TO THE EDITOR:
Preeclampsia complicates 5% to 7% of pregnancies and accounts for 30% of all preterm deliveries, resulting in neonatal intensive care unit admissions, increased health care cost, and neonatal morbidity and mortality.1 HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome is often considered a severe manifestation on the preeclampsia spectrum, affecting ∼0.1% to 0.2% of all pregnancies.2,3 Expectant management in HELLP syndrome carries significant maternal and fetal/neonatal risks, with an estimated 1% maternal mortality.4 Perinatal morbidity and mortality are most strongly associated with gestational age and birth weight. The exact pathogenesis of HELLP syndrome remains incompletely understood, limiting current management to supportive treatments, including fetal monitoring, steroids for fetal lung maturity, magnesium for seizure prophylaxis, management of hypertension, and ultimately, expedient delivery that results in iatrogenic preterm birth.5 Even with iatrogenic preterm birth, the disease often worsens until 72 hours post partum, putting women at continued risk of hypertension, stroke, seizure, liver and renal injury, hemorrhage, and coagulopathy.
In addition to features that define preeclampsia, HELLP syndrome is marked by thrombotic microangiopathy, drawing parallels to atypical hemolytic uremic syndrome (aHUS) or complement-mediated thrombotic microangiopathy, which is commonly diagnosed post partum and often preceded by a pregnancy complication, including preeclampsia, preterm birth, or fetal death.6-8 Pregnancy is a well-established trigger of complement activation.9,10 Complement is part of the innate immune system, functioning in defense against foreign pathogens, clearance of damaged cells, and removal of immune complexes, and is closely linked to coagulation and inflammatory pathways.11 The complement system plays critical dual roles in maintaining healthy pregnancy, combating infection and allowing tolerance of the semiallogeneic fetus; however, its careful regulation is equally critical.9,10,12 We previously identified rare germ line variants in genes regulating complement in ∼50% of women with HELLP syndrome, a frequency similar to that of aHUS.2 We further corroborated the central role of complement in HELLP syndrome pathogenesis using a functional, cell-based assay of complement and found increased complement activity in serum from 62% of pregnancies at HELLP syndrome diagnosis vs 16% of control pregnancies in the late third trimester. A single case report describes the successful use of the terminal complement inhibitor, eculizumab, to prolong pregnancy 17 days in a woman with preterm HELLP syndrome.13 Importantly, experience in aHUS and paroxysmal nocturnal hemoglobinuria demonstrate that eculizumab is safe in pregnancy. Although eculizumab can be detected in low levels in cord blood, levels are not high enough to affect complement in the neonate, nor there is significant eculizumab in newborn plasma.14 Eculizumab is not present in breast milk.15
Based on this evidence, we conducted a phase 1, open-label, single-center study to investigate eculizumab in preeclampsia with severe features/HELLP syndrome diagnosed at <30 weeks of gestation. Pregnant women at <30 weeks of gestation diagnosed with preeclampsia with severe features and laboratory abnormalities (hepatic abnormalities, thrombocytopenia, or kidney function impairment16) were eligible for participation. Women meeting the following criteria at screening were excluded: disseminated intravascular coagulopathy (International Society of Thrombosis and Haemostasis DIC score ≥ 5), stroke, eclamptic seizure, allergy to eculizumab, and previous diagnosis of aHUS, thrombotic thrombocytopenia purpura, or paroxysmal nocturnal hemoglobinuria. In addition, pregnancies with nonreassuring fetal status necessitating immediate delivery, nonviable fetus, or intrauterine fetal demise, as assessed before study entry, were ineligible for enrollment. Per protocol, participants received eculizumab (900 mg) weekly for a maximum of 4 doses via IV until delivery. All participants received meningococcal vaccination with Menactra and Bexsero and penicillin prophylaxis (penicillin V 500 mg twice daily) for 21 days after eculizumab. In penicillin-allergic participants, the protocol allowed for azithromycin 500 mg daily prophylaxis. The study was conducted in accordance with Good Clinical Practice Guidelines and the principles of the Declaration of Helsinki and approved by the Johns Hopkins Institutional Review Board. All participants provided written informed consent before enrollment.
The primary outcome was the change in aspartate aminotransferase, alanine aminotransferase, or platelet count from baseline at the time of study enrollment to 72 hours after eculizumab administration. Secondary outcomes included latency of pregnancy in days after eculizumab administration, maternal blood transfusions, and maternal postpartum length of stay.
The trial (www.ClinicalTrials.gov identifier: NCT04103489) was conducted from February 2021 to September 2023, and the study was completed 4 September 2023. Three women aged 22 to 30 years met the entry criteria and received a single dose of eculizumab 900 mg IV. Preeclampsia with severe features/HELLP syndrome was diagnosed between 25 weeks 6 days and 27 weeks 6 days of gestation. Demographic and baseline maternal and pregnancy characteristics, supportive measures for HELLP syndrome, and delivery and neonatal outcomes are described in Table 1. Cesarean delivery was performed between 25.5 and 59 hours after eculizumab administration, and the indication for delivery was based on fetal well-being in all cases. Furthermore, all fetuses had fetal growth restriction or <10% of estimated weight for gestational age. No maternal blood products were administered, and IV Pitocin was given after all cesarean deliveries, with an estimated blood loss between 750 and 1200 mL.
Maternal, fetal, and pregnancy characteristics
| . | Participant 1 . | Participant 2 . | Participant 3 . |
|---|---|---|---|
| Maternal age, y | 30 | 24 | 22 |
| Maternal race, ethnicity | White, non-Hispanic | Black, non-Hispanic | White, non-Hispanic |
| Maternal medical history | — | Subclinical hypothyroidism, obesity, and iron deficiency anemia | Type 1 diabetes and obesity |
| Obstetrical history | G1P0000 | G2P0010 | G2P1001, PIH |
| Antenatal course | Uncomplicated | Gestational diabetes, bacteriuria | COVID-19 infection |
| Gestational age at HELLP diagnosis | 27 wk, 6 d | 26 wk, 3 d | 25 wk, 6 d |
| Urine protein-to-creatinine ratio | 2.0 | 4.5 | 5.7 |
| Fetal presentation | Severe IUGR with IAEDF | Severe IUGR | IUGR |
| HTN | Severe | Severe | Severe |
| Supportive measures | Betamethasone, magnesium, and antihypertensives | Dexamethasone, magnesium, and antihypertensives | Betamethasone magnesium and antihypertensives |
| Time from eculizumab to delivery, h | 25.5 | 59 | 32 |
| Delivery mode | Low transverse cesarean delivery | Classical cesarean delivery | Mid-segment transverse cesarean delivery |
| Delivery indication | Nonreassuring fetal heart tracing | Nonreassuring fetal heart tracing | Nonreassuring fetal status, headache, and increased creatinine |
| Gestational age delivery | 28 wk, 0 d | 26 wk, 6 d | 27 wk, 1 d |
| EBL, mL | 760 | 900 | 1200 |
| Maternal LOS (postpartum day) | 4 | 4 | 4 |
| Apgar (1, 5) | 5, 7 | 1, 7 | 4, 8 |
| Birth weight, g | 780 | 740 | 710 |
| Fetal sex | F | M | F |
| Neonatal complications | Apnea of prematurity, bronchopulmonary dysplasia, anemia, and umbilical line–associated aortic thrombus | Apnea of prematurity, chronic lung disease, anemia, ventriculomegaly, poor enteral feeding, and steroid-induced adrenal insufficiency | Bronchopulmonary dysplasia, pulmonary HTN, anemia, steroid-induced adrenal insufficiency, and umbilical line–associated aortic thrombus |
| Neonatal interventions | CPAP, pRBC transfusions, TPN, heparin, and gastric tube | Intubation, gastric tube, and pRBC transfusions | Intubation, iNO, gastric tube, pRBC transfusions, and PDA ligation |
| NICU LOS, d | 50 | 87 | 500 |
| . | Participant 1 . | Participant 2 . | Participant 3 . |
|---|---|---|---|
| Maternal age, y | 30 | 24 | 22 |
| Maternal race, ethnicity | White, non-Hispanic | Black, non-Hispanic | White, non-Hispanic |
| Maternal medical history | — | Subclinical hypothyroidism, obesity, and iron deficiency anemia | Type 1 diabetes and obesity |
| Obstetrical history | G1P0000 | G2P0010 | G2P1001, PIH |
| Antenatal course | Uncomplicated | Gestational diabetes, bacteriuria | COVID-19 infection |
| Gestational age at HELLP diagnosis | 27 wk, 6 d | 26 wk, 3 d | 25 wk, 6 d |
| Urine protein-to-creatinine ratio | 2.0 | 4.5 | 5.7 |
| Fetal presentation | Severe IUGR with IAEDF | Severe IUGR | IUGR |
| HTN | Severe | Severe | Severe |
| Supportive measures | Betamethasone, magnesium, and antihypertensives | Dexamethasone, magnesium, and antihypertensives | Betamethasone magnesium and antihypertensives |
| Time from eculizumab to delivery, h | 25.5 | 59 | 32 |
| Delivery mode | Low transverse cesarean delivery | Classical cesarean delivery | Mid-segment transverse cesarean delivery |
| Delivery indication | Nonreassuring fetal heart tracing | Nonreassuring fetal heart tracing | Nonreassuring fetal status, headache, and increased creatinine |
| Gestational age delivery | 28 wk, 0 d | 26 wk, 6 d | 27 wk, 1 d |
| EBL, mL | 760 | 900 | 1200 |
| Maternal LOS (postpartum day) | 4 | 4 | 4 |
| Apgar (1, 5) | 5, 7 | 1, 7 | 4, 8 |
| Birth weight, g | 780 | 740 | 710 |
| Fetal sex | F | M | F |
| Neonatal complications | Apnea of prematurity, bronchopulmonary dysplasia, anemia, and umbilical line–associated aortic thrombus | Apnea of prematurity, chronic lung disease, anemia, ventriculomegaly, poor enteral feeding, and steroid-induced adrenal insufficiency | Bronchopulmonary dysplasia, pulmonary HTN, anemia, steroid-induced adrenal insufficiency, and umbilical line–associated aortic thrombus |
| Neonatal interventions | CPAP, pRBC transfusions, TPN, heparin, and gastric tube | Intubation, gastric tube, and pRBC transfusions | Intubation, iNO, gastric tube, pRBC transfusions, and PDA ligation |
| NICU LOS, d | 50 | 87 | 500 |
Severe HTN is defined as systolic blood pressure ≥160 mm Hg and/or diastolic blood pressure ≥110 mm Hg.
CPAP, continuous positive pressure ventilation; EBL, estimated blood loss; F, female; HTN, hypertension; IAEDF, intermittent absent end-diastolic flow; iNo, inhaled nitric oxide; IUGR, intrauterine growth restriction; LOS, length of stay; M, male; NICU, neonatal intensive care unit; PDA, patent ductus arteriosus; PIH, pregnancy induced hypertension; pRBC, packed red blood cell; TPN, total parenteral nutrition.
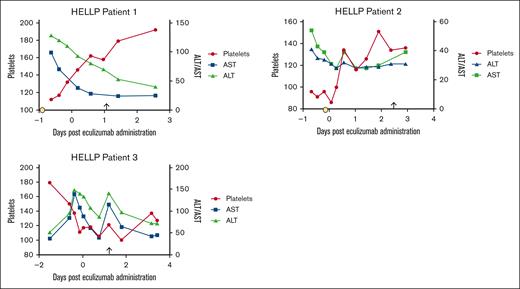
There was full compliance with the protocol, including meningococcal vaccination and prophylactic antibiotics. Maternal health and laboratory parameters improved in all patients (Figure 1; supplemental Table 1). None required intensive care unit admission. All women were discharged on postpartum day 4. There were no adverse events assessed as related to eculizumab administration by the investigators. There were no maternal infections, seizures, vascular events including strokes or venous thrombosis, or development of DIC. Participant 3 had worsening of creatinine (0.8-1.3 mg/dL) 1 day after eculizumab administration, which, in addition to nonreassuring fetal status, prompted delivery. Renal function resolved to baseline within 48 hours.
Transaminases and platelet count trends before and after eculizumab administration. Time 0 indicated the time of eculizumab administration. Delivery is noted with an arrow. Corticosteroid administration is represented by the yellow circle. In patient 3, corticosteroids were administered 6 days before eculizumab. The values of platelets (×109/L), AST (units per liter), and ALT (units per liter) are shown. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Transaminases and platelet count trends before and after eculizumab administration. Time 0 indicated the time of eculizumab administration. Delivery is noted with an arrow. Corticosteroid administration is represented by the yellow circle. In patient 3, corticosteroids were administered 6 days before eculizumab. The values of platelets (×109/L), AST (units per liter), and ALT (units per liter) are shown. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
There were no fetal or neonatal deaths. Delivery occurred between 26 weeks 6 days and 28 weeks 0 days of gestation age. Neonatal intensive care unit admissions ranged from 50 to 500 days. Up to age 3 years, 1 child had no significant developmental concerns, the second had communications delays, chronic lung disease, and ongoing gastric tube dependence, and the third child had global developmental delay with ventilator and gastric tube dependence. All neonatal complications were deemed related to prematurity and low birth weight (710-780 g) by the investigators. There were no neonatal infections during the first month of life; however, the neonatal intensive care unit course for infant 3 was later complicated by pneumonia and tracheitis, which were considered ventilator associated.
In conclusion, we report, to our knowledge, the first prospective study of complement inhibition for the management of early onset preeclampsia with severe features/HELLP syndrome. Eculizumab appears safe in terms of its effect on maternal clinical status and end organ function; however, because the time between eculizumab administration and delivery was short, fetal impact was likely small and preterm delivery was required in all cases. As far as the authors are aware, outside of case report data, this is, to our knowledge, the first study using eculizumab in early preterm HELLP syndrome. The limitations of this study include the small number of enrolled participants owing to the single-center nature of this study and the rarity of early preterm HELLP syndrome before 30 weeks that is not managed with urgent delivery. The small sample size and lack of a control arm limit conclusions about the efficacy of complement inhibition in terms of maternal parameters; however, our data support a larger, multicenter randomized study of eculizumab before 34 weeks of gestations on maternal outcomes, such as time to normalization of laboratory parameters, intensive care unit admissions, mortality, and length of stay. Additionally, all deliveries occurred for fetal indications, and continued evaluation of fetal safety is required. We hypothesize that earlier initiation of eculizumab in those at highest risk for HELLP syndrome development may be beneficial.
Acknowledgments: This work was supported by the National Institutes of Health Office of Research on Women’s Health Building Interdiscplinary Research Careers in Women's Health Award (1K12HD085845-01 [A.J.V. and G.F.G.]). G.F.G. is supported by the 2023 Haemostasis and Thrombosis Research Society Mentored Research Award from the Hemostasis and Thrombosis Research Society by an educational grant from Takeda.
The study drug was provided by Alexion Pharmaceuticals.
Contribution: A.J.V. designed the trial, enrolled participants, and collected data; G.F.G., R.A.B., and A.J.V. analyzed data; G.F.G. wrote the first draft of the manuscript; and A.J.V. and R.A.B. edited the manuscript.
Conflict-of-interest disclosure: G.F.G. has served on advisory boards for Alexion Pharmaceuticals and Apellis Pharmaceuticals. R.A.B. has received research funding from Alexion Pharmaceuticals. A.J.V. declares no competing financial interests.
Correspondence: Arthur Jason Vaught, Johns Hopkins University School of Medicine, 600 North Wolfe St, Phipps Building 228, Baltimore, MD 21287; email: avaught2@jhmi.edu.
References
Author notes
Deidentified data and protocol information contained in this study are available upon reasonable request from the corresponding author, Arthur Jason Vaught (avaught2@jhmi.edu).
The full-text version of this article contains a data supplement.