Key Points
AID-imprinted B cells overexpressing REL leads to both an exacerbated GC reaction and clonal expansion.
REL provides a long-term competitive advantage allowing for GCB persistence and continuous recirculation of AID-imprinted B cells.
Visual Abstract
In diffuse large B-cell lymphomas (DLBCLs), gains and amplifications of the 2p15-16 region, which always encompass the REL gene, are mostly restricted to the germinal center (GC) B-cell DLBCL subtype (GCB-DLBCL) for which c-Rel is the pivotal Rel/NF−κΒ subunit. Although REL plays a key role in the GC reaction, its contribution to GCB-DLBCLs remains unclear.
To understand the role of REL in the very first steps of GCB transformation, that is, when B cells with deregulated REL are competing with other B cells during chronic antigenic stimulation, we have created a dual-color mouse model that allows to induce REL in a limited pool of activation-induced cytidine deaminase (AID)-imprinted B cells after immunization and to differentially stain AID-imprinted B cells that overexpress REL or not. Dysregulation of REL in AID-imprinted B cells was associated with nuclear c-Rel overexpression in GCs 14 days after immunization. Dysregulation of REL at the GCB stage promoted GCB expansion, which was associated with both class-switch recombination and plasma cell differentiation. REL overexpression conferred a long-term competitive advantage, allowing GC persistence and continuous recirculation of REL-overexpressing B cells. IgHV dominance was increased at the messenger RNA level in REL-overexpressing B cells and clonal expansion was detected at the DNA level in some cases. Highlighting the role of the immune response, our results demonstrate the advantage conferred by REL in the GC competition and provide evidence that, as an oncogenic event of GCBs, its genetic deregulation induces the generation of a long-term pool of lymphoma precursor cells.
Introduction
c-Rel, the cellular equivalent of v-Rel,1 the oncogenic protein of reticuloendotheliosis virus, a causative agent of lymphoid tumors in poultry, gave its name to the Rel homology domain, the DNA-binding domain that characterizes the Rel/NF-κB transcription factor family.2 Rel/NF-κB transcription factors are composed of 5 subunits, RelA, c-Rel, RelB, p50, and p52, the 3 formers with a transcriptional activation domain. Constitutive NF-κΒ activation is the hallmark of several B-cell neoplasms, either indolent such as Waldenström macroglobulinemia, which harbors a MYD88 activating mutation in >90% cases or aggressive lymphomas such as Epstein-Barr virus related lymphoproliferative disorders with expression of the Epstein-Barr virus oncogenic protein LMP1 or diffuse large B-cell lymphomas (DLBCLs) with an activated phenotype. In this latter group, NF-κΒ activation is genetically related to mutations in the NF-κΒ activation track such as those of MYD88, CD79B, CARD11, or TNFAIP3.3
It has only recently been recognized that the 3 NF-κΒ subunits RelA, RelB, and c-Rel may have distinct and specific roles in B-cell lymphomagenesis, the latter being associated with DLBCLs with a germinal center (GC) B-cell phenotype (GCB-DLBCLs).4-6 In DLBCLs, 2p15-16 gains/amplifications, which include REL, are almost restricted to the GCB-DLBCL subtype,7,8 being found in 15% to 37% of cases,9 whereas it is almost never found in activated phenotype-DLBCLs.7 Some reports indicate that the minimal common region of 2p15-16 gains/amplifications always includes REL.10,REL gains/amplifications, which are the first genetic aberration of the Rel/NF-κΒ system reported in DLBCLs,11 are recurrently found in various B-cell cancers such as classical Hodgkin lymphoma, primary mediastinal B-cell lymphoma, or chronic lymphocytic leukemia.12 Such REL imbalances are also found in 20% to 35% of follicular lymphomas (FLs) in transformation, a poor prognosis FL subtype, close to and with a similar mutational pattern than GCB-DLBCLs.9 c-Rel does not contain a nuclear export signal and can accumulate in the nucleus upon chronic NF-κΒ activation,13 which explains why, in contrast to other NF-κΒ subunits, c-Rel overexpression is very likely to be sufficient to obtain the c-Rel effects.5,14,15 c-Rel plays a critical role in sustaining prolonged NF-κB responses in B cells and chronic B-cell receptor (BCR) stimulation results in nuclear c-Rel accumulation with upregulation of prosurvival genes, whereas nuclear RelA is almost undetectable under these conditions.14 The REL–/– mice exhibit reduced proliferation and activation of mature B cells in response to immunization associated with impaired GC formation.16 In normal B cells, c-Rel is a key transcription factor for GC reaction long-term maintenance.17 In GCs, c-Rel and c-Myc would be active in the same B cells and c-Rel would be involved in a metabolic program that would facilitate cell growth.18
To address the question of the role of REL at the very first steps of GCB transformation, we developed a dual-color mouse model that allows to induce c-Rel overexpression in a limited pool of activation-induced cytidine deaminase (AID)-imprinted B cells after immunization with a complex antigen and to differentially stain AID-imprinted B cells that overexpress REL or not.
Material and methods
Mouse models
The conditional REL-internal ribosome entry sequence (IRES)-yellow fluorescent protein (YFP) mouse model was generated for this study. The targeting vector pROSA-mREL-YFP was obtained by insertion of a 3521 pb AscI/AscI fragment containing the murine REL complementary DNA followed by an IRES for the expression of the YFP coding sequence (the REL-IRES-YFP transgene, synthetized by GeneCust, Mondorf-les-Bains, Luxembourg), into the previously published pROSA26-1 vector.19
The AID.Creert2-TOM model (thereafter called AID-TOM) was obtained by crossing previously published AID.Creert2 and Ai14 mouse models.20 AID-TOM mice are detailed in the supplemental Materials and methods. AID-TOM REL-IRES-YFP mice were crossed to induce the expression of the transgene in AID-imprinted B cells: the REL-AID mouse model.
B-cell purification, messenger RNA (mRNA) high-throughput sequencing and bioinformatic analyses are described in supplemental Materials and Methods.
All procedures were performed under an approved protocol according to European guidelines for animal experimentation (French national authorization number: 8708503 and French Ethics Committee registration number APAFIS#26105-2020061810023698 v1).
Tamoxifen induction and immunization
Immunization consisted of an intraperitoneal injection of 2.109 sheep red blood cells (SRBCs; Sarl Atlantis, Paris, France) on day 0 (D0) and monthly thereafter. Tamoxifen, purchased from Gibco (Gibco, Thermo Fisher Scientific, Illkirch-Graffenstaden, France), was administrated by gavage at 400 mg/kg on D0, D2, and D4.
Flow cytometry analysis from blood, lymphoid organs, and bone marrow
Blood samples were collected retro-orbitally. Spleen and lymph node cells were filtered on a 70 μm filter (Miltenyi Biotec SAS, Paris, France). Peritoneal lavage was performed with 3 mL of phosphate-buffered saline. Bones of both hind legs were rinsed with phosphate-buffered saline to collect the bone marrow cells. After red blood cell lysis, cells were labeled with fluorescent-conjugated monoclonal antibodies listed in supplemental Materials and Methods. Flow cytometry was performed on the CytoFLEX LX instrument (Beckman Coulter France, Villepinte, France). Results were analyzed using the Kaluza software (Beckman Coulter).
Immunofluorescence on isolated cells and tissue sections
CD19+ TOM+/YFP+ and TOM+ cells from REL-AID and AID-TOM mice were sorted on an Aria2 cell sorter (Becton Dickinson, Franklin Lakes, NJ). Cells were plated on poly-L-lysine coated slide and were fixed and permeabilized with reagents from the FOXP3-compatible kit according to the manufacturer’s protocol (eBioscience, San Diego, CA). The eFluor 660 monoclonal antibody 1RELAH5 against c-Rel was from eBioscience, was diluted at 1:50 and was incubated for 72 hours at 4°C, as was the isotype control (eBioscience). Cells were counterstained with DAPI (4′,6-diamidino-2-phenylindole). Fluorescence acquisition was done on the LSM880 confocal microscope (Carl Zeiss Microscopy GmbH, Jena, Germany).
Tissues were fixed in periodate–lysine–paraformaldehyde and frozen at −80°C according to Fra-Bido et al21 Ten micrometer tissue cryosections were either surface (peanut agglutinin [PNA], immunoglobulin D [IgD]) or intracellular (c-Rel and Ki67) labeled. For surface markers, sections were incubated with primary reagents for 1 hour: IgD-AF647 (1/100; BioLegend, San Diego, CA) or PNA-biotin (1/500; Vector Laboratories), followed by streptavidin-AF647 (1/1000; Thermo Fisher Scientific) for PNA. For c-Rel and Ki67 (1/500; Sigma-Aldrich-Merck), fixation/permeabilization and labeling were performed as described above for sorted cells.
Analysis of all fluorescence images was performed with the QuPath: Open source software.22
Statistical analysis
Statistical analyses were done with the GraphPad software (GraphPad Software, Boston, MA). These included Student t tests, Fisher exact tests, χ2 tests, Mann-Witney nonparametric test, Wilcoxon nonparametric paired tests, Kruskal-Wallis nonparametric test, and analysis of variance.
All procedures were performed under an approved protocol according to European guidelines for animal experimentation (French national authorization number: 8708503 and French Ethics Committee registration number APAFIS#26105-2020061810023698 v1).
Results
AID-imprinted B cells overexpressing REL or not can be differentially fate-mapped
To understand the consequences of REL constitutive overexpression at the GCB stage and beyond, we first generated the REL-IRES-YFP mouse model. Preceded by a STOP-Neomycin (STOP-Neo) cassette flanked by loxP sites and with a specific upstream KOZAK sequence that we previously used23 (refer to sequence in supplemental Materials and Methods), the native murine REL transgene was introduced in the Rosa26 locus on chromosome 6 (Figure 1A, top panel). The REL transgene is followed by an IRES to allow concomitant expression of the yellow fluorescent marker YFP. In parallel, we modified the tamoxifen-regulatable AID.Creert2 mouse model of Dogan et al.20 to introduce a sequence encoding the red tdTomato marker (TOM) preceded by a STOP cassette flanked by loxP sites in cis to AID.Creert2 allele at the Rosa26 locus (Figure 1A; supplemental Materials and Methods). Breeding REL-IRES-YFP with AID-TOM mice resulted in the dual-color REL-AID model. In this model, the 2 tdTomato and REL-YFP transgenes are both AID-inducible and tamoxifen-regulatable.

Characterization of the REL-AID mouse model. (A) Schematic representation of the REL-IRES-YFP and tdTomato inserts into the Rosa26 locus: the REL sequence was placed in frame with the IRES and the coding sequence for YFP. On the same chromosome 6 bearing tdTomato trangene, Creert2 was inserted into Aicda locus. (B) Schematic diagram of SRBC (red arrow) and tamoxifen (black arrow) administration protocol for mouse analysis (green arrow) at different time points after immunizations. Blood samples were collected on D14 and then each month, always before immunization boost (blue arrow). (C) Example of a flow cytometry biparametric YFP and tdTomato (TOM) histogram gated on live B220pos B cells. TOM+/YFP– and TOM+/YFP+ B cells are colored in red and green, respectively. PB cells were collected from an AID-TOM (left) or a REL-AID (right) mouse 14 day after immunization and tamoxifen gavage. Percentages of fluorescent cells are shown in each graph. (D) Relationship between the percentage of TOM+/YFP+ among total TOM+ B cells (x-axis) and that of TOM+ B cells among total B220+ B cells (y-axis) in REL-AID mice on D14. The correlation curve is shown in red. The value of the Pearson correlation coefficient r with its P value is shown in the graph. Each experiment has been done at least 4 times.

Characterization of the REL-AID mouse model. (A) Schematic representation of the REL-IRES-YFP and tdTomato inserts into the Rosa26 locus: the REL sequence was placed in frame with the IRES and the coding sequence for YFP. On the same chromosome 6 bearing tdTomato trangene, Creert2 was inserted into Aicda locus. (B) Schematic diagram of SRBC (red arrow) and tamoxifen (black arrow) administration protocol for mouse analysis (green arrow) at different time points after immunizations. Blood samples were collected on D14 and then each month, always before immunization boost (blue arrow). (C) Example of a flow cytometry biparametric YFP and tdTomato (TOM) histogram gated on live B220pos B cells. TOM+/YFP– and TOM+/YFP+ B cells are colored in red and green, respectively. PB cells were collected from an AID-TOM (left) or a REL-AID (right) mouse 14 day after immunization and tamoxifen gavage. Percentages of fluorescent cells are shown in each graph. (D) Relationship between the percentage of TOM+/YFP+ among total TOM+ B cells (x-axis) and that of TOM+ B cells among total B220+ B cells (y-axis) in REL-AID mice on D14. The correlation curve is shown in red. The value of the Pearson correlation coefficient r with its P value is shown in the graph. Each experiment has been done at least 4 times.
To induce a limited number of B cells in the context of complex repeated immune responses, our protocol consisted of an initial tamoxifen Creert2 induction together with chronic immunization with a complex T-dependent antigen, in this case SRBCs (Figure 1B). Using AID-TOM mice as a control, we intraperitoneally injected SRBCs on D0 together with tamoxifen gavage on D0, D2, and D4 (Figure 1B), which would favor initial GC formation.
As shown in Figure 1C-D, peripheral blood (PB) TOM+ B cells could be detected in both AID-TOM and REL-AID mice on D14, accounting for 0.79% to 2.14% of total PB B cells, with no significant differences between the 2 mouse strains. Because the Cre efficiency is inversely dependent on the size of the cassette to be excised between the loxP sites, not all B cells that had deleted the 870 bp long STOP cassette of the tdTomato transgene had also eliminated the 2540 bp long STOP-Neo cassette of the REL-IRES-YFP transgene. On D14, 28.5% ± 2.4% of TOM+ PB B cells were also YFP+ (Figure 1D). Therefore, with both YFP and tdTomato as reporters, this dual-color model allows for differentiated fate-mapping of TOM+/YFP+ (REL-positive) and TOM+/YFP– (REL-negative) B cells upon tamoxifen administration after AID-imprinting within the same animal.
Immunofluorescence analysis shows the relationships between c-Rel overexpressing cells and the GC reaction
To verify the relationship between YFP fluorescence and c-Rel expression at the protein level, 2 REL-AID and 2 AID-TOM mice were sacrificed on D14. TOM+/YFP+ and TOM+ CD19+ spleen B cells were sorted by fluorescence-activated cell sorter on 1 hand and 10 μm spleen cryosections were made on the other hand. Analysis of c-Rel immunolabeling by confocal microscopy is shown in supplemental Figure 1. c-Rel was overexpressed in TOM+/YFP+ CD19+ B cells when compared to TOM+ counterpart and was mostly colocalized with the DAPI DNA dye staining, indicating its nuclear localization. Immunofluorescence analysis of spleen adjacent sections is presented in Figure 2 and supplemental Figure 2. PNA and IgD labeling demonstrated that TOM fluorescence was almost exclusively found in GCs (Figure 2A-C). Most of GCs of REL-AID mice were also YFP+. Indeed, by flow cytometry, total TOM+ B cells ranged from 5% to 9% of CD19 B cells and TOM+/YFP+ cells ranged from 77% to 83% of TOM+ cells. Some GCs were predominantly colonized by TOM+/YFP cells (pointed by the red arrow in Figure 2B-C), which is consistent with the theory of clonal or oligoclonal foundation of GCs at the onset of a T-dependent immune response. C-Rel immunolabeling shows that c-Rel protein was overexpressed in GCs of REL-AID mice with an almost perfect overlap with YFP fluorescence (Figure 2D-E, middle panel), whereas c-Rel signal was much weaker in GCs of AID-TOM control mice (supplemental Figure 2).

Histological analysis of spleen section from REL-AID mouse model. Epifluorescence analysis of 10 μm adjacent spleen cryosections for YFP and tdTomato (TOM) fluorescence together with fluorescent immunolabeling of PNA (A), IgD (B), c-Rel (REL) (C), as well c-Rel isotypic control (Iso) and Ki67 (D) for a REL-AID mouse. All cryosections were counterstained with DAPI. DAPI, YFP, and tdTomato fluorescence were colorized in blue, green, and red, respectively. Fluorescence of PNA, IgD, c-Rel, Iso, and Ki67 markers was colorized in white. For panels A-C, the merged fluorescence for Dapi/YFP/tdTomato/antibody fluorescence is shown in the large image on the left, whereas the small images on the right show the separate fluorescence for the antibody, YFP, and tdTomato (top, middle, and bottom image, respectively). In panels B and C, the red arrow points on a GC predominantly colonized by TOM+/YFP– B cells. (D) shows the fluorescence labeling of the GC pointed by the yellow arrow on panel C. The top images are for c-Rel Iso, c-Rel (REL), and Ki67. Below are images for separate fluorescence of YFP and tdTomato. The bottom image merges Dapi, antibody, YFP, and tdTomato fluorescence. Scale bars are inserted in each merged image.

Histological analysis of spleen section from REL-AID mouse model. Epifluorescence analysis of 10 μm adjacent spleen cryosections for YFP and tdTomato (TOM) fluorescence together with fluorescent immunolabeling of PNA (A), IgD (B), c-Rel (REL) (C), as well c-Rel isotypic control (Iso) and Ki67 (D) for a REL-AID mouse. All cryosections were counterstained with DAPI. DAPI, YFP, and tdTomato fluorescence were colorized in blue, green, and red, respectively. Fluorescence of PNA, IgD, c-Rel, Iso, and Ki67 markers was colorized in white. For panels A-C, the merged fluorescence for Dapi/YFP/tdTomato/antibody fluorescence is shown in the large image on the left, whereas the small images on the right show the separate fluorescence for the antibody, YFP, and tdTomato (top, middle, and bottom image, respectively). In panels B and C, the red arrow points on a GC predominantly colonized by TOM+/YFP– B cells. (D) shows the fluorescence labeling of the GC pointed by the yellow arrow on panel C. The top images are for c-Rel Iso, c-Rel (REL), and Ki67. Below are images for separate fluorescence of YFP and tdTomato. The bottom image merges Dapi, antibody, YFP, and tdTomato fluorescence. Scale bars are inserted in each merged image.
Therefore, as shown in Figure 1B, 3 cohorts of AID-TOM and REL-AID mice were setup. Initial tamoxifen gavage and SRBC immunizations were identical for the 3 cohorts. Then, the first cohort, named short-term cohort, consisted of mice sacrificed 10 days after the second SRBC boost. In the second cohort, called mid-term cohort, mice were sacrificed 10 to 15 days after the fifth SRBC boost. In the third cohort, the long-term cohort, mice were monthly reimmunized with SRBCs from month 1 to 4. Then, mice were kept free of antigenic stimulation for 5 to 8 additional months, being sacrificed between month 9 and month 12. Blood samples were collected monthly for both the mid-term and the long-term cohorts.
Transcriptome analysis reveals the c-Rel overexpressing B-cell characteristics
To check whether TOM+/YFP+ (REL-positive) and TOM+/YFP– (REL-negative) B cells could differ in terms of gene expression, the REL-AID and AID-TOM mice were analyzed after the second SRBC boost (Figure 2A-B). To specifically evaluate the expression of the transgenes, reads aligned to the sequence of YFP, tdTomato, and KOZAK-REL junction were specifically counted. As shown in Figure 2C, expression of tdTomato was strictly restricted to AID-imprinted B cells and was among the most differentially expressed genes between AID-imprinted and non–AID-imprinted B cells (log2[fold change] = 6.03, adjusted P value <<10-6). Both YFP and KOZAK-REL were the 2 most differentially expressed genes between TOM+/YFP+ and TOM+/YFP– AID-imprinted B cells (log2[fold change] = 13.15 and 12.23 respectively, adjusted P value <<10-6 for both) (supplemental Tables 1 and 2). As shown in supplemental Figure 3, all TOM+ AID-imprinted B-cell samples exhibited a similar GCB signature with AID expression.
We next focused on the genes specifically up or down regulated in TOM+/YFP+ (REL-positive) B cells when compared to their TOM+/YFP– and TOM+ counterparts (Figure 3C). A set of 1480 genes, 649 down and 831 up, was selected with a fold change of 2, and both an adjusted P value and an false discovery rate of 1% (supplemental Table 2). Based on this set of genes, 3 main groups of samples could be identified after principal component analysis, the group of non-AID-imprinted B cells, the group of TOM+ (REL-negative) B cells and the group of TOM+/YFP+ (REL-positive) B cells (supplemental Figure 4). Unsupervised clustering of the 30 B-cell samples was in full agreement with the principal component analysis and all TOM+/YFP+ (REL-positive) samples were in the same cluster (Figure 3C). Five gene clusters were identified, from clusters C1 to C5 (supplemental Table 3 for the gene composition of each cluster).

mRNA sequencing analysis of AID-TOM and REL-AID mouse spleen (SP) B cells after the second SRBC boost according to the expression of the tdTomato and YFP transgenes. (A) Schematic representation of the mouse immunization protocol. AID-TOM and REL-AID mice were submitted to a tamoxifen gavage at day D0, D2, D4 together with an SRBC immunization at d0. SRBC immunization boosts were done at M1 and M2. Animals were sacrificed 10 days after immunization. (B) Color code of the 5 CD19+ B-cell subsets sorted by fluorescence-activated cell sorter for RNA extraction and mRNAseq (see ”Materials and methods” and “Results”): TOM+ and TOM– cells from AID-TOM mice, and TOM–/YFP–, TOM+/YFP– and TOM+/YFP+ cells from REL-AID mice. In total, 30 B-cell samples from 14 mice were collected that were a priori classified as non–AID-imprinted (TOM–, n = 6 and TOM–/YFP–, n = 8) and AID-imprinted B cells (TOM+, n = 6; TOM+/YFP–, n = 3, and TOM+/YFP+, n = 7). (C) Color heatmaps for expression of tdTomato, YFP, and KOZAK-REL junction and for differential gene expression between TOM+/YFP+ and TOM+/YFP– plus TOM+ B-cell samples. After unsupervised clustering of both genes and samples, 5 clusters of genes were identified, numbered C1 to C5 from the bottom to the top. On the left side, some key functions related to Gene Ontology annotations are shown for each cluster. On the right side are highlighted some key genes for each cluster. The number n of genes is given for each cluster in the heat map. Being immediately adjacent, the YFP and KOZAK-REL transgenes are in bold and underlined and are placed at their exact position in the clustering. (D) Frequency of the most abundant IgHV segment for each B-cell sample (ie, mRNA IgHV dominance). Mean and standard error of the mean are shown for each group of samples by a red and 2 black lines, respectively. ∗Mann Whitney P <.05; ∗∗∗Mann Whitney P <10–3. mRNAseq, mRNA high-throughput sequencing. SD, standard deviation.

mRNA sequencing analysis of AID-TOM and REL-AID mouse spleen (SP) B cells after the second SRBC boost according to the expression of the tdTomato and YFP transgenes. (A) Schematic representation of the mouse immunization protocol. AID-TOM and REL-AID mice were submitted to a tamoxifen gavage at day D0, D2, D4 together with an SRBC immunization at d0. SRBC immunization boosts were done at M1 and M2. Animals were sacrificed 10 days after immunization. (B) Color code of the 5 CD19+ B-cell subsets sorted by fluorescence-activated cell sorter for RNA extraction and mRNAseq (see ”Materials and methods” and “Results”): TOM+ and TOM– cells from AID-TOM mice, and TOM–/YFP–, TOM+/YFP– and TOM+/YFP+ cells from REL-AID mice. In total, 30 B-cell samples from 14 mice were collected that were a priori classified as non–AID-imprinted (TOM–, n = 6 and TOM–/YFP–, n = 8) and AID-imprinted B cells (TOM+, n = 6; TOM+/YFP–, n = 3, and TOM+/YFP+, n = 7). (C) Color heatmaps for expression of tdTomato, YFP, and KOZAK-REL junction and for differential gene expression between TOM+/YFP+ and TOM+/YFP– plus TOM+ B-cell samples. After unsupervised clustering of both genes and samples, 5 clusters of genes were identified, numbered C1 to C5 from the bottom to the top. On the left side, some key functions related to Gene Ontology annotations are shown for each cluster. On the right side are highlighted some key genes for each cluster. The number n of genes is given for each cluster in the heat map. Being immediately adjacent, the YFP and KOZAK-REL transgenes are in bold and underlined and are placed at their exact position in the clustering. (D) Frequency of the most abundant IgHV segment for each B-cell sample (ie, mRNA IgHV dominance). Mean and standard error of the mean are shown for each group of samples by a red and 2 black lines, respectively. ∗Mann Whitney P <.05; ∗∗∗Mann Whitney P <10–3. mRNAseq, mRNA high-throughput sequencing. SD, standard deviation.
Cluster C1 corresponds to genes downregulated in REL-positive samples, whereas they were specifically overexpressed in their REL-negative counterpart. Gene Ontology annotations associated to these genes included MHC, cooperation between T and B lymphocytes, and apoptosis induction.
Being immediately adjacent, KOZAK-REL and YFP genes were found in cluster C2, this cluster corresponding to genes specifically overexpressed in REL-positive B cells only. Other overexpressed genes belonging to this cluster evoked the Ig gene transcription and the DNA methylation.
Cluster C3 contains genes that were expressed in TOM+/YFP– (REL-negative) B cells when compared to non–AID-imprinted B cells and that were even more overexpressed in TOM+/YFP+ (REL-positive) B cells. Cluster C3 was enriched in genes belonging to the BCL6 driven GC-up gene set enrichment analysis M6510 signature,24 with genes involved in cell proliferation, immunoglobulin class switching and plasma cell (PC) differentiation (supplemental Figure 5).
Cluster C4 consists in genes expressed in non–AID-imprinted B cells and in a subset of 4 of 7 TOM+/YFP+ (REL-positive) B cells and downregulated in TOM+ (REL-negative) B cells. Epigenetics and DNA repair were the main functions associated to this cluster.
Finally, cluster C5 corresponds to downregulated genes in AID-imprinted B cells. These genes were more strongly downregulated in REL-positive B cells. Related functions encompassed the cell death and apoptosis, the downregulated genes in GC B cells, as well as the genes involved in cooperation between T and B lymphocytes interactions and the BCR pathways.
An mRNA IgHV dominance was then calculated by first counting paired-end reads aligned to the same IgHV segment and then identifying the IgHV segment with the highest proportion of reads. As shown in Figure 3D, mRNA IgHV dominance was increased in all AID-imprinted cells, consistent with the notion that GCBs are often oligoclonal. Interestingly, mRNA IgHV dominance was significantly increased in TOM+/YFP+ B cells. Although the differences between groups were not significant (Kruskal-Wallis test, P = .076), somatic hyper mutation of dominant mRNA IgHV segments was found only in TOM+ or TOM+/YFP–-sorted B cells (supplemental Figure 6, upper panel), ie, in AID-imprinted B cells that did not overexpress c-Rel, which may mean that REL overexpressing B cells were less subjected to antigen selection pressure and/or the number of cyclical reentry into the dark zone was lower. In that view, the repertoire of dominant IgHV segments in TOM+/YFP+ and in TOM+ or TOM+/YFP– B cells were different (supplemental Figure 6, lower panel).
Fully consistent with immunofluorescence analyses, these results first demonstrate that expression of the tdTomato transgene was specifically restricted to B cells that were a priori labeled as AID-imprinted B cells from either AID-TOM or REL-AID mice. In full agreement with the fact that the Creert2 activity depends on the Aicda gene promoter, Aicda gene expression was increased in TOM+ B cells, which exhibited a GCB signature. Secondly, both REL and YFP transgenes were specifically expressed in TOM+/YFP+ B cells. REL-positive B cells not only exhibited an exacerbated GCB signature (clusters C3 and C5), but were also very likely to have increased the immunoglobulin class switch activity (downregulation of Ighd and Ighm and upregulation of Ighg1 and Ighg3 with increased transcription of various IgHV segments), to be committed to PC differentiation, to be protected from apoptosis and to exhibit altered T-cell interactions. Third, the mRNA IgHV dominance was increased in REL-positive B cells that indicates a trend to oligoclonality.
Long-term competitive advantage of REL-overexpressing B cells when compared to their REL-negative counterpart
Monthly analysis of blood samples showed that, after initial tamoxifen induction of Creert2 activity in AID-TOM control mice, the levels of PB TOM+ B cells moderately decreased despite SRBC immunization boost (Figure 4A). Then, TOM+ B cell percentages remained stable as long as mice were repeatedly immunized, implying an equilibrium or a stationary state between blood entry and exit of TOM+ B cells. This would indicate a continuous recirculation and/or production of TOM+ B cells in secondary lymphoid organs. Subsequently, TOM+ B cells gradually declined to near-zero levels as immunization boosts ended, revealing the dependence of TOM+ B-cell production on the immune response. Contrary to AID-TOM mice, REL-AID mice exhibited stable levels of PB TOM+ B cells not only during the immunization period but also for up to 2 months after cessation of immunization before declining (Figure 4A). This shows that REL overexpression led to an increase in production (blood entry increase) and/or survival (blood exit decrease) of TOM+ B cells.

Relationships between the kinetics of PB TOM+ and TOM+/YFP+ with CD80 expression. (A) Box plot of the mean percentage (± standard error of the mean) of PB TOM+ cells among live B cells (CD19+ B220+/−) in AID-TOM and REL-AID mice over time post-tamoxifen induction. The dashed red line indicates the end of immunization boost. The P value of a 2-way analysis of variance (ANOVA) for REL effect is shown. (B) Relationship between the percentage of TOM+/YFP+ among total TOM+ B cells (x-axis) and that of TOM+ B cells among total B220+ B cells (y-axis) in REL-AID mice at month 2 (top graph) and month 12 (bottom graph). The correlation curve is shown in red. The values of the Pearson correlation coefficient r and of its P values are given in each graph. (C) Distribution of the percentage of TOM+/YFP+ B cells among total TOM+ B cells over time in REL-AID mice. The red dot represents the mouse with the highest percentage and green dot the mouse with the lowest percentage. The dashed red line indicates the end of immunization boost. Mean, standard error of the mean, and number n of mice are indicated above the graph. The P value of the 1-way ANOVA for time effect is shown. (D) CD80 expression levels in TOM+/YFP– B cells from either AID-TOM and REL-AID mice as well as TOM+/YFP+ B cells from REL-AID mouse model. Mean and SEM are shown for each B-cell sample by a red and 2 black lines, respectively. Mann Whitney test P values are shown. Each experiment has been done at least 4 times. MFI, mean fluorescence intensity.

Relationships between the kinetics of PB TOM+ and TOM+/YFP+ with CD80 expression. (A) Box plot of the mean percentage (± standard error of the mean) of PB TOM+ cells among live B cells (CD19+ B220+/−) in AID-TOM and REL-AID mice over time post-tamoxifen induction. The dashed red line indicates the end of immunization boost. The P value of a 2-way analysis of variance (ANOVA) for REL effect is shown. (B) Relationship between the percentage of TOM+/YFP+ among total TOM+ B cells (x-axis) and that of TOM+ B cells among total B220+ B cells (y-axis) in REL-AID mice at month 2 (top graph) and month 12 (bottom graph). The correlation curve is shown in red. The values of the Pearson correlation coefficient r and of its P values are given in each graph. (C) Distribution of the percentage of TOM+/YFP+ B cells among total TOM+ B cells over time in REL-AID mice. The red dot represents the mouse with the highest percentage and green dot the mouse with the lowest percentage. The dashed red line indicates the end of immunization boost. Mean, standard error of the mean, and number n of mice are indicated above the graph. The P value of the 1-way ANOVA for time effect is shown. (D) CD80 expression levels in TOM+/YFP– B cells from either AID-TOM and REL-AID mice as well as TOM+/YFP+ B cells from REL-AID mouse model. Mean and SEM are shown for each B-cell sample by a red and 2 black lines, respectively. Mann Whitney test P values are shown. Each experiment has been done at least 4 times. MFI, mean fluorescence intensity.
The increased production and/or survival of TOM+ B cells in REL-AID mice was due to TOM+/YFP+ double-positive, that is, to REL overexpressing B cells (Figure 4B-C). Indeed, percentages of YFP+ cells within the TOM+ B-cell fraction were strongly correlated with those of TOM+ cells among total PB B cells at month 2 and 12 (Figure 4B), which is in contrast with the result at D14 (Figure 1D). Figure 4C shows the progressive but rapid, consistent, and persistent accumulation of YFP+ B cells within the TOM+ B-cell compartment, even when immunization boosts ceased. Compared to TOM+ B cells from AID-TOM mice or TOM+/YFP– B cells from REL-AID mice, TOM+/YFP+ B cells overexpressed CD80 (Figure 4D), a marker of NF-κΒ that is known to be increased upon c-Rel activation.25
B-cell expansion was then studied at the site of injection and in lymph nodes, spleen, and bone marrow (Figure 5A-B). For the mid-term cohort, even if heterogeneous, the proportion of TOM+ B cells was increased in the peritoneum, that is, at the SRBC injection site, when compared to the spleen, lymph nodes, and bone marrow in AID-TOM control mice (Figure 5C), again indicating the role of antigen stimulation in promoting AID-imprinted B cells. This phenomenon was increased in REL-AID mice in the peritoneum in the spleen, lymph nodes and bone marrow, and most of these TOM+ B cells were also YFP+ (Figure 5C). One REL-AID mouse of this cohort, marked with an arrow in Figure 5C, developed a diffuse large B-cell lymphoma with almost 100% TOM+/YFP+ B cells (Figure 5C, lower panel).
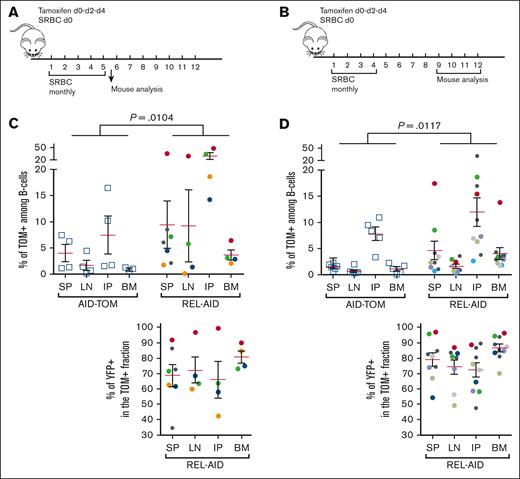
Relationship between the levels of TOM+ and TOM+/YFP+ B cells in secondary lymphoid organs and in the BM. (A-B) Schematic representation of the induction and immunization protocol for analysis either mid-term (A) or long-term (B) cohorts. (C-D) Analysis of TOM+ and TOM+/YFP+ B cells in secondary lymphoid organs for the mid-term (C) and long-term (D) cohorts for AID-TOM control (left) and REL-AID mice (right) in the SP, LN, BM, and in the IP. Top: percentage of TOM+ B cells among total live B cells (CD19+ B220+/−). Bottom: percentage of TOM+/YFP+ B cells among TOM+ B cells. In panel C, the case with a diffuse aggressive B-cell lymphoma is indicated by an arrow. Mean and SEM are shown by a red and 2 black lines, respectively. The P value of a 2-way ANOVA for REL effect is shown on the top of the graph. Each experiment has been done at least 4 times. BM, bone marrow; LN, lymph nodes; IP, peritoneum.

Relationship between the levels of TOM+ and TOM+/YFP+ B cells in secondary lymphoid organs and in the BM. (A-B) Schematic representation of the induction and immunization protocol for analysis either mid-term (A) or long-term (B) cohorts. (C-D) Analysis of TOM+ and TOM+/YFP+ B cells in secondary lymphoid organs for the mid-term (C) and long-term (D) cohorts for AID-TOM control (left) and REL-AID mice (right) in the SP, LN, BM, and in the IP. Top: percentage of TOM+ B cells among total live B cells (CD19+ B220+/−). Bottom: percentage of TOM+/YFP+ B cells among TOM+ B cells. In panel C, the case with a diffuse aggressive B-cell lymphoma is indicated by an arrow. Mean and SEM are shown by a red and 2 black lines, respectively. The P value of a 2-way ANOVA for REL effect is shown on the top of the graph. Each experiment has been done at least 4 times. BM, bone marrow; LN, lymph nodes; IP, peritoneum.
For the long-term cohort, while persisting in the peritoneum, TOM+ B cells almost disappeared from both spleen, lymph nodes, and bone marrow of AID-TOM control mice (Figure 5D). In contrast, TOM+ B cells persisted in spleen, lymph nodes, and bone marrow, albeit at lower levels and more heterogeneously than immediately after immunization, and remained higher in the peritoneum of REL-AID mice. Again, most TOM+ B cells from REL-AID mice were also YFP+ (Figure 5D). In addition, even if not all mice could be tested and numbers are small, an IgHV monoclonality was detected only on DNA samples from B cells of REL-AID mice (supplemental Figure 7). For these cases, histological examination of spleen did not reveal any diffuse lymphomatous aspect (not shown).
Collectively, these results demonstrate that recirculation of AID-imprinted B cells is markedly dependent on chronic immunization. They also strongly support a significant long-term competitive advantage of B cells that have benefited from a REL overexpression event when compared to their REL-negative counterpart.
REL-overexpressing GCBs show a competitive advantage with an increase in both immunoglobulin class switching and PC differentiation.
The splenic B-cell composition of REL-positive (TOM+/YFP+) and REL-negative (TOM+/YFP–) B cells was analyzed in the mid-term cohort (Figure 6A). Live CD95+ CD38low GCBs (gated on CD19+ B220+/−) were significantly increased in TOM+ B cells of REL-AID mice when compared to their AID-TOM control (Figure 6B-C). This GC B-cell fraction was specifically enriched in REL-positive B cells, reaching almost 90% of total TOM+ B cells, even in mice with a rather low percentage of YFP+ cells among total TOM+ B cells (Figure 6D). Indeed, the GL7 positive GCB fraction was enhanced in REL-positive B cells (supplemental Figure 8). The increase in GCBs in the TOM+ fraction was also found in REL-AID mice of the long-term cohort, whereas these GC TOM+ B cells almost disappeared in AID-TOM controls mice (supplemental Figure 9).

Analysis of splenic GC, IgG1 class switched B cells, and PC differentiation after the fifth immunization boost. (A) Schematic representation of the induction and immunization protocol for the mid-term cohort. (B) Strategy for gating TOM+ B cells from TOM-AID mice (upper panel) and TOM+/YFP− and TOM+/YFP+ from REL-AID mice (lower panel) as well as GCBs from total CD19+ live B cells (CD19+ B220+/−). (C) Percentage of GCBs among TOM+ B cells in AID-TOM control and REL-AID mice. Mean and SEM are shown by a red and 2 black lines, respectively. The P value of the Mann-Whitney test is shown. (D) Percentage of TOM+/YFP+ B cells in the total TOM+ (left) or in the GCB (right) fraction in REL-AID mice. Each line connects the points for 1 mouse. The P value of the paired Wilcoxon test is shown. (E) Example of gating on IgM−/IgD− double-negative (DN) B cells in both AID-TOM (upper) and REL-AID (lower) mice. (F) Analysis of DN B cells in both AID-TOM and REL-AID mice. Upper: ratio of DN/IgM+ cells among total TOM+ B cells in AID-TOM and REL-AID mice. Lower: percentages of YFP+ B cells among TOM+ B cells in the total TOM+ and in the DN B-cell fraction in REL-AID mice (each line connects the points for 1 mouse). The P values of the Mann-Whitney (upper) and paired Wilcoxon test (lower) are shown. (G) Example IgG1 class switched B-cells gating for AID-TOM (upper) and REL-AID (lower) mice. (H) Analysis of IgG1+ B cells in both AID-TOM and REL-AID mice. Left: percentages of IgG1+ (also IgM− and IgD−) B cells among TOM+ B cells in AID-TOM and REL-AID mice. Right: percentages of YFP+ B cells among total TOM+ and IgG1+ B cells (each line connects the points for 1 mouse). The P values of the Mann-Whitney (upper) and paired Wilcoxon test (lower) are shown. Each experiment has been done at least 4 times.

Analysis of splenic GC, IgG1 class switched B cells, and PC differentiation after the fifth immunization boost. (A) Schematic representation of the induction and immunization protocol for the mid-term cohort. (B) Strategy for gating TOM+ B cells from TOM-AID mice (upper panel) and TOM+/YFP− and TOM+/YFP+ from REL-AID mice (lower panel) as well as GCBs from total CD19+ live B cells (CD19+ B220+/−). (C) Percentage of GCBs among TOM+ B cells in AID-TOM control and REL-AID mice. Mean and SEM are shown by a red and 2 black lines, respectively. The P value of the Mann-Whitney test is shown. (D) Percentage of TOM+/YFP+ B cells in the total TOM+ (left) or in the GCB (right) fraction in REL-AID mice. Each line connects the points for 1 mouse. The P value of the paired Wilcoxon test is shown. (E) Example of gating on IgM−/IgD− double-negative (DN) B cells in both AID-TOM (upper) and REL-AID (lower) mice. (F) Analysis of DN B cells in both AID-TOM and REL-AID mice. Upper: ratio of DN/IgM+ cells among total TOM+ B cells in AID-TOM and REL-AID mice. Lower: percentages of YFP+ B cells among TOM+ B cells in the total TOM+ and in the DN B-cell fraction in REL-AID mice (each line connects the points for 1 mouse). The P values of the Mann-Whitney (upper) and paired Wilcoxon test (lower) are shown. (G) Example IgG1 class switched B-cells gating for AID-TOM (upper) and REL-AID (lower) mice. (H) Analysis of IgG1+ B cells in both AID-TOM and REL-AID mice. Left: percentages of IgG1+ (also IgM− and IgD−) B cells among TOM+ B cells in AID-TOM and REL-AID mice. Right: percentages of YFP+ B cells among total TOM+ and IgG1+ B cells (each line connects the points for 1 mouse). The P values of the Mann-Whitney (upper) and paired Wilcoxon test (lower) are shown. Each experiment has been done at least 4 times.
REL-positive B-cell enrichment was not found for IgM+ IgD– B cells (Figure 6E; supplemental Figure 10). Including class switched B cells, the IgM/IgD double-negative B-cell subset was increased in REL-AID mice when compared to controls (Figure 6E-F). The enrichment in REL-positive B cells in this subset was moderate although significant. But IgM/IgD double-negative B cells also contain GCBs that have downregulated their BCR or have a nonfunctional or damaged BCR. Thus, we also looked at the expression of the IgG1 isotype. Although heterogeneous, percentages of IgG1 class switched B cells were increased in REL-AID mice and these IgG1 B cells were enriched in REL-positive B cells (Figure 6G-H).
Gating on PCs with the CD138 marker revealed that PC differentiation was rather heterogeneous in REL-AID mice, although not significantly increased when compared to TOM-AID controls (Figure 7A-B). Nevertheless, almost all PCs from REL-AID mice were derived from the REL-positive B-cell fraction.

Analysis of splenic PCs shortly after immunization. (A) Example of gating on PCs in AID-TOM (upper) and REL-AID (lower) mice from the mid-term cohort (also refer to Figure 5A). Red colored cells are TOM+ B cells only and green colored cells are TOM+YFP+ B cells (same gating of the first 2 cytogram in Figure 5B). (B) Percentages of PCs among total TOM+ B cells in AID-TOM and REL-AID mice (upper) and percentages of YFP+ among TOM+ B cells in the total TOM+ and in the PC fraction in REL-AID mice (each line connects the points for 1 mouse) (lower). Mean and standard error of the mean are shown by a red and 2 black lines, respectively. The P values of the Mann-Whitney (upper) and paired Wilcoxon (lower) test are shown. ns, nonsignificant.

Analysis of splenic PCs shortly after immunization. (A) Example of gating on PCs in AID-TOM (upper) and REL-AID (lower) mice from the mid-term cohort (also refer to Figure 5A). Red colored cells are TOM+ B cells only and green colored cells are TOM+YFP+ B cells (same gating of the first 2 cytogram in Figure 5B). (B) Percentages of PCs among total TOM+ B cells in AID-TOM and REL-AID mice (upper) and percentages of YFP+ among TOM+ B cells in the total TOM+ and in the PC fraction in REL-AID mice (each line connects the points for 1 mouse) (lower). Mean and standard error of the mean are shown by a red and 2 black lines, respectively. The P values of the Mann-Whitney (upper) and paired Wilcoxon (lower) test are shown. ns, nonsignificant.
Discussion
We developed the dual-color REL-AID model to get closer to what is most likely to occur in the true life of an emerging tumor B cell that has recently been hit by an initial genetically transforming event such as REL gains in GCs. REL overexpression in GCB-DLBCLs is directly correlated with REL gains but is rather modest, being about 1.5-fold change in most cases.5 Therefore, we first chose to keep our REL transgene under the control of the native Rosa26 promoter to mimic a weak but continuous deregulation of c-Rel. Second, Creert2 expression depended on AID regulation to mainly target the GCB stage. Third, Creert2 activity was transiently induced by tamoxifen gavage together with immunization with a complex antigen. Fourth, the few REL-positive B cells generated after tamoxifen gavage were left in competition with all other REL unhit B cells for a long time in the context of a complex and chronic immune response.
This competition would begin rather early within the GCs and would continue later in post-GC differentiation stages. Indeed, 14 days after immunization, most GCs were colonized by TOM+/YFP+ B cells that overexpressed c-Rel at the protein level. But some GCs were predominantly colonized by TOM+/YFP– B cells, which could be interpreted as an oligoclonal GC foundation by some TOM+/YFP– B cells mainly with few accompanying TOM+/YFP+ B cells before GCB competition took place. In addition to the GC topographical specificity of YFP and tdTomato fluorescence as well as of c-Rel overexpression. mRNA sequencing demonstrated the specific expression of the tdTomato, and of both YFP and REL transgenes in TOM+ and TOM+/YFP+ B cells, respectively. It also suggests that REL-positive B cells may have an exacerbated GCB signature when compared to their TOM+/YFP– counterpart, with increased immunoglobulin class switching and PC differentiation. The finding that that IgHV clonal dominance was increased in TOM+/YFP+ B cells at the mRNA level, whereas these cells did not exhibit an hypermutated Igvh pattern when compared to TOM+/YFP– B cells, indicates that, with less need to be antigen selected and/or to reenter the dark zone, c-Rel would favor oligoclonal expansion of GCB.
To find that the small pool of REL-positive AID-imprinted B cells was favored in vivo for GC reaction and then for class switch recombination and PC differentiation is consistent with the results of Kober-Hasslacher et al.15 In the model of these authors, the REL transgene was under the dependence of a strong cytomegalovirus early enhancer/chicken β actin promoter. REL overexpression was obtained constitutively in either all CD19pos or all switch-engaged GCB, after breeding with CD19-Cre or Cγ1Cre mice. The phenotype of these mice demonstrated a positive effect of c-Rel on GC B-cell expansion, resulting in increased PC differentiation with production of autoantibodies. In our model, we demonstrate a clear competitive advantage in vivo for the long-term maintenance and recirculation of REL-positive B cells during and after chronic immunization when compared to their REL-negative counterpart. This REL effect on the recirculating B cells would favor long-term survival of post-GC recirculating long-lived B cells. In addition to highlighting the role of chronic antigenic stimulation, our results also indicate that part of this competitive advantage may very well be related to the long-term persistence of REL-positive GCB production in secondary lymphoid organs.
Most B-cell transformation scenarios imply a primary event that allows escape from homeostasis and then accumulation of mutations over time until clinical emergence of the B-cell tumor. Resulting in deregulation of Bcl2, the primary transforming event in FLs is the t(14;18)(q32;q21) translocation. These t(14;18)pos B cells can be detected at a very low frequency in most adults. However, much of these t(14;18)pos B cells will never transform. Clonal FL lymphomagenesis is due to the accumulation of secondary mutations such as those in CREBBP, TNFSRF14, EZH2, or KMT2D over decades.26 These secondary mutations are also commonly found in GCB-DLBCLs with the REL signature.5 IgHV clonality analysis showed clonal B-cell expansion in 2 REL-AID mice. This feature and the mRNA IgHV dominance increase in REL-AID mice would be in agreement with the notion that REL-positive B cells would contribute to the generation of a pool of lymphoma precursor cells. We indeed found that REL-positive B-cell signature was markedly enriched in genes from the BCL6 driven GC-up gene set enrichment analysis M6510 signature issued from the paper of Pasqualucci et al,24 that is, in genes clearly associated with the development of AID-dependent mouse DLBCLs.
Initially proposed by Staudt27 and recently reinvigorated by Pasqualucci and Klein,4 REL amplification would act very early in the evolution of, as cited, “the tumor-precursor cell to a bona fide lymphoma.” Our findings provide the first experimental evidence consistent with this hypothesis, suggesting that, as an oncogenic hit, genetic deregulation of c-Rel expression is sufficient by itself to favor the emergence of a pool of lymphoma precursor GCBs, raising the question of REL-associated secondary events for GCB transformation.
Acknowledgments
The authors thank the 3 reviewers for the experimental suggestions. The authors thank reviewer 1 for having inspired the title of the manuscript. This work was supported by Association pour la Recherche contre le Cancer, contract no. ARCPJA2021060003884, Ligue Nationale Contre le Cancer, Equipe Labelisée Ligue 2016-2021, convention no. EL2016.LNCC/JeF and Comités Limousins de la Ligue Contre le Cancer, contract no. MM/CFn41.
Authorship
Contribution: L.P., K.F., D.S., O.M., T.M., Q.L., C.O., and C.C. performed research; C.V.-F. and N.F. designed the vector for REL transgenesis and supervised the embryonnic stem cell transgenesis for the REL-YFP mouse model; M.C. designed the AID-TOM model and edited the manuscript; J.F. wrote the manuscript; and N.F. designed and performed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nathalie Faumont, UMR CRIBL CNRS7276/INSERM1262, Centre de Biologie et de Recherche en Santé, CHU et Faculté de Médecine de Limoges, Av Bernard Descottes, 87025 Limoges Cedex, France; email: nathalie.faumont@unilim.fr.
References
Author notes
L.P., K.F., and D.S. contributed equally and are joint first authors.
All primary RNA-sequencing data have been uploaded on the Gene Expression Omnibus data repository with the accession number GSE282304.
Original data are available on request from the corresponding author, Nathalie Faumont (nathalie.faumont@unilim.fr).
The full-text version of this article contains a data supplement.

