TO THE EDITOR:
Teclistamab and talquetamab are the first US Food and Drug Administration–approved B-cell maturation antigen × CD3 and G protein–coupled receptor, family C, group 5, member D × CD3 bispecific antibodies (bsAbs), respectively, for the treatment of triple-class–exposed patients with relapsed/refractory multiple myeloma after 4 prior lines, based on the pivotal MajesTEC-1 and MonumenTAL-1 studies.1,2 Teclistamab and talquetamab are associated with unique adverse effects (AEs) including cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS). In MajesTEC-1, 72.1% of patients experienced CRS (most grade 1-2 with 1 grade 3 event).1 In the MonumenTAL-1 study, CRS occurred in 77% and 80% of patients who received weekly and biweekly dosing of talquetamab, respectively (most grade 1-2 except for 1 grade 3 event).3 To mitigate CRS and ICANS, the package insert recommends initiating therapy with step-up dosing (SUD), including premedication and inpatient monitoring during the SUD period.
Other notable toxicities associated with teclistamab and talquetamab include infections and myelosuppression, as well as skin, nail, oral, and taste disorders with talquetamab. Dose delays are the primary method for managing these toxicities. For delays >28 days, the US Food and Drug Administration–approved label recommends restarting teclistamab at SUD1 with premedication and hospitalization for 48 hours after administration of all SUDs. For talquetamab, the package insert recommends restarting weekly talquetamab at SUD2 and biweekly talquetamab at SUD3 if dose delay is 29 to 56 days. For delays >56 days, talquetamab is recommended to restart with SUD1.
Herein, we present our single center experience of forgoing reinitiation of SUD for patients with prolonged dose delay (>28 days) of teclistamab and talquetamab and present the incidence of CRS and/or ICANS in these patients after restarting therapy.
Between 29 November 2022 to 31 July 2024, 96 patients received teclistamab and 40 patients received talquetamab outside of a clinical trial at Memorial Sloan Kettering. There were 37 patients who had prolonged dose-delay events: 4 reinitiated SUD per drug label, 30 omitted SUD, and 3 reinitiated SUD per drug label and omitted SUD at different time points. Among the 33 patients who restarted therapy without SUD, 26 received teclistamab after a >28-day delay, 6 received talquetamab after dose delay of 29 to 56 days, and 1 received talquetamab after a >56-day delay. There were 62 prolonged dose-delay events without repeat SUD. The median dose-delay interval was 35 days (range, 29-121) overall with a median of 35 days (range, 29-121) and 33 days (range, 29-63) for teclistamab and talquetamab, respectively.
The median age was 69 years (interquartile range, 63-77) with 54% female and a median of 7 prior lines of therapy (interquartile range, 4-9), including 16 (43%) who received prior B-cell maturation antigen–directed therapy. There were no significant differences in baseline characteristics between patients who received or omitted repeat SUD after prolonged dose delay (Table 1). For the 33 patients who reinitiated without SUD, 23 patients (70%) experienced CRS, and 4 (12%) had ICANS, including 3 with both CRS and ICANS during the initial SUD period. Most CRS events were grade 1 (57%) and grade 2 (35%), except for 1 grade 3 event (4%). All ICANS events were limited to grade 1 (50%) and grade 2 (50%). The median time on bsAbs before dose delay was 280 days (range, 30-579) for teclistamab and 127 days (range, 29-211) for talquetamab. Patients received a median of 19 (range, 1-43) and 6 (range, 1-15) treatment doses of teclistamab and talquetamab, respectively, prior to prolonged dose delay. None of the prolonged dose-delay events occurred during the initial SUD period. Twenty-one patients were on less frequent dosing of teclistamab. Common reasons for prolonged delay include scheduling/travel/patient preference (23/62 [37%]), hospitalization (12/62 [19%]), infection without hospitalization (11/62 [18%]), management of a second primary malignancy (3/62 [5%]), and cytopenias without infection (3/62 [5%]). Two patients receiving talquetamab required prolonged delay due to talquetamab-associated AEs.
Patient and disease characteristics
| Characteristic . | Overall population (N = 37) . | No SUD at reinitiation (n = 30) . | SUD at reinitiation (n = 7)∗ . | P value . |
|---|---|---|---|---|
| Median age (IQR), y | 69 (63-77) | 70 (63-77) | 65 (63-78) | .6 |
| Sex, female, n (%) | 20 (54) | 15 (50) | 5 (71) | .4 |
| ECOG performance status, no./total no. (%) | >.9 | |||
| 0 | 4/21 (19) | 4/18 (22) | 0 | |
| 1 | 17/21 (81) | 14/18 (78) | 3/3 (100) | |
| ISS stage, no./total no. (%) | .5 | |||
| I | 10/23 (43) | 8/19 (45) | 2/4 (50) | |
| II | 7/23 (30) | 5/19 (26) | 2/4 (50) | |
| III | 6/23 (26) | 6/19 (32) | 0 | |
| Cytogenetic risk, no./total no. (%) | .8 | |||
| Standard risk | 15/35 (43) | 13/29 (45) | 2/6 (33) | |
| High risk† | 20/35 (57) | 16/29 (55) | 4/6 (67) | |
| Isotype | .2 | |||
| IgG kappa | 21 (57) | 18 (60) | 3 (43) | |
| IgG lambda | 6 (16) | 6 (20) | 0 (0) | |
| IgA kappa | 4 (11) | 2 (6.7) | 2 (29) | |
| IgA lambda | 4 (11) | 3 (10) | 1 (14) | |
| Kappa light chain | 1 (2.7) | 1 (3.3) | 0 (0) | |
| Lambda light chain | 1 (2.7) | 0 | 1 (14) | |
| Extramedullary disease,‡ no./total no. (%) | ||||
| At diagnosis | 13/30 (43) | 11/26 (42) | 2/4 (50) | >.9 |
| Within 3 mo before bispecific | 13/36 (36) | 11/29 (38) | 2/7 (29) | >.9 |
| CNS involvement | 1 (2.7) | 0 (0) | 1∗ (14) | .2 |
| Baseline CrCl of <30 mL/min, no./total no. (%) | 6/36 (17) | 5/29 (17) | 1/7 (14) | >.9 |
| Triple-class refractory, n (%) | 31 (84) | 25 (83) | 6 (86) | >.9 |
| Penta-drug refractory, n (%) | 9 (24) | 7 (23) | 2 (29) | >.9 |
| Median prior lines (range) | 7 (4-9) | 7 (4-9) | 8 (5-9) | .7 |
| Median time from diagnosis to bsAb initiation (IQR), mo | 78 (58-109) | 82 (48-113) | 68 (60-109) | .8 |
| Prior BCMA-directed therapy, n (%) | 16 (43) | 12 (40) | 4 (57) | .4 |
| ADC | 9 (24) | 7 (23) | 2 (29) | >.9 |
| CAR-T | 10 (27) | 7 (23) | 3 (43) | .4 |
| Bispecific | 2 (5) | 2 (7) | 0 (0) | >.9 |
| Prior non-BCMA TCR therapy, n (%) | >.9 | |||
| FcRH5 bsAb | 2 (5.4) | 2 (6.7) | 0 | |
| GPRC5D bsAb | 1 (2.7) | 1 (3.3) | 0 | |
| Current therapy, n (%) | >.9 | |||
| Teclistamab | 29 (78) | 23 (77) | 6∗ (86) | |
| Talquetamab | 8 (22) | 7 (23) | 1∗ (14) |
| Characteristic . | Overall population (N = 37) . | No SUD at reinitiation (n = 30) . | SUD at reinitiation (n = 7)∗ . | P value . |
|---|---|---|---|---|
| Median age (IQR), y | 69 (63-77) | 70 (63-77) | 65 (63-78) | .6 |
| Sex, female, n (%) | 20 (54) | 15 (50) | 5 (71) | .4 |
| ECOG performance status, no./total no. (%) | >.9 | |||
| 0 | 4/21 (19) | 4/18 (22) | 0 | |
| 1 | 17/21 (81) | 14/18 (78) | 3/3 (100) | |
| ISS stage, no./total no. (%) | .5 | |||
| I | 10/23 (43) | 8/19 (45) | 2/4 (50) | |
| II | 7/23 (30) | 5/19 (26) | 2/4 (50) | |
| III | 6/23 (26) | 6/19 (32) | 0 | |
| Cytogenetic risk, no./total no. (%) | .8 | |||
| Standard risk | 15/35 (43) | 13/29 (45) | 2/6 (33) | |
| High risk† | 20/35 (57) | 16/29 (55) | 4/6 (67) | |
| Isotype | .2 | |||
| IgG kappa | 21 (57) | 18 (60) | 3 (43) | |
| IgG lambda | 6 (16) | 6 (20) | 0 (0) | |
| IgA kappa | 4 (11) | 2 (6.7) | 2 (29) | |
| IgA lambda | 4 (11) | 3 (10) | 1 (14) | |
| Kappa light chain | 1 (2.7) | 1 (3.3) | 0 (0) | |
| Lambda light chain | 1 (2.7) | 0 | 1 (14) | |
| Extramedullary disease,‡ no./total no. (%) | ||||
| At diagnosis | 13/30 (43) | 11/26 (42) | 2/4 (50) | >.9 |
| Within 3 mo before bispecific | 13/36 (36) | 11/29 (38) | 2/7 (29) | >.9 |
| CNS involvement | 1 (2.7) | 0 (0) | 1∗ (14) | .2 |
| Baseline CrCl of <30 mL/min, no./total no. (%) | 6/36 (17) | 5/29 (17) | 1/7 (14) | >.9 |
| Triple-class refractory, n (%) | 31 (84) | 25 (83) | 6 (86) | >.9 |
| Penta-drug refractory, n (%) | 9 (24) | 7 (23) | 2 (29) | >.9 |
| Median prior lines (range) | 7 (4-9) | 7 (4-9) | 8 (5-9) | .7 |
| Median time from diagnosis to bsAb initiation (IQR), mo | 78 (58-109) | 82 (48-113) | 68 (60-109) | .8 |
| Prior BCMA-directed therapy, n (%) | 16 (43) | 12 (40) | 4 (57) | .4 |
| ADC | 9 (24) | 7 (23) | 2 (29) | >.9 |
| CAR-T | 10 (27) | 7 (23) | 3 (43) | .4 |
| Bispecific | 2 (5) | 2 (7) | 0 (0) | >.9 |
| Prior non-BCMA TCR therapy, n (%) | >.9 | |||
| FcRH5 bsAb | 2 (5.4) | 2 (6.7) | 0 | |
| GPRC5D bsAb | 1 (2.7) | 1 (3.3) | 0 | |
| Current therapy, n (%) | >.9 | |||
| Teclistamab | 29 (78) | 23 (77) | 6∗ (86) | |
| Talquetamab | 8 (22) | 7 (23) | 1∗ (14) |
ADC, antibody-drug conjugate; BCMA, B-cell maturation antigen; CAR-T, chimeric antigen receptor T cell; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; FcRH5, Fc receptor homolog 5; GPRC5D, G protein–coupled receptor, family C, group 5, member D; IgG/A, immunoglobulin G/A; IQR, interquartile range; ISS, International Staging System; TCR, T-cell redirecting therapy.
Includes 3 patients who reinitiated bsAb with and without SUD at different timepoints.
Include gain/amp 1q, del(17p), t(4:14), t(14;16), and/or t(14;20).
Included soft-tissue plasmacytomas not associated with bone.
Despite prolonged dose delays, there were no CRS or ICANS events for any patient who restarted therapy after >28-day dose delay without repeat SUD, and all reinitiation treatment doses were given in an outpatient setting. There were 2 patients who had a fever within 7 days after restarting teclistamab, but fevers were attributed to active infection (1 from COVID-19 and 1 from pneumonia). After prolonged dose delay, 26 (42%) doses were resumed without premedication, and 20 (32%) doses were given with acetaminophen, diphenhydramine, and/or corticosteroids as premedication, specifically for bsAbs. One patient received tocilizumab premedication before restarting teclistamab. The remaining doses were given concurrently with either IV immunoglobulin replacement (14 [23%]) or with IV pentamidine (2 [3%]), which required premedication with acetaminophen, diphenhydramine, corticosteroids, and/or famotidine due to institutional protocol and/or prior reactions.
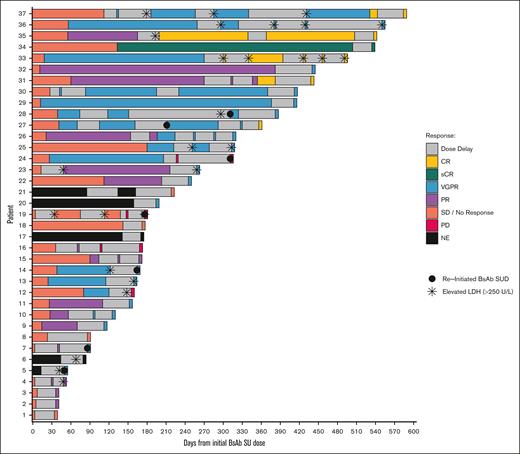
Responses before and after prolonged dose-delay events and reinitiation of therapy are summarized in Figure 1. Table 2 summarizes the M-protein and serum free light chains before reinitiation of bsAb after prolonged dosing intervals. Before restarting bsAb, 4 patients had M-spike >1.5 g/dL and involved serum free light chains >20 mg/dL. Most patients without repeat SUD maintained the same response or had improved response at bsAb reinitiation, but 2 patients had progressive disease at reinitiation with rise in serum myeloma markers.
A swimmer plot for patients treated with teclistamab or talquetamab, colored by treatment response according to International Myeloma Working Group response criteria, prior to prolonged dose-delay event(s) and after reinitiation of bispecific antibody therapy. CR, complete response; LDH, lactate dehydrogenase; NE, not evaluable; PD, progressive disease; PR, partial response; sCR, stringent complete response; SD, stable disease; VGPR, very good partial response.
A swimmer plot for patients treated with teclistamab or talquetamab, colored by treatment response according to International Myeloma Working Group response criteria, prior to prolonged dose-delay event(s) and after reinitiation of bispecific antibody therapy. CR, complete response; LDH, lactate dehydrogenase; NE, not evaluable; PD, progressive disease; PR, partial response; sCR, stringent complete response; SD, stable disease; VGPR, very good partial response.
Serum myeloma markers at time of bsAb reinitiation without SUD
| Myeloma serum marker . | Overall events N = 62 . |
|---|---|
| M-protein, g/dL | |
| Mean (SD) | 0.19 (0.53) |
| Median (IQR) | 0.00 (0.00-0.10) |
| Range | 0.00-2.74 |
| Free kappa light chain, mg/dL∗ | |
| Mean (SD) | 4.45 (25.83) |
| Median (IQR) | 0.08 (0.08-0.42) |
| Range | 0.08-208.23 |
| Free lambda light chain, mg/dL∗ | |
| Mean (SD) | 1.06 (4.96) |
| Median (IQR) | 0.15 (0.15-0.16) |
| Range | 0.15-38.08 |
| Myeloma serum marker . | Overall events N = 62 . |
|---|---|
| M-protein, g/dL | |
| Mean (SD) | 0.19 (0.53) |
| Median (IQR) | 0.00 (0.00-0.10) |
| Range | 0.00-2.74 |
| Free kappa light chain, mg/dL∗ | |
| Mean (SD) | 4.45 (25.83) |
| Median (IQR) | 0.08 (0.08-0.42) |
| Range | 0.08-208.23 |
| Free lambda light chain, mg/dL∗ | |
| Mean (SD) | 1.06 (4.96) |
| Median (IQR) | 0.15 (0.15-0.16) |
| Range | 0.15-38.08 |
SD, standard deviation.
Patients who had serum free light chain levels below the limit of detection were entered as the lower limit of normal in the reference range.
Although package inserts for teclistamab and talquetamab recommend restarting with SUDs after prolonged dose delays, we were able to safely reinitiate treatment doses without SUD or inpatient monitoring for most of our patients with prolonged dose delays. In our cohort, we did not observe CRS or ICANS after restarting bsAbs despite 73% of patients having had CRS and/or ICANS with initial SUD. Notably, most of the patients in our cohort received premedication with acetaminophen, diphenhydramine, and/or corticosteroids before restarting treatment dose bsAbs, in part due to concomitant administration of IV immunoglobulin or pentamidine. Our real-world data analysis demonstrated that repeat SUD and inpatient monitoring after prolonged dose delay of bsAbs may not be needed, reflecting substantial potential savings in time, cost, and resources. The small number of patients from a single center is a limitation of our study, and validation with a larger, more diverse patient cohort may be needed. Because these patients received all their treatments at our center, they were followed closely for AEs. However, given the inherent limitations of retrospective studies, there is the potential for underreporting of AEs.
In support of our findings, van de Donk et al4 recently demonstrated that reinitiating teclistamab after prolonged dose interval of ≤62 days does not require SUD based on population pharmacokinetics modeling and retrospective analysis of MajesTEC-1 for 61 patients with prolonged dosing intervals (>28 days). Only 2 patients experienced CRS after restarting teclistamab with SUD. The European Medicines Agency recently updated guidance on restarting teclistamab to reflect these findings, highlighting the evolving landscape of clinical recommendations for safe administration of bsAb and supporting our current institutional practice.
Patients should still be closely monitored for CRS and ICANS when reinitiating teclistamab or talquetamab after prolonged dose delay. There may be patient subgroups who may benefit from repeat SUD and close monitoring after prolonged dose delay. Future studies are needed to further characterize this patient population and refine clinical practice. Our study provides clinically important information for treating physicians and patients to make an individualized decision regarding forgoing SUD after prolonged dose delays, especially as we move toward less frequent dosing of bsAb therapy to mitigate side effects.
Acknowledgment: Funding support for this publication was provided by Memorial Sloan Kettering National Institutes of Health/National Cancer Institute Cancer Center Support grant P30 CA008748.
Contribution: C.R.T., A.W., and S.Z.U. conceived and designed the study; A.W., T.S., and C.R.T. curated the data; C.R.T., A.W., D.N., and A.D. analyzed, verified, and interpreted the data; C.R.T. and A.W. wrote the first draft of the manuscript; and all authors reviewed the manuscript, had full access to the data in the study, and had final responsibility for the decision to submit for publication.
Conflict-of-interest disclosure: C.R.T. reports research funding from Janssen and Takeda; personal fees from MJH Life Sciences; and has received honoraria for consultancy/participating in the advisory boards for Janssen and Sanofi. T.S. reports receiving honoraria from Roche-Genentech. K. Maclachlan reports grant support from American Society of Hematology, Multiple Myeloma Research Foundation, and International Myeloma Society. M.H. reports research funding from GlaxoSmithKline (GSK), BeiGene, AbbVie, and Daiichi Sankyo, and has received honoraria for consultancy/participated in the advisory boards for Curio Science LLC, Intellisphere LLC, Bristol Myers Squibb (BMS), Janssen, and GSK. S.M. reports research funding from the National Cancer Institute, Janssen Oncology, BMS, Allogene Therapeutics, Fate Therapeutics, Caribou Therapeutics, and Takeda Oncology; has received consulting fees from EviCore, Optum, Bio Ascend, Janssen Oncology, BMS, AbbVie, HMP Education, and Legend Biotech; and has received honoraria from OncLive, Physician Education Resource, MJH Life Sciences, and Plexus Communications. H. Hassoun reports grants from Celgene, Takeda, and Janssen, outside the submitted work. A.L. reports nonfinancial support from Pfizer; grants and personal fees from Janssen, outside the submitted work; serves on the data safety monitoring board for Arcellx; and also has a patent US20150037346A1 with royalties paid. U.A.S. reports research support from Celgene/BMS and Janssen (to the institution); nonfinancial research support from Sabinsa pharmaceuticals and M&M Labs (to the institution); and personal fees from, Janssen Biotech, Sanofi, BMS, and i3 Health, outside the submitted work. H. Hashmi reports consultancy for Karyopharm, Amgen, and Janssen. G.L.S. receives research funding from Janssen, Amgen, BMS, Beyond Spring; serves on the data safety monitoring board for Arcellx; and receives research funding to the institution from Janssen, Amgen, BMS, Beyond Spring, and GPCR. M.S. served as a paid consultant for McKinsey & Company, Angiocrine Bioscience Inc, and Omeros Corporation; received research funding from Angiocrine Bioscience Inc, Omeros Corporation, Amgen Inc, BMS, and Sanofi; served on ad hoc advisory boards for Kite (a Gilead company) and Miltenyi Biotec; and received honoraria from i3 Health, Medscape, Cancer Network, and IDEOlogy. H.J.L. has served as a paid consultant for AbbVie, Immix Biopharma, Legend Biotech, Alexion, Prothena, and has received research funding from Nexcella, Janssen, Alexion, Protego, and Prothena. S.A.G. reports personal fees from and advisory role with (scientific advisory board) Actinium, Celgene, BMS, Sanofi, Amgen, Pfizer, GSK, Jazz, Janssen, Omeros, Takeda, and Kite, outside the submitted work. N.K. reports research funding through Amgen, Janssen, Epizyme, AbbVie; consults for Clinical Care Options, OncLive, and Intellisphere Remedy Health; and participated in advisory board for Janssen and MedImmune. S.Z.U. received research funding from Amgen, AbbVie, Array Biopharma, BMS, Celgene, Gilead, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDx, and Takeda; is a consultant to AbbVie, Amgen, BMS, Celgene, Edo Pharma, Genentech, Gilead, GSK, Gracell, Janssen, Oncopeptides, Pfizer, Sanofi, Seattle Genetics, Secura Bio, SkylineDx, Takeda, and TeneoBio; and is a speaker for Amgen, BMS, Janssen, and Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Carlyn Rose Tan, Myeloma Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 530 E 74th St, New York, NY 10021; email: tanc4@mskcc.org; and Alice Wang, Department of Pharmacy, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10021; email: wanga8@mskcc.org.
References
Author notes
C.R.T. and A.W. are joint first authors.
Deidentified individual participant data that underlie the reported results will be made available 3 months after publication for a period of 1 year after the publication date if requests are made through emails to the corresponding authors, Carlyn Rose Tan (tanc4@mskcc.org) and Alice Wang (wanga8@mskcc.org).