 did not change (Figure 5E; supplemental Figure 5A). H3K4me2 and H3K4me3 peaks at SAMD1 motifs (GC-rich regions) were segregated based on occupancy of SAMD1 in control cells. Whereas SAMD1-occupied sites contained higher H3K4me2 and H3K4me3 levels than non–SAMD1-bound regions, SAMD1 knockout increased H3K4me2 and decreased H3K4me3 at these sites (Figure 5F; supplemental Figure 5B). The number of significantly changing (P < .01) individual H3K4me2 sites with and without SAMD1 occupancy revealed that H3K4me2 signal increased at 3044 (of 10 366 total) sites after SAMD1 knockout, compared with only 547 sites at which H3K4me2 signal was decreased (Figure 5G). H3K4me3 signal increased at 7617 (of 8881 total) sites and decreased at 2719 sites (supplemental Figure 5C). Nearest gene annotation of SAMD1-occupied H3K4me2 sites that were increasing after SAMD1 knockout revealed a gene signature (based on gene set enrichment analysis) consistent with erythroid cells (Figure 5H). Decreasing H3K4me3 peaks did not annotate to any specific phenotype (supplemental Figure 5D).
SAMD1 alters the chromatin landscape of erythroid progenitors. (A) Western blot of histone 3 methylation marks and total histone 3 at differentiation day 12. (B) Densitometry analysis of the histone 3 methylation marks. Relative to total histone 3 (n = 8). (C) Western blot of SAMD1, LSD1, and β-actin in HUDEP-2 cells. (D) Representative flow cytometry plot of anti-CD71 PE and anti-CD235a APC of sgControl sgSAMD1 HUDEP-2 cells. (E) Violin plot of all H3K4me2 peak signals in scramble (sgControl) and SAMD1 knockout (sgSAMD1) HUDEP-2 cells (2-tailed unpaired Student t test). (F) Scatterplot of H3K4me2 peak signal at CpG islands overlapping with SAMD1 or not. (G) Scatter plot of peak signal at overlapping H3K4me2 peaks in sgControl and sgSAMD1 HUDEP-2 cells divided into overlapping with a SAMD1 peak or not. Significantly changing peaks calculated by manorm using H3K4me2 read density at peak.38 (H) GSEA of annotated significantly changing (P < .05) H3K4me2 peaks overlapping with SAMD1. (I) Chromatin occupancy profile and normalized scores from CUT&RUN of SAMD1, LSD1, and H3K4me2 at the SLC4A1, SP3, and BCL11A loci in sgControl and sgSAMD1 HUDEP-2 cells. Δ(sgSAMD1 – sgControl) track indicates the change in H3K4me2 after SAMD1 knockout. Red box indicates the normalized score region (n = 2; 2-tailed paired Student t test). (J) Normalized RNA-seq of sgControl and sgSAMD1 primary erythroid progenitors at day 12 of erythroid differentiation (n = 4). RNA-seq counts were normalized based on reads per kilobase per million mapped reads (TPM). (K) Enrichr analysis of annotated H3K4me2 peaks increasing upon SAMD1 knockout, using data from the GWAS catalog. Error bars represent SD. ∗∗∗P < .001; ∗∗∗∗P < .0001. GSEA, gene set enrichment analysis; GWAS, genome-wide association study; NES, normalized enrichment score; ns, not significant.
SAMD1 alters the chromatin landscape of erythroid progenitors. (A) Western blot of histone 3 methylation marks and total histone 3 at differentiation day 12. (B) Densitometry analysis of the histone 3 methylation marks. Relative to total histone 3 (n = 8). (C) Western blot of SAMD1, LSD1, and β-actin in HUDEP-2 cells. (D) Representative flow cytometry plot of anti-CD71 PE and anti-CD235a APC of sgControl sgSAMD1 HUDEP-2 cells. (E) Violin plot of all H3K4me2 peak signals in scramble (sgControl) and SAMD1 knockout (sgSAMD1) HUDEP-2 cells (2-tailed unpaired Student t test). (F) Scatterplot of H3K4me2 peak signal at CpG islands overlapping with SAMD1 or not. (G) Scatter plot of peak signal at overlapping H3K4me2 peaks in sgControl and sgSAMD1 HUDEP-2 cells divided into overlapping with a SAMD1 peak or not. Significantly changing peaks calculated by manorm using H3K4me2 read density at peak.38 (H) GSEA of annotated significantly changing (P < .05) H3K4me2 peaks overlapping with SAMD1. (I) Chromatin occupancy profile and normalized scores from CUT&RUN of SAMD1, LSD1, and H3K4me2 at the SLC4A1, SP3, and BCL11A loci in sgControl and sgSAMD1 HUDEP-2 cells. Δ(sgSAMD1 – sgControl) track indicates the change in H3K4me2 after SAMD1 knockout. Red box indicates the normalized score region (n = 2; 2-tailed paired Student t test). (J) Normalized RNA-seq of sgControl and sgSAMD1 primary erythroid progenitors at day 12 of erythroid differentiation (n = 4). RNA-seq counts were normalized based on reads per kilobase per million mapped reads (TPM). (K) Enrichr analysis of annotated H3K4me2 peaks increasing upon SAMD1 knockout, using data from the GWAS catalog. Error bars represent SD. ∗∗∗P < .001; ∗∗∗∗P < .0001. GSEA, gene set enrichment analysis; GWAS, genome-wide association study; NES, normalized enrichment score; ns, not significant.
SAMD1 knockout caused locus-specific changes to H3K4me2 levels at multiple erythroid gene promoters. Among these, H3K4me2 levels increased at the band 3 anion transport protein (SLC4A1), Sp3 transcription factor (SP3), and BCL11A loci (Figure 5I). Decreased H3K4me3 and H3K4me2 were observed at loci encoding FLNA and MAP2K7, for example (supplemental Figure 5E). H3K4me2/3 changes after SAMD1 knockout corresponded to changes in mRNA levels of SLC4A1 (1.3-fold up), BCL11A (1.4-fold up), and FLNA (1.5-fold down), but not SP3 or MAP2K7 (Figures 2I and 5J; supplemental Figure 5F). In genome-wide association studies, SAMD1-occupied sites at which the H3K4me2 signal increased after SAMD1 knockout contain single-nucleotide polymorphisms associated with red blood cell features (Figure 5K). Overall, our results reveal that SAMD1 coordinates H3K4me2/3 methylation, possibly via LSD1 activity.
An increase in H3K4me2 levels indicates a role for SAMD1 in LSD1 activity. Our CUT&RUN results revealed SAMD1 and LSD1 overlapped at 75% of SAMD1 binding sites (8845; Figure 6A). SAMD1 knockout increased H3K4me2 signal at SAMD1 and LSD1 peaks to a higher degree than H3K4me2 peaks in which only SAMD1 is bound (Figure 6B). Interestingly, H3K4me3 levels decreased more at SAMD1/LSD1 co-occupied sites than SAMD1-only sites (supplemental Figure 5G).
SAMD1 opposes and promotes LSD1 activity in erythroid progenitors. (A) Venn diagram of SAMD1 and LSD1 binding sites in scramble HUDEP-2 cells. (B) Change in H3K4me2 peak signal between sgControl and sgSAMD1 HUDEP-2 cells in the presence or absence of LSD1 (2-tailed unpaired Student t test). (C) Chromatin occupancy profile from CUT&RUN of SAMD1 and H3K4me2 at the GATA2, RUNX1, SPI1, and TAL1 loci in sgControl and sgSAMD1 HUDEP-2 cells (2-tailed paired Student t test). (D) Normalized RNA-seq of sgControl and sgSAMD1 primary erythroid progenitors at day 12 of erythroid differentiation (n = 4). RNA-seq counts were normalized based on reads per kilobase per million mapped reads (TPM). (E) mRNA levels of SLC4A1 and GATA2 after 24 hours of either dimethyl sulfoxide alone, 10 nM or 100 nM of GSK-LSD1 treatment in sgControl and sgSAMD1 HUDEP-2 cells (n = 6; 1-way analysis of variance). Not all statistically significant values in panel E are indicated. Error bars represent SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
SAMD1 opposes and promotes LSD1 activity in erythroid progenitors. (A) Venn diagram of SAMD1 and LSD1 binding sites in scramble HUDEP-2 cells. (B) Change in H3K4me2 peak signal between sgControl and sgSAMD1 HUDEP-2 cells in the presence or absence of LSD1 (2-tailed unpaired Student t test). (C) Chromatin occupancy profile from CUT&RUN of SAMD1 and H3K4me2 at the GATA2, RUNX1, SPI1, and TAL1 loci in sgControl and sgSAMD1 HUDEP-2 cells (2-tailed paired Student t test). (D) Normalized RNA-seq of sgControl and sgSAMD1 primary erythroid progenitors at day 12 of erythroid differentiation (n = 4). RNA-seq counts were normalized based on reads per kilobase per million mapped reads (TPM). (E) mRNA levels of SLC4A1 and GATA2 after 24 hours of either dimethyl sulfoxide alone, 10 nM or 100 nM of GSK-LSD1 treatment in sgControl and sgSAMD1 HUDEP-2 cells (n = 6; 1-way analysis of variance). Not all statistically significant values in panel E are indicated. Error bars represent SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
At loci encoding transcription factors essential for HSPC maintenance and differentiation, for example the GATA2 promoter; GATA2 −77 kilobase enhancer; and the RUNX1, TAL1, and PU.1 (SPI1) promoters, SAMD1 knockout decreased H3K4me2 levels (Figure 6C). Other than GATA2 mRNA, SAMD1 knockout did not change expression levels of hematopoietic transcription factors in erythroid-committed CD34+ HSPCs (Figures 2I and 6D). Because H3K4me2 is associated with poised/active transcription, H3K4me2 methylation changes at loci essential for hematopoietic lineage commitment suggest a role for SAMD1 in hematopoiesis
To evaluate whether SAMD1-mediated gene expression was dependent on the activity of LSD1, we treated sgControl and sgSAMD1 HUDEP-2 cells with an enzymatic inhibitor of LSD1, GSK-LSD1.39 In SAMD1 knockout cells treated with the LSD1 inhibitor, the SAMD1-repressed SLC4A1 mRNA levels were 2.3-fold higher than in sgControl cells (Figure 6E). Conversely, LSD1 inhibition decreased SLC4A1 expression in both sgControl (1.8-fold) and sgSAMD1 (1.9-fold; Figure 6E). Distinct LSD1-dependent and -independent activities of SAMD1 were observed at other SAMD1-repressed genes, BCL11A, SP3, and KDM7A (supplemental Figure 6A). SAMD1’s ability to promote GATA2 expression was unaffected by LSD1 inhibition (Figure 6E). Similar results were observed at other SAMD1-activated genes FLNA and MAPK3 (supplemental Figure 6B). These data support models of SAMD1 transcriptional regulation that are both dependent and independent of LSD1 activity.
Samd1 inhibits HSPC differentiation in vivo
To examine in vivo Samd1 function, we transplanted equal numbers of control shRNA-infected CD45.1+ cells with CD45.2+ HSPCs infected with shSamd1 or control shRNA into irradiated CD45.1+ recipient mice (Figure 7A). At 16 weeks after transplant, Samd1 mRNA was 2.7-fold lower in shSamd1 CD45.2+ HSPCs vs control infections (Figure 7B). We observed a 30% increase (P = .008) in the peripheral blood CD45.2+ fraction of the mature hematopoietic cell types after Samd1 knockdown (Figure 7C; supplemental Figure 7A). shSamd1-infected hematopoietic stem cells also contributed to higher numbers of B cells (2.3-fold), granulocytes (2.9-fold), and monocytes (2.4-fold) at 16 weeks after transplant than shControl-infected cells (Figure 7D). We also noted a 4.1-fold increase in granulocytes and a 3.6-fold decrease in T cells in Samd1 knockdown–transplanted HSPCs, indicating a myeloid bias with Samd1 loss (Figure 7E).
Samd1 inhibits HSPC differentiation in vivo. (A) Schematic outlining the in vivo competitive transplant assay. (B) Samd1 mRNA in shRNA-expressing GFP+ CD45.2+ bone marrow at week 16 (n = 4). Normalized to 18s rRNA. (C) Quantitation of total GFP+ CD45.2+ peripheral blood (PB) at weeks 4, 8, 12, and 16 after transplant as determined by flow cytometry (n = 11). (D) Quantitation of GFP+ CD45.2+ B cells (CD19+), T cells (Thy1.2+), granulocytes (GR-1+ CD11b+), and monocytes (CD11b+) from PB at weeks 4, 8, 12, and 16 after transplant as determined by flow cytometry (n = 11). (E) Cell-type make-up of CD45.2+ fraction of PB at weeks 4, 8, 12, and 16 as determined by flow cytometry (n = 11). (F) Quantitation of CD71+ erythroid progenitors from 16 weeks after transplant bone marrow (CD45.2+ GFP+) as determined by flow cytometry (n = 4). (G) Quantitation of Kit MFI of CD71+ erythroid progenitors from 16 weeks after transplant bone marrow (CD45.2+ GFP+) as determined by flow cytometry (n = 4). (H) Quantitation of total CD45.2+ (GFP+) bone marrow at week 16 of transplant as determined by flow cytometry (n = 4). (I) Proposed model of SAMD1 mechanism in erythropoiesis. Error bars represent SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 (2-tailed unpaired Student t test). CT, control; KD, knockdown.
Samd1 inhibits HSPC differentiation in vivo. (A) Schematic outlining the in vivo competitive transplant assay. (B) Samd1 mRNA in shRNA-expressing GFP+ CD45.2+ bone marrow at week 16 (n = 4). Normalized to 18s rRNA. (C) Quantitation of total GFP+ CD45.2+ peripheral blood (PB) at weeks 4, 8, 12, and 16 after transplant as determined by flow cytometry (n = 11). (D) Quantitation of GFP+ CD45.2+ B cells (CD19+), T cells (Thy1.2+), granulocytes (GR-1+ CD11b+), and monocytes (CD11b+) from PB at weeks 4, 8, 12, and 16 after transplant as determined by flow cytometry (n = 11). (E) Cell-type make-up of CD45.2+ fraction of PB at weeks 4, 8, 12, and 16 as determined by flow cytometry (n = 11). (F) Quantitation of CD71+ erythroid progenitors from 16 weeks after transplant bone marrow (CD45.2+ GFP+) as determined by flow cytometry (n = 4). (G) Quantitation of Kit MFI of CD71+ erythroid progenitors from 16 weeks after transplant bone marrow (CD45.2+ GFP+) as determined by flow cytometry (n = 4). (H) Quantitation of total CD45.2+ (GFP+) bone marrow at week 16 of transplant as determined by flow cytometry (n = 4). (I) Proposed model of SAMD1 mechanism in erythropoiesis. Error bars represent SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 (2-tailed unpaired Student t test). CT, control; KD, knockdown.
In posttransplant bone marrow after 16 weeks, the CD45.2+ fraction was 2.8-fold higher in shSamd1 knockdown vs competitor (Figure 7F). The percentage of CD45.2+ CD71+ cells were similarly overrepresented 2.7-fold compared with controls (Figure 7G). Cell surface Kit was 2.4-fold lower in Samd1 knockdown cells vs competitor (Figure 7H). We also evaluated CD45.2+ progenitors in the bone marrow. CD45.2+ hematopoietic stem cells, LSKs were slightly elevated (supplemental Figure 7B-C). Kit MFI was not altered in the LSKs (supplemental Figure 7D). CD45.2+ LSKs contained more MEPs, granulocyte-monocyte progenitors (GMPs), common lymphoid progenitors (CLPs) and common myeloid progenitors (CMPs) than CD45.1+ competitors (supplemental Figure 7E), suggesting that Samd1 is coordinating HSPC expansion/differentiation in in vivo contexts. Samd1 mechanisms of gene regulation in HSPCs may be valuable targets for improving stem cell functionalities after transplantation.
To evaluate whether SAMD1 regulates GATA2 levels in undifferentiated HSPCs, mRNA was isolated from sgControl and sgSAMD1 human CD34+ HSPCs after a 6-day expansion. GATA2 expression was similar between the 2 groups at both the protein and mRNA level (supplemental Figure 8A-B). Other transcription factors associated with stem/progenitor cells also did not change between sgControl and sgSAMD1 CD34+ HSPCs (supplemental Figure 8B).
Discussion
Some of our findings regarding SAMD1 function in HSPCs are consistent with a working model of transcriptional repression in which SAMD1 cooperates with LSD1.13,40 However, SAMD1 can both upregulate and downregulate gene expression in LSD1-dependent and LSD1-independent manners, and its activities can vary from locus to locus (Figure 7I). Moreover, the transcriptional outcomes of SAMD1 loss in HSPCs at specific sites (eg, its role in GATA2 regulation) are distinct from outcomes after erythroid commitment. We attribute the distinct phenotypes elicited by LSD1 and SAMD1 loss of function to distinct activities and/or occupancy sites on chromatin. For example, LSD1 and SAMD1 co-occupy the 1S promoter and −77 enhancer of the GATA2 locus. However, SAMD1 knockout decreases H3K4me2 at the −77 enhancer whereas LSD1 knockdown increases H3K4me2 at the same site,41 suggesting that either SAMD1 is repressing LSD1 activity/occupancy or recruiting cofactors that counteract LSD1 activity/occupancy. Likewise, SAMD1 can promote GATA2 expression independent of LSD1 activity.
Our data highlight genome-wide and locus-specific mechanisms through which transcription factors enable and refine the roles of other transcription factors or epigenetic readers. SAMD1 interacts with itself through a SAM domain and with the SAM protein SFMBT1, which was identified in a list of SAM proteins expressed in erythroid progenitors.12,42 Whereas SFMBT1 has no known role in erythropoiesis, it facilitates LSD1 activities, resulting in transcriptional repression.42 Thus, SAMD1, LSD1, and SFMBT1 may form a complex to coordinate H3K4 methylation for a specific set of erythroid genes. Dozens of proteins are co-occupied with SAMD1, including GATA1, MYC, and MAX, with known roles in erythropoiesis, which may promote, oppose, or have no influence on SAMD1-LSD1 activity at specific sites. A plethora of additional positive and negative regulatory factors are known to coordinate LSD1 cell-type and locus-specific activities including CoREST and L3MBTL3.43 Because of roles in demethylation of repressive marks, LSD1 also promotes gene activation.44,45 SAMD1 functions are consistent with both mechanisms, contributing to foundational knowledge regarding context-dependent transcription factor regulation.46-48
During erythropoiesis, poised H3K4me2+/H3K4me3− promoters lose their H3K4me2 mark genome-wide coinciding with gene repression at select sites.37 Whereas SAMD1 knockout increased erythroid differentiation, LSD1 loss-of-function studies have shown its requirement for erythroid lineage commitment.6,7,26 SAMD1 and LSD1 may work together to modulate H3K4 methylation at poised promoters in HSPCs to maintain the progenitor pool39,49 but act in oppositional or distinct manners during differentiation. Loss of SAMD1 increases H3K4me2 levels genome wide and at erythroid-specific genes including BCL11A, SLC4A1, and SP3.20 However, we observed that SAMD1 occupancy correlated with higher H3K4me2 levels at a subset of loci that are critical for HSPC activities (eg, GATA2, TAL1, and PU.1). Distinct SAMD1 activities within cellular contexts of self-renewal and differentiation are consistent with previous SAMD1 work in ES cells, in which a much larger cellular and molecular phenotype of SAMD1 loss emerged after differentiation.12
The discovery of SAMD1 as a new coordinator of histone methylation at vital HSPC regulatory cis elements reflects an exciting new path of investigation given the correlations between H3K4 methylation status and hematologic disease states. The SAMD1-LSD1–occupied distal region of GATA2 is a crucial cis regulatory element driving hematopoietic development and deregulated in myelodysplasia.50,51 Both SAMD1 and LSD1 are commonly upregulated in acute myeloid leukemia, and high expression is correlated with poor prognosis.12 A generalizable role for SAMD1 in hematopoiesis offers new avenues for increasing HSPC activity to improve transplantation therapeutics. We established bimodal SAMD1 functions in altering chromatin landscapes to direct transcription during progenitor cell transitions.
Acknowledgments
The authors thank the Bresnick laboratory for kindly providing the anti-GATA2 antibody.
This study was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (R01 HL155439); funding for K.J.H and S.B. from the Nebraska Center for Molecular Target Discovery and Development (1P20GM121316); and an F31 predoctoral fellowship for Meg Schaefer (1F31HL172634). S.B. is supported by the NIH/National Institute of General Medical Sciences (NIGMS; R00 GM138920). The authors are grateful to the core facilities at University of Nebraska Medical Center (UNMC). Most notably, the Flow Cytometry Research Facility, which is supported by the Nebraska Research Initiative and The Fred and Pamela Buffett Cancer Center's (FPBCC) National Cancer Institute Cancer Support Grant. The UNMC Genomics Core Facility receives partial support from the NIGMS IDeA Networks of Biomedical Research Excellence (INBRE) (P20GM103427), as well as the National Cancer Institute and the FPBCC Support Grant (P30CA036727).
These contents are the sole responsibility of the authors and do not necessarily represent the official views of the NIH.
Authorship
Contribution: M.A.S. conceptualized and designed research, conducted research, analyzed data, performed bioinformatics analyses, and wrote the manuscript; V.R.D. conducted research and performed bioinformatics analyses; S.L.G., Y.Z., P.R., S.R., and L.C. conducted research and contributed to methodology; S.B. contributed to methodology and aided in bioinformatics analyses; R.L. aided in study design and provided Samd1 antibody; and K.J.H. conceptualized and designed research, conducted research, analyzed data, supervised the research, and revised and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kyle J. Hewitt, Department of Genetics, Cell Biology and Anatomy, University of Nebraska Medical Center, 985805 Nebraska Medical Center, S Saddle Creek Dr, Omaha, NE 68198; email: kyle.hewitt@unmc.edu.
References
Author notes
The full-text version of this article contains a data supplement.



![SAMD1 directs the transcription of erythroid progenitors. (A) Heat map of CUT&RUN signal showing SAMD1 enrichment at SAMD1 peaks compared with IgG in HUDEP-2 cells (n = 11 798). (B) Annotation of SAMD1 peaks at genomic features in HUDEP-2 cells. (C) Annotation of SAMD1 peaks at significantly changing genes from the RNA-seq of sgSAMD1 vs sgControl erythroid progenitors. (D) Enriched motif at SAMD1-bound peaks. (E) Venn diagram of SAMD1 occupancy sites in HUDEP-2 and ES cells. (F) Table of co-occupied transcription factors with SAMD1 occupancy sites from publicly available chromatin immunoprecipitation (ChIP) data in HUDEP-2, K562, and primary erythroid progenitors. The signal for each peak is derived from 2 replicates. (G) CUT&RUN chromatin occupancy of SAMD1 at the L3MBTL3, GATA2, and BCL11A loci in HUDEP-2 cells. (H) Quantitative ChIP quantitative polymerase chain reaction (qPCR) at the L3MBTL3, GATA2, and BCL11A promoters in HUDEP-2 cells infected with scramble guides in IgG and SAMD1 ChIP ( = 4). (I) Normalized RNA-seq of sgControl and sgSAMD1 primary erythroid progenitors at day 12 of erythroid differentiation ( = 4). RNA-seq counts were normalized based on reads per kilobase per million mapped reads (transcripts per million [TPM]). (J) Western blot of GATA2 and actin in day-12 CD34+ progenitors. Error bars represent standard deviation (SD). ∗P < .05; ∗∗P < .01 (2-tailed unpaired Student t test). IgG, immunoglobulin G; UTR, untranslated region.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/9/15/10.1182_bloodadvances.2024015627/2/m_blooda_adv-2024-015627-gr2.jpeg?Expires=1764990886&Signature=iDytrxzXP4LC-cZeZa~3IUfU15Pal5WvNXn4W9SpkkiXK2MPDAfMnK-fbmSjFwYsPxljd5rOdnAQRq7hAMnrnX-SLOpHw2Dbr1c90vRZiXANMzbOsdcgmrmJr6hCgHKId01VVwW7kDyyMKgislVjCVi8s8RIGqwLkOHPk~5T0H2~hCZtNerFl8~ojr8PkCj7TKkdeQFAStszsV0l7l0mLfsBBnZFfl0qgxhHWefh9hZVhjTd8vx-5rAoQIcLUCMI47B-bYXRIqRQKcd0DwDcIYBRNShCN0y7P7e~I9K9OuDdPErHhtXSSBuAqDMqXTK~MypluMxbYadBa0y~BUqL7Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)