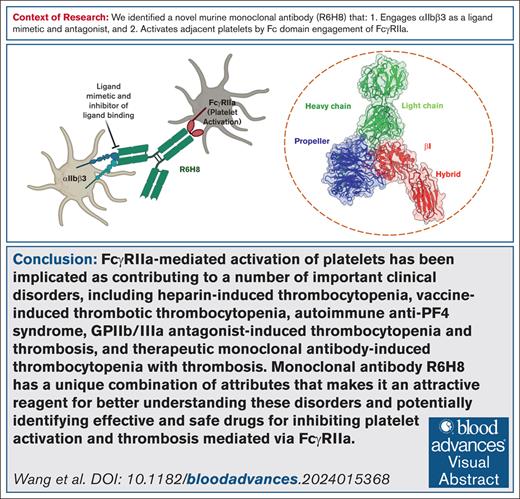
Key Points
mAb R6H8 is an αIIbβ3 ligand mimetic/antagonist that induces an activating conformational change in αIIbβ3.
R6H8 also engages FcγRIIa, activating platelets.
Visual Abstract
To produce a murine monoclonal antibody (mAb) that binds to glycoprotein IIb/IIIa (αIIbβ3) and inhibits clot retraction (CR), we immunized mice with human platelets and tested hybridoma supernatants for their ability to bind to αIIbβ3 and inhibit CR. The immunoglobulin G1 (IgG1) mAb R6H8 completely inhibited CR at 20 μg/mL. Paradoxically, at 5 μg/mL, R6H8 initiated platelet aggregation and induced P-selectin expression, fibrinogen binding, and PAC-1 binding. At 20 μg/mL, however, R6H8 completely inhibited aggregation induced by thrombin PAR-1 receptor activating peptide SFLLRN (T6; 25 μg/mL) and T6-induced fibrinogen and PAC-1 binding to platelets. Platelet aggregation induced by R6H8 was inhibited by mAb IV.3, which blocks the FcγIIa receptor (FcγRIIa), and the Fab fragment of R6H8 did not induce platelet aggregation, suggesting that R6H8 binds to both αIIbβ3 and FcγRIIa. Cryogenic electron microscopy analysis of the R6H8 Fab–αIIbβ3 complex revealed that R6H8 (1) binds to the αIIbβ3 RGD binding pocket via an Arg-Tyr-Asp (RYD) sequence in its heavy chain complementarity-determining region 3; (2) interacts with β3 Asp126, producing a reorientation of Asp126 and loss of the adjacent to metal ion-dependent adhesion site Ca2+; and (3) initiates swing-out of the β3 hybrid domain. We conclude that R6H8 is an αIIbβ3 ligand-mimetic mAb that activates platelets via FcγRIIa at low concentrations and potently inhibits platelet aggregation and CR at high concentrations. R6H8 simulates the actions of a number of pathological antibodies, including platelet-activating antibodies developed after therapy with αIIbβ3 inhibitors and platelet-activating antibodies in heparin-induced thrombocytopenia and vaccine-induced immune thrombotic thrombocytopenia. As such, it may be a valuable reagent for better understanding these disorders and identifying potential therapies.
Introduction
Platelet integrin receptor αIIbβ3 is required for platelets to bind fibrinogen to support platelet aggregation and to interact with fibrin to retract fibrin clots.1 Platelets of patients with Glanzmann thrombasthenia, who lack functional αIIbβ3 receptors, are not able to aggregate or retract clots.2 We sought to produce a murine monoclonal antibody (mAb) that binds to αIIbβ3 in a manner that inhibits clot retraction. To that end, we immunized mice with human platelets and performed a screening assay for antibodies in culture supernatant that were potent inhibitors of clot retraction. We found one supernatant (R6H8) that met these criteria and then prepared subclones and purified the antibody. Surprisingly, we found that the antibody was capable of inducing platelet aggregation at low concentrations and inhibiting aggregation at high concentrations. Cryogenic electron microscopy (EM; cryo-EM) revealed that the antibody is a ligand mimetic, binding directly to the αIIbβ3 RGD-binding pocket.3 The activation of platelets induced by the antibody was demonstrated to rely on the binding of the antibody to the FcγIIa receptor (FcγRIIa). Thus, the antibody simulates the actions of several pathological antibodies, including a subpopulation of platelet-activating antibodies developed after therapy with glycoprotein IIb/IIIa (αIIbβ3) inhibitors (GPIs), and antibodies responsible for producing heparin-induced thrombocytopenia, vaccine-induced immune thrombotic thrombocytopenia, and related disorders. As such, the antibody provides a reagent for better understanding these disorders and identifying potential therapies.
Methods
Reagents and mAbs
The PAR1-activating peptide SFLLRN (T6) was obtained from AnaSpec. Alexa Fluor488-labeled fibrinogen, Alexa Fluor488 protein labeling kit, and phycoerythrin (PE)-conjugated anti-human/mouse P-selectin antibody were purchased from Invitrogen. FITC-conjugated mAb PAC-1 was obtained from BD Biosciences.
mAbs 10E5 (anti-αIIbβ3; αIIb cap domain),3 and 7E3 (anti-αIIbβ3; β3 I domain)4 were produced in our laboratory. mAb PMI-1 (anti-αIIbβ3; αIIb calf-2 domain) was a generous gift of Mark Ginsberg (University of California, San Diego, CA).5 Anti-human CD32 mAb clone IV.3 was obtained from StemCell Technologies. Ligand-induced binding site (LIBS) mAb AP5 (anti-αIIbβ3; β3 plexin-semaphorin-integrin domain) was a kind gift of Peter Newman (Versiti, Milwaukee, WI).6
Platelet preparation from peripheral blood
Blood was obtained from healthy volunteers who provided informed consent under a protocol approved by the Rockefeller University Institutional Review Board and was prepared as described in the supplemental Materials and Methods.
Clot retraction, αIIbβ3 enzyme-linked immunoassay (ELISA), platelet aggregation, and flow cytometry
Production and isotype determination of mAb R6H8
BALB/c mice were immunized with human platelets separated from acid-citrate-dextrose-anticoagulated whole blood as previously described8 and boosted with purified αIIbβ3 before splenocytes were obtained and fused with a nonsecretory hybridoma cell line (supplemental Materials and Methods). The isotype of colony R6H8 was determined to be immunoglobulin G1-kappa (IgG1-kappa) using an antibody isotyping kit (Roche), and the Fab fragment was prepared using a mouse IgG1 Fab and F(ab')2 preparation kit (Thermo Scientific).
Miniaturized clot retraction assay for mAb screening
A miniaturized clot retraction assay was developed to screen hybridoma supernatants in 96-well microtiter plates (final volume of 100 μL) as described in the supplemental Materials and Methods.
αIIbβ3–R6H8 Fab complex formation and negative-stain EM
Full-length αIIbβ3 purified from platelets was mixed with R6H8 Fab at a 1:4 molar ratio in buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4, 150 mM NaCl, 2 mM CaCl2, and 1 mM MgCl2), incubated for 1 hour at 4°C, and subjected to size-exclusion chromatography on a Superose 6 Increase column. Column fractions containing αIIbβ3–R6H8 Fab complexes were identified by sodium-dodecyl-sulfate polyacrylamide gel electrophoresis analysis. Negative-stain EM was performed as previously described,9 with details in the supplemental Materials and Methods. Particles were automatically picked with Blob Picker in CryoSPARC,10 which was used for all further image processing, and 2-dimensional (2D) classification (supplemental Figure 1) was performed as described in the supplemental Materials and Methods.
Cryo-EM sample preparation and data collection
A 3.5-μL aliquot of the αIIbβ3–R6H8 Fab complex was applied to a freshly glow-discharged 400-mesh graphene oxide (GO) copper grid (R1.2/1.3) and analyzed as described in the supplemental Materials and Methods. Cryo-EM data collection statistics are provided in supplemental Table 1.
Cryo-EM image processing
A total of 13 711 image stacks were collected, motion corrected, dose weighted, summed, and binned over 2×2 pixels in MotionCor2.11 Particle picking, 2D and 3D classification, 3D refinement and reconstruction, and model building (supplemental Figure 2) were performed as described in the supplemental Materials and Methods.
Model building and refinement
To build the model of apo αIIbβ3, the propeller, βI, and hybrid domains were extracted from a crystal structure of the αIIbβ3 ectodomain (Protein Data Bank, 3FCS) and fitted as a rigid body into the EM density map using CHIMERA.12 The model was improved as described in the supplemental Materials and Methods. The final model includes the αIIb propeller domain (residues 1-451), the β3 βI and hybrid domains (residues 59-432), the R6H8 Fab heavy chain (residues 1-224), and the R6H8 light chain (residues 1-217). The refinement statistics are listed in supplemental Table 1.
Statistical analysis
Results are expressed as means ± standard deviation, with the number of observations indicated as n. Data were analyzed using GraphPad Prism 6.07. Student t test was used to assess whether the means of 2 populations were significantly different. Differences were considered significant at P value ≤.05.
Results
mAb R6H8
Screening of hybridoma supernatants identified IgG antibodies that bind to αIIbβ3 and inhibit clot retraction
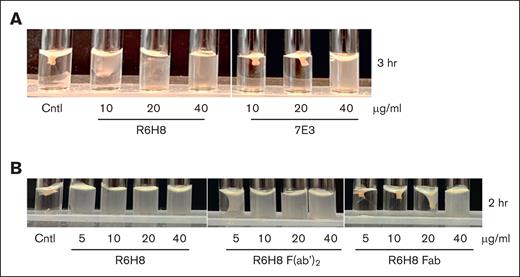
Culture media from 115 of 301 hybridomas that were secreting IgG when grown in semisolid medium were found to contain IgG that bound to purified full-length αIIbβ3 and so were selected for further testing. After additional selection, 11 produced partial inhibition, and 4 produced complete inhibition of clot retraction. One of the latter hybridomas (R6H8) was subcloned, and the antibody was purified. The purified antibody was a potent inhibitor of clot retraction, producing nearly complete inhibition for up to 3 hours at 20 μg/mL (Figure 1A). For comparison, mAb 7E3, which is a potent inhibitor of clot retraction,7,13 required 40 μg/mL to achieve similar inhibition. The Fab fragment of R6H8 was substantially less potent than either the intact IgG or the F(ab')2 fragment, requiring 40 μg/mL to achieve the same inhibition at 2 hours as 10 μg/mL of either the intact R6H8 or the F(ab')2 fragment of R6H8 (Figure 1B).
mAb R6H8 is a potent inhibitor of clot retraction, but potency requires bivalent binding. (A) Washed platelets (3 × 108 platelets per mL) were incubated with the indicated concentration of mAb R6H8 or 7E3 IgG for 20 minutes at room temperature. Clot retraction was initiated by adding platelets treated as above to an aggregation cuvette containing 2 mM CaCl2 and 0.2 U/mL thrombin, as indicated in “Methods.” Shown are representative images of 4 independent experiments. (B) Washed platelets (3 × 108 platelets per mL) were incubated with the indicated concentration of mAb R6H8, R6H8 F(ab')2, or R6H8 Fab for 20 minutes at room temperature. Clot retraction was initiated by adding platelets treated as above to an aggregation cuvette containing 2 mM CaCl2 and 0.2 U/mL thrombin. Shown are representative images of 3 independent experiments. Cntl, control (untreated) platelets.
mAb R6H8 is a potent inhibitor of clot retraction, but potency requires bivalent binding. (A) Washed platelets (3 × 108 platelets per mL) were incubated with the indicated concentration of mAb R6H8 or 7E3 IgG for 20 minutes at room temperature. Clot retraction was initiated by adding platelets treated as above to an aggregation cuvette containing 2 mM CaCl2 and 0.2 U/mL thrombin, as indicated in “Methods.” Shown are representative images of 4 independent experiments. (B) Washed platelets (3 × 108 platelets per mL) were incubated with the indicated concentration of mAb R6H8, R6H8 F(ab')2, or R6H8 Fab for 20 minutes at room temperature. Clot retraction was initiated by adding platelets treated as above to an aggregation cuvette containing 2 mM CaCl2 and 0.2 U/mL thrombin. Shown are representative images of 3 independent experiments. Cntl, control (untreated) platelets.
mAb R6H8 induces platelet aggregation at low concentrations and inhibits it at high concentrations, but induces secretion and exposure of the LIBS mAb AP5 epitope in a monotonic, concentration-dependent fashion
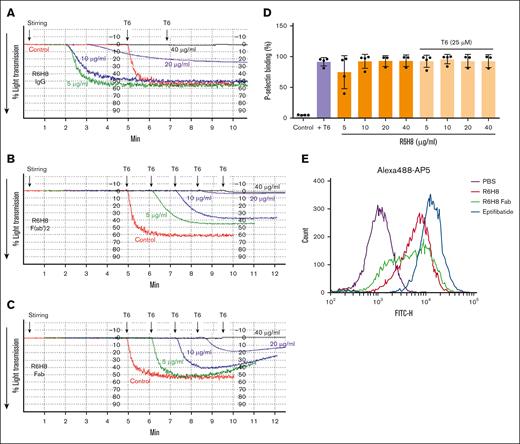
When washed platelets, untreated or treated with R6H8 for 20 minutes, were placed in an aggregometer, the untreated control sample did not undergo aggregation when stirred for 5 minutes at 37°C, but it did undergo brisk aggregation when the PAR1-activating peptide (T6) was added at the 5-minute time point (Figure 2A). Unexpectedly, R6H8 alone at 5, 10, and 20 μg/mL initiated platelet aggregation in a reverse concentration-dependent manner after the onset of stirring, without the addition of any agonist. In sharp contrast, at 40 μg/mL, R6H8 did not initiate platelet aggregation, and it completely inhibited aggregation induced by T6. Unlike R6H8 IgG, neither the F(ab')2 nor the Fab fragment of R6H8 induced platelet aggregation at low concentrations (Figure 2B-C). The F(ab')2 fragment inhibited T6-induced platelet aggregation at concentrations starting at 5 μg/mL and produced nearly complete inhibition at 40 μg/mL, whereas the Fab fragment partially inhibited aggregation starting at 10 μg/mL and also produced nearly complete inhibition at 40 μg/mL. Similar results were obtained with platelet-rich plasma (supplemental Figure 4).
R6H8 demonstrates effects on platelet aggregation that vary based on concentration and whether the intact R6H8 IgG or fragments of R6H8 are tested. (A) Washed platelets (2 × 108 platelets per mL) were treated with the indicated dose of the mAb for 20 minutes at room temperature. Stirring at 37°C was initiated in an aggregometer, and changes in light transmission were continuously measured. After 5 minutes, nonaggregated platelets were activated with 25-μM T6. Data shown are representative of at least 3 experiments. (B-C) Neither mAb R6H8 F(ab')2 nor Fab induced agonist-independent platelet aggregation, but they did block T6-induced platelet aggregation in a concentration-dependent fashion. Washed platelets (2 × 108 platelets per mL) were treated with the indicated dose of R6H8 F(ab')2 (B) or Fab (C) for 20 minutes at room temperature. Platelets were then transferred to an aggregometer and stirring at 37°C was initiated. After ∼5 minutes, platelets were activated with 25-μM T6. Data shown are representative of at least 3 experiments. (D) Washed platelets in HEPES-buffered modified Tyrode's solution (1 × 108 platelets per mL) were treated with the indicated concentrations of R6H8 for 20 minutes at room temperature and then incubated with PE-labeled anti–P-selectin antibody for another 20 minutes. Samples were then diluted and analyzed by flow cytometry. Control platelets were used to set a gate at ∼1% to 2% positive events. P-selectin binding percentage (%) represents positive events. (E) Washed platelets were incubated with 20 μg/mL R6H8, 40 μg/mL R6H8-Fab, or 1-μM eptifibatide for 15 minutes at 22°C, and then 10 μg/mL Alexa488-labeled AP5 was added for 15 minutes and the binding of AP5 was detected by flow cytometry. FITC-H, fluorescence.
R6H8 demonstrates effects on platelet aggregation that vary based on concentration and whether the intact R6H8 IgG or fragments of R6H8 are tested. (A) Washed platelets (2 × 108 platelets per mL) were treated with the indicated dose of the mAb for 20 minutes at room temperature. Stirring at 37°C was initiated in an aggregometer, and changes in light transmission were continuously measured. After 5 minutes, nonaggregated platelets were activated with 25-μM T6. Data shown are representative of at least 3 experiments. (B-C) Neither mAb R6H8 F(ab')2 nor Fab induced agonist-independent platelet aggregation, but they did block T6-induced platelet aggregation in a concentration-dependent fashion. Washed platelets (2 × 108 platelets per mL) were treated with the indicated dose of R6H8 F(ab')2 (B) or Fab (C) for 20 minutes at room temperature. Platelets were then transferred to an aggregometer and stirring at 37°C was initiated. After ∼5 minutes, platelets were activated with 25-μM T6. Data shown are representative of at least 3 experiments. (D) Washed platelets in HEPES-buffered modified Tyrode's solution (1 × 108 platelets per mL) were treated with the indicated concentrations of R6H8 for 20 minutes at room temperature and then incubated with PE-labeled anti–P-selectin antibody for another 20 minutes. Samples were then diluted and analyzed by flow cytometry. Control platelets were used to set a gate at ∼1% to 2% positive events. P-selectin binding percentage (%) represents positive events. (E) Washed platelets were incubated with 20 μg/mL R6H8, 40 μg/mL R6H8-Fab, or 1-μM eptifibatide for 15 minutes at 22°C, and then 10 μg/mL Alexa488-labeled AP5 was added for 15 minutes and the binding of AP5 was detected by flow cytometry. FITC-H, fluorescence.
In contrast to its biphasic effects on platelet aggregation, R6H8 IgG produced a monotonic, concentration-dependent effect in inducing the platelet release reaction, as judged by P-selectin expression, starting at concentrations as low as 5 μg/mL and reaching levels similar to that produced by 25 μM T6 (Figure 2D). Even though R6H8 inhibited T6-induced platelet aggregation, it did not affect the induction of P-selectin produced by T6, even at 40 μg/mL.
To assess whether the binding of R6H8 mimicked the effect of ligand binding, we tested the effect of R6H8 IgG on the exposure of the epitope for the LIBS mAb AP5 on the β3 plexin-semaphorin-integrin domain.14 R6H8 IgG dramatically increased AP5 binding, being nearly as effective as the small-molecule inhibitor eptifibatide, which is known to expose the AP5 epitope (Figure 2E).15
R6H8 induces both fibrinogen and mAb PAC-1 binding to platelets at low concentrations; at high concentrations, it inhibits fibrinogen and PAC-1 binding induced by T6
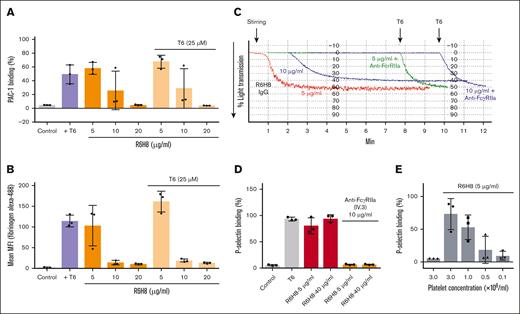
R6H8 at 5 to 10 μg/mL, but not at higher concentrations (20 μg/mL), initiated fibrinogen and PAC-1 binding to platelets (Figure 3A-B). At higher concentrations, it was a potent inhibitor of both fibrinogen and PAC-1 binding initiated by T6.
mAb R6H8 activates platelets via simultaneously binding αIIbβ3 and engaging FcγRIIa on nearby platelets and inhibits platelet aggregation by blocking ligand binding to integrin αIIbβ3. (A-B) Washed platelets in HEPES-buffered modified Tyrode's solution (1 × 108 platelets per mL) were treated with the indicated concentrations of R6H8 for 20 minutes at room temperature, and then incubated with FITC-labeled anti–PAC-1 antibody (25 μg/mL) (A) or 200 μg/mL Alexa488-human fibrinogen with or without T6 (25 μM) (B) for another 20 minutes. Samples were then diluted and analyzed by flow cytometry. Control platelets were used to set a gate at ∼1% to 4% positive events. PAC-1 binding percentage (%) represents PAC-1 positive events inside the gate. Results are of 3 independent experiments, reported as mean ± standard deviation (SD). (C) Washed platelets (2 × 108 platelets per mL) were treated with 10 μg/mL of anti- FcγRIIa (mAb VI.3) for 20 minutes at room temperature, followed by a 20-minute treatment with the indicated dose of mAb R6H8. Platelets were then transferred to the aggregometer, and stirring was initiated. After ∼8 minutes, nonaggregated platelets were activated with 25-μM T6. Data shown are representative of at least 3 similar experiments. (D) Washed platelets (1 × 108 platelets per mL) were treated with 10 μg/mL of anti-FcγRIIa mAb IV.3 for 20 minutes at room temperature, followed by a 20-minute treatment with the indicated dose of mAb R6H8. Platelets were then treated with PE-labeled anti–P-selectin antibody for another 20 minutes. Samples were then diluted and analyzed by flow cytometry. (E) Washed platelets were diluted in HEPES-buffered modified Tyrode's solution to the indicated concentration and incubated for 20 minutes with 5 μg/mL of mAb R6H8. Platelets samples were then incubated for another 20 minutes with PE–P-selectin antibody. Control platelets were used to set a gate at ∼1% to 2% positive events. P-selecting binding percentage (%) represent positive events with fluorescence values ± SD. MFI, mean fluorescence intensity.
mAb R6H8 activates platelets via simultaneously binding αIIbβ3 and engaging FcγRIIa on nearby platelets and inhibits platelet aggregation by blocking ligand binding to integrin αIIbβ3. (A-B) Washed platelets in HEPES-buffered modified Tyrode's solution (1 × 108 platelets per mL) were treated with the indicated concentrations of R6H8 for 20 minutes at room temperature, and then incubated with FITC-labeled anti–PAC-1 antibody (25 μg/mL) (A) or 200 μg/mL Alexa488-human fibrinogen with or without T6 (25 μM) (B) for another 20 minutes. Samples were then diluted and analyzed by flow cytometry. Control platelets were used to set a gate at ∼1% to 4% positive events. PAC-1 binding percentage (%) represents PAC-1 positive events inside the gate. Results are of 3 independent experiments, reported as mean ± standard deviation (SD). (C) Washed platelets (2 × 108 platelets per mL) were treated with 10 μg/mL of anti- FcγRIIa (mAb VI.3) for 20 minutes at room temperature, followed by a 20-minute treatment with the indicated dose of mAb R6H8. Platelets were then transferred to the aggregometer, and stirring was initiated. After ∼8 minutes, nonaggregated platelets were activated with 25-μM T6. Data shown are representative of at least 3 similar experiments. (D) Washed platelets (1 × 108 platelets per mL) were treated with 10 μg/mL of anti-FcγRIIa mAb IV.3 for 20 minutes at room temperature, followed by a 20-minute treatment with the indicated dose of mAb R6H8. Platelets were then treated with PE-labeled anti–P-selectin antibody for another 20 minutes. Samples were then diluted and analyzed by flow cytometry. (E) Washed platelets were diluted in HEPES-buffered modified Tyrode's solution to the indicated concentration and incubated for 20 minutes with 5 μg/mL of mAb R6H8. Platelets samples were then incubated for another 20 minutes with PE–P-selectin antibody. Control platelets were used to set a gate at ∼1% to 2% positive events. P-selecting binding percentage (%) represent positive events with fluorescence values ± SD. MFI, mean fluorescence intensity.
Platelet aggregation induced by R6H8 is inhibited by blocking platelet FcγRIIa
Platelet aggregation induced by mAb R6H8 at 5 μg/mL was inhibited by a mAb IV.3, which blocks the FcγRIIa receptor (anti-FcγRIIa), and the platelets retained their ability to aggregate in response to T6 (Figure 3C). Increasing the concentration of R6H8 to 10 μg/mL decreased the initial aggregation response; this aggregation was also inhibited by the antibody to FcγRIIa without diminishing the subsequent response to T6. To assess whether engagement of FcγRIIa is necessary for P-selectin exposure, we analyzed the effect of inhibiting FcγRIIa with mAb IV.3 on P-selectin expression induced by R6H8. We found that mAb IV.3 inhibited the exposure of P-selectin (Figure 3D). This supports the hypothesis that the engagement of FcγRIIa is required for the activation leading to P-selectin exposure.
Platelet dilution studies support interplatelet rather than intraplatelet engagement of FcγRIIa to achieve platelet activation
To assess whether R6H8 engages both αIIbβ3 and FcγRIIa on the same platelet or whether it engages αIIbβ3 on one platelet and FcγRIIa on another platelet (intraplatelet or interplatelet activation), we reasoned that the former mechanism would not be affected by decreasing the concentration of platelets in the suspension, whereas the latter would result in decreased activation. Using P-selectin expression as an index of platelet activation, we found that decreasing the platelet concentration resulted in a stepwise reduction in R6H8-induced expression of P-selectin, suggesting that R6H8 activates platelets via an interplatelet rather than an intraplatelet mechanism (Figure 3E).
Negative-stain EM analysis of the αIIbβ3–R6H8 Fab complex indicates that R6H8 binding induces αIIbβ3 to adopt the extended-open conformation
Negative-stain EM images of full-length αIIbβ3 purified from platelets showed a monodispersed particle population, with the particles varying in size and shape (supplemental Figure 1A). After picking and classifying ∼10 000 particles into 100 classes, the class averages showed the integrin in conformations varying from the V-shaped bent-closed conformation to the extended-closed conformation (supplemental Figure 1B-C). We then imaged the αIIbβ3–R6H8 Fab complex and observed an increase in the size of the particles compared to full-length αIIbβ3 alone (supplemental Figure 1D). We again classified ∼10 000 particles into 100 classes (supplemental Figure 1E-F). Several of the class averages showed only the integrin headpiece with an associated bilobal or circular density, consistent with the side view or face view of a bound Fab, respectively. The integrin legs are not resolved in these averages, suggesting that the integrins adopted the extended-open conformation, in which the legs tend to be very flexible and are thus often averaged out. Other averages showed the integrin in the canonical V-shaped bent-closed conformation without bound Fab. Collectively, these results suggest that R6H8 binding activates αIIbβ3 and induces it to adopt an extended-open conformation.
Cryo-EM analysis of the αIIbβ3–R6H8 Fab complex indicates that R6H8 is a ligand-mimetic that induces swing-out of the β3 hybrid domain and a unique loss of the ADMIDAS metal ion
Cryo-EM analysis resulted in a model of apo αIIbβ3 at 3.3-Å resolution and the αIIbβ3–R6H8 Fab complex at 2.8-Å resolution 3D structures (Figures 4 and 5; supplemental Figures 2 and 3; supplemental Table 1). Both models include αIIb residues 1 to 451, comprising the β-propeller domain, and β3 residues 59 to 432, comprising the βI and hybrid domains, and the model for the αIIbβ3–R6H8 Fab complex also includes R6H8 residues 1 to 224 from the heavy chain and residues 1 to 217 from the light chain.
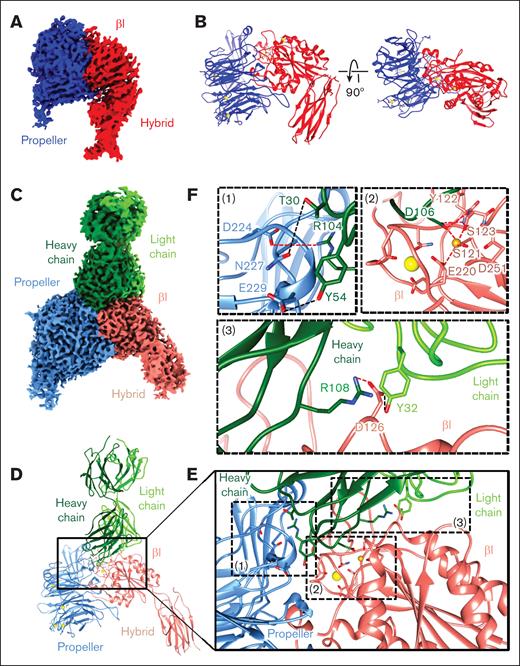
Analysis of αIIbβ3 alone and in complex with R6H8 Fab, revealing a ligand-mimetic mode of binding. (A-D) Cryo-EM analysis of αIIbβ3 by itself (A-B) and in complex with R6H8 Fab (C-D). Shown are the density maps colored by domains (panels A,C) and models in ribbon representation (panels B,D). (E) Enlarged view of the region marked in panel D showing the interaction interface between the αIIbβ3 headpiece and the R6H8 Fab. (F) Zoomed-in views of the regions marked and labeled (1) to (3) in panel E. Residues involved in the interactions are shown as sticks. The synergistic metal ion binding site and MIDAS cations are shown as yellow and gold spheres, respectively. Charge interactions are indicated by red dashed lines and hydrogen bonds by black dashed lines.
Analysis of αIIbβ3 alone and in complex with R6H8 Fab, revealing a ligand-mimetic mode of binding. (A-D) Cryo-EM analysis of αIIbβ3 by itself (A-B) and in complex with R6H8 Fab (C-D). Shown are the density maps colored by domains (panels A,C) and models in ribbon representation (panels B,D). (E) Enlarged view of the region marked in panel D showing the interaction interface between the αIIbβ3 headpiece and the R6H8 Fab. (F) Zoomed-in views of the regions marked and labeled (1) to (3) in panel E. Residues involved in the interactions are shown as sticks. The synergistic metal ion binding site and MIDAS cations are shown as yellow and gold spheres, respectively. Charge interactions are indicated by red dashed lines and hydrogen bonds by black dashed lines.
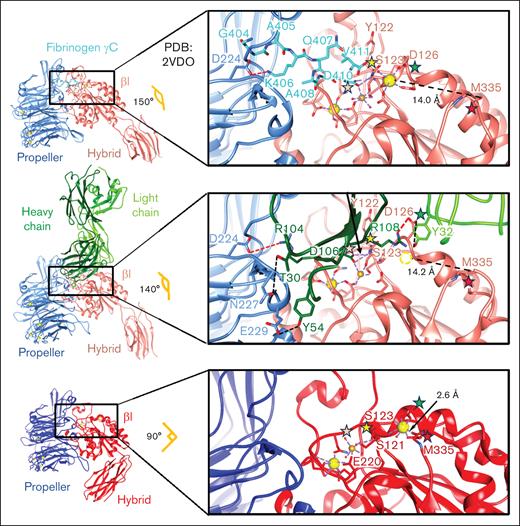
Structural comparison of αIIbβ3 by itself and in complex with the fibrinogen γC peptide and the R6H8 Fab, showing the ligand-mimetic binding of the R6H8 Fab. Atomic models of αIIbβ3 in complex with fibrinogen γC peptide (top), αIIbβ3-R6H8 Fab complex (middle), and apo αIIbβ3 (bottom). Each structure is colored based on individual subunits. The angles between the βI and hybrid domains in the 3 structures are indicated. Each inset shows the detailed interactions in the RGD-binding pocket. The pink asterisk indicates that D106 from the CDR3 of R6H8 Fab heavy chain and D410 from the fibrinogen γC peptide both directly coordinate the MIDAS Mg2+ by replacing the water molecule in the apo structure. The yellow asterisk indicates that S123 from the βI domain interacts with MIDAS Mg2+ directly in the fully extended conformation, whereas in the apo state, S123 interacts with the MIDAS Mg2+ via a water bridge. The green asterisk indicates that the dramatic change in orientation of the S126 side chain induced by R6H8 Fab binding as a result of its interaction with R6H8 Fab D108 (CDR3 heavy chain) and Y32 (CDR1 light chain) leads to loss of the ADMIDAS Ca2+ (dotted sphere). The red asterisk indicates that the loss of the ADMIDAS Ca2+ results in loss of its interaction with M335’s backbone carbonyl, which leads to swing-out of the hybrid domain. Residues involved in binding the synergistic metal ion binding site Ca2+ (yellow sphere), MIDAS Mg2+ (gold sphere), and ADMIDAS Ca2+ (yellow sphere) are shown as sticks. Interactions in synergistic metal ion binding site, MIDAS, and ADMIDAS regions are indicated with dotted purple lines. Charge interactions are indicated with dotted red lines, whereas hydrogen bonds are indicated with dotted black lines. The distances between the M335 carbonyl and the ADMIDAS Ca2+ are indicated. PDB, Protein Data Bank.
Structural comparison of αIIbβ3 by itself and in complex with the fibrinogen γC peptide and the R6H8 Fab, showing the ligand-mimetic binding of the R6H8 Fab. Atomic models of αIIbβ3 in complex with fibrinogen γC peptide (top), αIIbβ3-R6H8 Fab complex (middle), and apo αIIbβ3 (bottom). Each structure is colored based on individual subunits. The angles between the βI and hybrid domains in the 3 structures are indicated. Each inset shows the detailed interactions in the RGD-binding pocket. The pink asterisk indicates that D106 from the CDR3 of R6H8 Fab heavy chain and D410 from the fibrinogen γC peptide both directly coordinate the MIDAS Mg2+ by replacing the water molecule in the apo structure. The yellow asterisk indicates that S123 from the βI domain interacts with MIDAS Mg2+ directly in the fully extended conformation, whereas in the apo state, S123 interacts with the MIDAS Mg2+ via a water bridge. The green asterisk indicates that the dramatic change in orientation of the S126 side chain induced by R6H8 Fab binding as a result of its interaction with R6H8 Fab D108 (CDR3 heavy chain) and Y32 (CDR1 light chain) leads to loss of the ADMIDAS Ca2+ (dotted sphere). The red asterisk indicates that the loss of the ADMIDAS Ca2+ results in loss of its interaction with M335’s backbone carbonyl, which leads to swing-out of the hybrid domain. Residues involved in binding the synergistic metal ion binding site Ca2+ (yellow sphere), MIDAS Mg2+ (gold sphere), and ADMIDAS Ca2+ (yellow sphere) are shown as sticks. Interactions in synergistic metal ion binding site, MIDAS, and ADMIDAS regions are indicated with dotted purple lines. Charge interactions are indicated with dotted red lines, whereas hydrogen bonds are indicated with dotted black lines. The distances between the M335 carbonyl and the ADMIDAS Ca2+ are indicated. PDB, Protein Data Bank.
The αIIbβ3–R6H8 Fab complex structure was notable for the following features: (1) The R6H8 heavy-chain residue Arg104 in complementarity-determining region 3 (CDR3) forms an electrostatic interaction with αIIb Asp224 (Figure 4C-F, inset 1), mimicking the interaction of the Arg residue in RGD-containing compounds and related ligands; (2) The R6H8 heavy-chain residues Thr30 in CDR1 and Tyr54 in CDR2 form hydrogen bonds with αIIb Asn227 and Glu229, respectively (Figure 4C-F, inset 1); (3) The R6H8 heavy-chain residue Asp106 in CDR3 displaces a water molecule and coordinates the MIDAS Mg2+, which is the same interaction that occurs with Asp residues in fibrinogen and RGD-containing compounds and related ligands (Figure 4C-F, inset 2); (4) The β3 β1-α1 loop moves toward the MIDAS as the backbone nitrogen of the β3 residue Ser123 in the βI domain interacts with the carboxyl group of R6H8 heavy-chain residue Asp106, resulting in β3 Ser123 making a direct interaction with the MIDAS Mg2+ rather than interacting through an intermediate water molecule, again simulating the effect of ligand binding to β3;3 and (5) The R6H8 heavy-chain residue Arg108 in CDR3 and light-chain residue Tyr32 in CDR1 interact with β3 residue Asp126 in the βI domain, leading to reorientation of Asp126 away from the ADMIDAS and the loss of its coordination with the ADMIDAS Ca2+ (Figure 4C-F, inset 3). This, in turn, leads to the loss of the ADMIDAS Ca2+ itself, breakage of the interaction between the Met335 backbone carbonyl and the ADMIDAS Ca2+, and the swing-out movement of the β3 hybrid domain away from the MIDAS as the β3 α7 helix moves down (Figure 5, middle).
Figure 5 highlights the similarities and differences between the binding of R6H8 Fab and the fibrinogen γC 400-411 peptide to αIIbβ3.16 The key residues for the fibrinogen γC peptide are Lys406, which forms a charge interaction with αIIb propeller residues Asp224, and Asp410, which directly coordinates to the MIDAS Mg2+ cation and further interacts with the backbone nitrogen of Ser123 through a hydrogen bond (Figure 5, top). In the αIIbβ3–R6H8 Fab complex, the R6H8 Fab heavy-chain residues Arg104 and Asp106 in CDR3 play comparable roles, leading to reorganization of the MIDAS and swing-out of the hybrid domain, changing its angle with the βI domain from 90° in the apo integrin (Figure 5, bottom) to 140° in the R6H8-bound integrin (Figure 5, middle), which is similar to the 150° angle in the fully extended state of the γC peptide-bound integrin (Figure 5, top). The major differences in binding are the additional interactions of R6H8 Fab with αIIb propeller residues Asn227 and Glu229 and the loss of the ADMIDAS Ca2+ as a result of R6H8 Fab binding to and reorienting β3 residue Asp126 away from the ADMIDAS Ca2+. These structural data indicate that R6H8 Fab acts as a ligand-mimetic, with high affinity for the αIIbβ3 RGD-binding pocket and the ability to induce swing-out of the hybrid domain to the extended-open conformation.
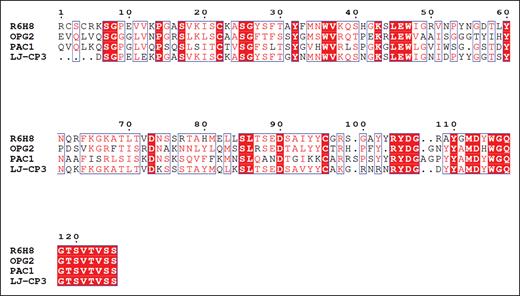
The sequence of the R6H8 heavy-chain CDR3 contains an RYD sequence, which is the same sequence present in all 3 of the other reported αIIbβ3 ligand-mimetic antibodies
Similar to the 3 αIIbβ3 ligand-mimetic antibodies previously reported, R6H8 has an Arg-Tyr-Asp (RYD) sequence in its CDR3 (Figure 6).17-19 Although previous reports inferred that this sequence was responsible for the ligand-mimetic binding to the receptor using peptide inhibition and biochemical approaches, we now provide structural evidence that the RYD sequence in R6H8 is responsible for binding to the RGD-binding site in αIIbβ3.
Comparison of the amino acid sequences of the variable domains of the heavy chains of mAbs R6H8, OPG2, PAC-1, and LJ-CP3. Multiple-sequence alignment of R6H8, OPG2, PAC-1, and LJ-CP3. The multiple-sequence alignment of individual heavy chains was calculated with the program Clustal Omega66 and visualized in Espript.67 The highly conserved amino acid residues are presented as white letters on a red background. Amino acid residues that have comparable chemical and physical properties are presented as red letters within blue frames. The RYDG sequence, which begins at amino acid 105 in mAb R6H8, is conserved in all 4 CDR3 sequences.
Comparison of the amino acid sequences of the variable domains of the heavy chains of mAbs R6H8, OPG2, PAC-1, and LJ-CP3. Multiple-sequence alignment of R6H8, OPG2, PAC-1, and LJ-CP3. The multiple-sequence alignment of individual heavy chains was calculated with the program Clustal Omega66 and visualized in Espript.67 The highly conserved amino acid residues are presented as white letters on a red background. Amino acid residues that have comparable chemical and physical properties are presented as red letters within blue frames. The RYDG sequence, which begins at amino acid 105 in mAb R6H8, is conserved in all 4 CDR3 sequences.
Discussion
We report on a novel murine mAb to αIIbβ3 with 2 unusual features: acting as a ligand-mimetic and activating platelets to undergo both secretion and aggregation via its Fc domain engaging platelet FcγRIIa. Three αIIbβ3 ligand-mimetic antibodies have previously been reported, but none demonstrated the ability to activate platelets. Shattil et al reported on the IgM κ murine mAb PAC-1, which was paradoxically produced by immunizing the mouse with platelets from a patient with Glanzmann thrombasthenia.17-19 Its binding required platelet activation, which could be initiated by a variety of agonists. PAC-1 inhibited platelet aggregation, and the CDR3 of its heavy chain contained the RYD sequence; a series of mutations of this sequence led to the conclusion that the RYD sequence mimicked the RGD sequence in αIIbβ3 ligands, making it an activation-dependent, ligand-mimetic antibody. Niiya reported on the murine IgG1 mAb LJ-CP3,20 and Tomiyama et al reported on the IgG1 kappa murine mAb OP-G2,21 both of which recognize αIIbβ3 and bind to both unactivated and activated platelets. The binding of both antibodies was inhibited by the peptide RGDW, raising the possibility that they are also ligand-mimetics. This was supported by the finding that both antibodies also contain an RYD sequence in their heavy-chain CDR3 domains (Figure 6).22 The crystal structure of OP-G2 showed that the RYD sequence was at the tip of a long loop, with structural similarity to the fibronectin loop containing the RGD sequence.23,24 Tomiyama et al also noted that all 3 antibodies appear to derive from the murine heavy-chain D-gene DSP2.10, which has the sequence YYRYDG.22 R6H8 contains this exact sequence, as does PAC-1, whereas the other antibodies differ at the first and/or second amino acid.
Murine mAbs directed against platelet glycoproteins have been found to activate platelets via FcγRIIa, with mAbs to CD9 most commonly showing this property; however, some antibodies directed against GPIb and GPIV also have this property.25-32 In contrast, most mAbs developed to αIIbβ3 have not caused platelet activation, but rare ones have had this property.28,30,32 In particular, Horsewood et al reported on mAb Raj-1, which activated platelets by binding to both αIIbβ3 and FcγRIIa.30 This antibody did not, however, inhibit ligand binding to αIIbβ3. Similarly, Bachelot et al and Rubinstein et al reported on the murine mAb P256, directed against αIIbβ3, which induced platelet activation and aggregation; but it appeared to operate only in part via interactions with FcγRIIa because mAb IV.3 only partially inhibited aggregation, and the F(ab')2 fragment initiated partial aggregation, whereas the Fab fragment did not induce aggregation.33-35 Of note, FcγRIIa with the R131 polymorphism bound the antibody more tightly, and platelets with the FcγRIIa R131 polymorphism had brisker aggregation responses.34 They did not, however, localize the αIIbβ3 binding site, and unlike R6H8, the P256 antibody did not inhibit platelet aggregation. Li et al described mAb 9D2, which reacted with human αIIbβ3 but did not inhibit human platelet aggregation; they found that it induced P-selectin expression on the platelets of some but not all human donors, and the activation was inhibited by mAb IV.3, raising the possibility that it was selective for either FcγRIIa R131 or H131.36 The orientation of the antibody on αIIbβ3 probably plays an important role in its ability to induce platelet activation as demonstrated by the finding by Huang et al that the nonactivating anti-αIIbβ3 mAb AP2 could become an activating mAb if the platelets were pretreated with eptifibatide or select snake venoms known to induce the high-affinity ligand-binding conformation of αIIbβ3.37 Separately, FcγRIIa has been shown to be topographical close to αIIbβ3 and play an important role in αIIbβ3-mediated outside-in activation, although the precise mechanism that links αIIbβ3 to FcγRIIa during this process has not been established.38-40 mAb R6H8 is an IgG1, and this isotype is known to bind to both the human FcγRIIa-H131 and FcγRIIa-R131 variants, with a higher affinity for the FcγRIIa-R131 variant.41
Activation of the platelet FcγRIIa is also the final common pathway for a variety of disorders associated with platelet activation and thrombosis (Table 1). These include heparin-induced thrombocytopenia, vaccine-induced immune thrombotic thrombocytopenia, autoimmune heparin-induced thrombocytopenia, autoimmune anti–platelet factor 4 (PF4) syndrome, paraprotein-associated chronic thrombocytopenia/thrombosis, and GPI-associated thrombocytopenia with thrombosis.42-51 Antibodies to PF4 play an important role in all of these disorders except GPI-associated thrombocytopenia with thrombosis, a rare complication of therapy with tirofiban or eptifibatide, in which antibodies to the αIIbβ3-drug complex are able to signal through FcγRIIa.45 Antibody complexes with PF4 tetramers, enhanced in size by heparin binding to PF4 in the case of heparin-induced thrombocytopenia, have generally been proposed to crosslink platelet FcγRIIa as the mechanism for triggering platelet activation, but Buka et al have reported that PF4 may bind to the platelet c-myeloproliferative leukemia virus oncogene (Mpl) receptor, in which case the antibodies may be spanning between the PF4−c-Mpl complex and FcγRIIa on the same or a nearby platelet.52 Activation of platelet FcγRIIa by circulating immune complexes has also been implicated in the thrombotic complications associated with autoimmune disorders, including systemic lupus erythematosus and antiphospholipid syndrome,49 and may also contribute to the activated platelet signature identified in patients with long COVID-19.53
Pathological disorders associated with FcγRIIA activation
| Disorder . | PF4 participation . | Heparin participation . | Vaccine participation . | Antecedent viral infection/surgery . |
|---|---|---|---|---|
| Heparin-induced thrombocytopenia | + | + | – | – |
| Vaccine-induced thrombotic thrombocytopenia | + | – | + | – |
| Autoimmune heparin-induced thrombocytopenia | + | + | – | – |
| Autoimmune anti-PF4 syndrome | + | – | – | + |
| Paraprotein-associated chronic thrombocytopenia/thrombosis | + | – | – | – |
| GPI-induced thrombocytopenia with platelet activation/thrombosis | – | – | – | – |
| Therapeutic mAb-induced thrombocytopenia/thrombosis | – | – | – | – |
| mAb-drug conjugate–induced thrombocytopenia and liver/macrophage injury | – | – | – | – |
| Thrombotic cardiovascular disease | – | – | – |
| Disorder . | PF4 participation . | Heparin participation . | Vaccine participation . | Antecedent viral infection/surgery . |
|---|---|---|---|---|
| Heparin-induced thrombocytopenia | + | + | – | – |
| Vaccine-induced thrombotic thrombocytopenia | + | – | + | – |
| Autoimmune heparin-induced thrombocytopenia | + | + | – | – |
| Autoimmune anti-PF4 syndrome | + | – | – | + |
| Paraprotein-associated chronic thrombocytopenia/thrombosis | + | – | – | – |
| GPI-induced thrombocytopenia with platelet activation/thrombosis | – | – | – | – |
| Therapeutic mAb-induced thrombocytopenia/thrombosis | – | – | – | – |
| mAb-drug conjugate–induced thrombocytopenia and liver/macrophage injury | – | – | – | – |
| Thrombotic cardiovascular disease | – | – | – |
Several therapeutic mAbs have been associated with thrombosis linked to FcγRIIa-mediated platelet activation. Inhibition of the interaction of CD40 ligand (CD40L) with CD40 is an attractive therapeutic approach for treating autoimmune disorders, but initial attempts to develop mAbs to target this interaction were thwarted by a high rate of arterial thrombosis in patients and nonhuman primates.54-56 Langer et al demonstrated that the binding of an anti-CD40L mAb to trimeric CD40L triggered platelet activation and aggregation via platelet FcγRIIa. This group went on to show that it could induce thrombosis in mice with human platelet FcγRIIa,57,58 while others showed that platelets release soluble trimeric CD40L when activated.59 To avoid platelet activation via FcγRIIa, investigators focused on disrupting the interaction between CD40 and CD40L have developed the following: (1) a bivalent nonantibody Tn3 scaffold of ∼90 amino acids with loops corresponding to immunoglobulin complementarity-determining regions selected for inhibiting CD40L-CD40 fused to albumin to prolong the half-life;60 (2) an mAb to CD40L that underwent Fc engineering to abrogate stimulation via the FcγRIIa;61 and (3) a polyethylene glycol-conjugated anti-CD40L Fab fragment.62,63 These agents have shown clinical promise in several disorders without producing thrombosis. The anti-vascular endothelial growth factor (VEGF) mAb bevacizumab, which also increased the risk of thrombosis, was shown to activate platelets via FcγRIIa in the presence of VEGF, and this was augmented by heparin, which binds to VEGF.64 Antibody-drug conjugates have also led to thrombocytopenia and variable liver and monocyte damage when antibody-drug conjugate aggregates activate platelets via FcγRIIa, followed by drug delivery to the liver cells, and macrophages that clear the antibody-coated platelets.65,66 Finally, the level of expression of FcγRIIa on platelets has been associated with the response of platelets to agonists and the risk of thrombotic cardiovascular disease events among patients at high risk.67-69
The cryo-EM structure of the αIIbβ3–R6H8 Fab complex is notable for 3 features. First, unlike RGD peptides or the fibrinogen γ-chain C-terminal peptide, R6H8 uses amino acids from all 3 heavy-chain CDRs to make multiple interactions with αIIb. Thus, in addition to a charge interaction with αIIb Asp224 (with CDR3 R104), similar to that made by the RGD and fibrinogen γ-chain (with Lys406) peptides, R6H8 also uses its CDR1 Thr30 to interact with Asn227 and its CDR2 Tyr54 to interact with αIIb Asp229. Second, R6H8 Asp106 coordinates the MIDAS Mg2+ in a manner similar to that of the Asp residues in the RGD and fibrinogen γ-chain (with Asp411) peptides. Third, R6H8 makes a novel set of interactions involving heavy-chain CDR3 Arg108 and light-chain CDR1 Tyr32 with β3 Asp126 that displaces it so that it no longer coordinates the ADMIDAS Ca2+. This, in turn, leads to the loss of the ADMIDAS Ca2+. This is analogous to the loss of the MIDAS Mg2+ when the small-molecule RUC-2 binds to the Glu220 that coordinates the MIDAS Mg2+.70
In summary, we have produced a novel mAb with the unusual properties of activating platelets at low antibody concentrations via engaging the platelet FcγRIIa receptor on nearby platelets, acting as a ligand-mimetic in engaging the RGD-binding site, inducing the conformational change associated with high-affinity ligand binding, and inhibiting ligand binding at high concentrations. This unique combination of attributes makes it an attractive reagent for better understanding the structural requirements for αIIbβ3 antagonist drug-induced mAbs to αIIbβ3 that produce platelet activation and thrombosis45 and for potentially identifying effective and safe drugs for inhibiting platelet activation and thrombosis mediated via binding to and signaling through FcγRIIa.
Acknowledgments
The authors thank Suzanne Rivera for outstanding administrative assistance.
This work was supported, in part, by National Institutes of Health (NIH), National Heart, Lung, and Blood Institute grants HL19278 and K01-HL169359-01; NIH, National Center for Advancing Translational Sciences grant UL1TR001866; The Kellen Women’s Entrepreneurship Fund; the Robertson Therapeutic Development Fund at Rockefeller University; and the Stavros Niarchos Foundation (SNF) as part of its grant to the SNF Institute for Global Infectious Disease Reseach at The Rockefeller University.
Authorship
Contribution: J.W., L.B., and B.S.C. conceptualized the study; J.W. performed cryogenic electron microscopy experimental procedures; L.B., L.W., and J.L. performed antibody screening; L.B. and L.W. performed experimental procedures; J.W., L.B., L.W., and J.L. acquired data; J.W. and L.B. analyzed data; T.W. designed, oversaw, and analyzed electron microscopy studies; J.W., L.B., T.W., and B.S.C. wrote the manuscript; B.S.C. supervised the project; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lorena Buitrago, The Rockefeller University, 1230 York Ave, Hospital Building, Room 611, New York, NY 10065; email: lbuitrago@rockefeller.edu.
References
Author notes
J.W. and L.B. contributed equally to this work.
Two cryogenic electron microscopy density maps of apo αIIbβ3 and αIIbβ3 in complex with R6H8 Fab have been deposited in the Electron Microscopy Data Bank (accession codes EMD-46701 and EMD-46695), and the atomic coordinates of apo αIIbβ3 and αIIbβ3 in complex with R6H8 Fab have been deposited in the Protein Data Bank (accession codes 9DAX and 9DAO).
Original data are available on request from the corresponding author, Lorena Buitrago (lbuitrago@rockefeller.edu).
The full-text version of this article contains a data supplement.