Key Points
Canagliflozin significantly increased hematocrit levels compared with placebo, even in patients with erythrocytosis.
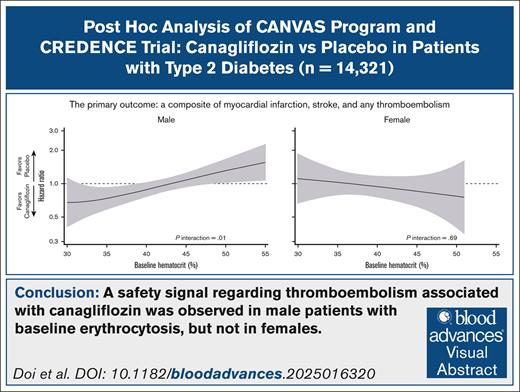
Baseline hematocrit levels modified the effect of canagliflozin on thromboembolism, indicating harm in males with erythrocytosis.
Visual Abstract
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are increasingly recognized as a common cause of drug-induced erythrocytosis. SGLT2 inhibitor–induced erythropoiesis may increase blood viscosity and precipitate thromboembolism, particularly in patients with preexisting erythrocytosis. We conducted a post hoc pooled analysis of patient-level data from the randomized, double-blind, placebo-controlled Canagliflozin Cardiovascular Assessment Study program and the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation trial, which assessed the safety and efficacy of canagliflozin in patients with type 2 diabetes mellitus. The primary outcome, a composite of myocardial infarction (MI), stroke, and any thromboembolism, was evaluated using sex-specific Cox models, with baseline hematocrit as an effect modifier. Among participants with available baseline hematocrit values (98.5% [14 321/14 543]), 35% were female. Canagliflozin significantly increased hematocrit levels compared with placebo even in patients with erythrocytosis (males > 49%; females > 48%) and increased the proportion of individuals with erythrocytosis at 1 year (males, 16.9% vs 5.5%; females, 5.2% vs 1.0%). Overall, canagliflozin did not alter the risk of the primary outcome in either males or females. However, in males, baseline hematocrit levels modified the treatment effect on the primary outcome, whether assessed categorically (anemia, normal, and erythrocytosis) or continuously with fractional polynomial (FP) analysis (P interaction < .05). FP analysis showed treatment benefits in anemic males but show harm in those with erythrocytosis, primarily driven by an increased risk of MI. Meanwhile, no heterogeneity was seen in females for these outcomes. In conclusion, canagliflozin may pose a safety concern for thromboembolism in males with erythrocytosis at baseline, warranting further investigations. These trials were registered at www.ClinicalTrials.gov as #NCT01032629, #NCT01989754, and #NCT02065791.
Introduction
Compelling evidence supports the use of sodium-glucose cotransporter 2 (SGLT2) inhibitors (SGLT2is) in individuals with heart failure, kidney disease, and type 2 diabetes mellitus (T2DM) at an elevated risk of atherosclerotic cardiovascular (CV) disease.1-8 Therefore, international guidelines recommend the utilization of SGLT2is in this population.9-12 Several mediation analyses in large clinical trials of SGLT2i consistently emphasized the pivotal role of increased red blood cell indices in cardiorenal protection provided by SGLT2is.13-18 Notably, the increases in hemoglobin and hematocrit levels associated with SGLT2i treatment are not solely caused by hemoconcentration from diuretic effects but rather attributed to enhanced erythropoiesis, similar to the responses to hypoxia-inducible factor prolyl hydroxylase enzyme inhibitors.19,20
Large-scale observational studies have identified associations between elevated hemoglobin and hematocrit levels and adverse CV outcomes.21-24 In addition, patients with erythrocytosis, who often present with multiple CV risk factors such as diabetes, obesity, hypertension, metabolic syndrome, dyslipidemia, and smoking,22,25 may be considered appropriate candidates for SGLT2i therapy. The hematology community has recently recognized SGLT2i as a significant causative agent of erythrocytosis.26 Several reports have raised concerns about SGLT2i-induced erythropoiesis, with some cases necessitating phlebotomy and others documenting occurrences of thromboembolism potentially linked to erythrocytosis.27-35
Recent meta-analyses have demonstrated reduced 3-point major adverse CV events (MACEs) by SGLT2is, primarily through its beneficial effects on heart failure.36-38 Meanwhile, this agent has a neutral impact on the incidence of ischemic events, such as myocardial infarction (MI), stroke, and thromboembolism.36-39 Theoretically, by increasing blood viscosity, erythrocytosis may hinder optimal blood supply to vital organs and increase the risk of thrombus formation. Hence, SGLT2is could present adverse effects, particularly thromboembolism, in patients with erythrocytosis. Currently, data on the safety and efficacy of SGLT2is in patients with erythrocytosis are lacking. Our study aimed to investigate whether baseline hematocrit levels modify the effects of SGLT2is, with an emphasis on safety concerns in patients with erythrocytosis.
Methods
Study design and participants
This was a post hoc pooled data analysis using individual participant data from the Canagliflozin Cardiovascular Assessment Study (CANVAS) program (CANVAS and Canagliflozin cardiovascular Assessment Study-Renal)2 and the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial.4 Detailed information on study design, participant characteristics, randomized treatments, and major findings from the CANVAS program and the CREDENCE trial has been previously published.2,4 Briefly, both studies were randomized, double-blinded, placebo-controlled, multicenter trials designed to assess the efficacy of canagliflozin in patients with T2DM. The CANVAS program included patients with T2DM and a hemoglobin A1c (HbA1c) of 7.0% to 10.5% who were aged ≥30 years and had a history of atherosclerotic vascular disease or who were aged ≥50 years with ≥2 risk factors for CV disease. The CREDENCE trial included patients with T2DM and HbA1c of 6.5% to 12.0% who were aged ≥30 years, had an estimated glomerular filtration rate of 30 to <90 mL/min per 1.73 m2, had a urine albumin-creatinine ratio of 300 to 5000 mg/g, and received renin-angiotensin system blockade. All trial protocols received approval from the ethics committees at each site (ClinicalTrials.gov identifiers: NCT01032629, NCT01989754, and NCT02065791) and adhered to the principles outlined in the Declaration of Helsinki. A written informed consent was obtained from all study participants. Clinical data from the CANVAS program and the CREDENCE trial were accessed through the Yale University Open Data Access Project (http://yoda.yale.edu/).
Hematocrit measurements and the definition of categories by hematocrit
Hematocrit concentrations were measured at the prespecified visit of each trial (supplemental Table 1). To account for differences in visit schedules and follow-up periods across studies, we used data from baseline, 1-year, and 2-year assessments, which were commonly collected in all studies. The participants were classified into 3 categories based on hematocrit levels: erythrocytosis, normal, and anemia. Based on the 2016 World Health Organization classification, erythrocytosis was defined as hematocrit levels >49% in males or >48% in females.22,40 A hematocrit level of <39% in males and <36% in females was categorized as anemia.41,42 Levels within these thresholds were considered normal.
Outcomes
The primary outcome was a composite of MI (fatal and nonfatal), stroke (fatal and nonfatal), and any thromboembolism. The secondary outcomes included MI (fatal and nonfatal), stroke (fatal and nonfatal), any thromboembolism, arterial thromboembolism, venous thromboembolism, MACE (CV death, nonfatal MI, or nonfatal stroke), CV death, death from any cause, hospitalization for heart failure, and a composite kidney outcome (end-stage kidney disease, doubling of serum creatinine level, or renal death). Except for arterial, venous, or any thromboembolism, all outcomes were adjudicated by independent adjudication committee members in each trial. Arterial, venous, and any thromboembolism were identified based on adverse events reported by the investigators, using the MedDRA (Medical Dictionary for Regulatory Activities) preferred terms.
Statistical analysis
Participant characteristics stratified by baseline hematocrit categories and sex were summarized as medians (interquartile ranges) or percentages. The effect of canagliflozin relative to placebo on hematocrit levels over time was investigated using mixed-effects models for repeated measure. Unless otherwise noted, this model incorporated the fixed effects of the treatment arm, trial, trial visit, treatment-by-visit interaction, and baseline value-by-visit interaction. An unstructured covariance matrix used accounting for within-patient correlation. The canagliflozin groups were analyzed as a combined entity because a dose-dependent effect on hematocrit levels was not observed in the CANVAS trial, where participants were randomly assigned in a 1:1:1 ratio to canagliflozin 100 mg, 300 mg, or placebo. Moreover, effect modification by baseline hematocrit categories was tested by adding 2-way and 3-way interaction terms among treatment, visit, and baseline hematocrit categories. Logistic regression models with random intercept for each trial were used to estimate the odds of erythrocytosis or anemia at 1 or 2 years with canagliflozin vs placebo. For all canagliflozin groups vs placebo in the intention-to-treat population, the outcomes were depicted using the Kaplan-Meier curve and analyzed using stratified Cox regression models with a stratification variable of trial. Heterogeneity was assessed by adding the interaction term between baseline hematocrit categories and treatment to the Cox model. Furthermore, to enhance statistical power and avoid setting arbitrary thresholds, we used a multivariate fractional polynomial interaction approach to assess the interaction between continuous hematocrit values and treatment assignment.43 Log cumulative hazard plots were used to visually assess the proportional hazard assumption. Poisson regression models adjusted for trial effects were used in cases where violations of proportionality were detected. Sex-specific analyses were performed considering the sex differences in the distribution of hematocrit levels (supplemental Figure 1) and hematocrit response by SGLT2i.44 All statistical tests were 2-tailed, and P values of < .05 were considered statistically significant. No adjustments were made for multiplicity. Analyses were performed using the Stata/SE18 statistical software package (StataCorp LLC, College Station, TX).
Results
Baseline characteristics
Baseline hematocrit levels were available for 98.5% (14 321/14 543) of participants in the CANVAS program and the CREDENCE trial. Among these participants, females accounted for 35% (5046/14 321). For both sexes, the median age and median follow-up were 63 and 2.4 years, respectively. The median hematocrit level was 43% and 40% in males and females, respectively (Table 1; supplemental Figure 1). As baseline hematocrit levels increased, the participants tended to be younger and current smokers, had a shorter duration of diabetes, and had a history of CV disease and MI. Individuals with higher hematocrit levels also exhibited higher diastolic blood pressure, glycated hemoglobin, total cholesterol, low-density lipoprotein cholesterol, and triglyceride levels, but they were more likely to have lower systolic blood pressure and preserved estimated glomerular filtration rate. These trends were similar across both sexes. Those exhibiting elevated hematocrit levels were less likely to use angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, diuretics, and insulin in both sexes (Table 1). Baseline characteristics were similar between the 2 treatment arms across baseline hematocrit status, for both males and females (supplemental Tables 2 and 3).
Baseline characteristics of the participants according to baseline hematocrit levels and sex
| Characteristics . | Male . | Female . | ||||
|---|---|---|---|---|---|---|
| Anemia∗ . | Normal . | Erythrocytosis† . | Anemia∗ . | Normal . | Erythrocytosis† . | |
| N = 1656 . | N = 7095 . | N = 524 . | N = 742 . | N = 4215 . | N = 89 . | |
| Age, y | 64 (57-69) | 63 (56-68) | 60 (53-66) | 62 (56-68) | 64 (56-68) | 60 (56-66) |
| Race, n (%) | ||||||
| White | 1085 (66) | 5431 (77) | 401 (77) | 436 (59) | 3271 (78) | 69 (78) |
| Black or African American | 72 (4) | 193 (3) | 17 (3) | 70 (9) | 196 (5) | 3 (3) |
| Asian | 392 (24) | 1059 (15) | 50 (10) | 188 (25) | 446 (11) | 1 (1) |
| Other‡ | 107 (6) | 412 (6) | 56 (11) | 48 (6) | 302 (7) | 16 (18) |
| Current smoker, n (%) | 213 (13) | 1351 (19) | 160 (31) | 45 (6) | 605 (14) | 27 (30) |
| History of hypertension, n (%) | 1539 (93) | 6452 (91) | 477 (91) | 693 (93) | 3945 (94) | 84 (94) |
| History of CV disease, n (%) | 1028 (62) | 4645 (65) | 341 (65) | 375 (51) | 2276 (54) | 49 (55) |
| History of MI, n (%) | 332 (20) | 1988 (28) | 149 (28) | 99 (13) | 743 (18) | 20 (22) |
| History of cerebrovascular, n (%) | 281 (17) | 1223 (17) | 98 (19) | 129 (17) | 864 (20) | 14 (16) |
| Diabetes duration, y | 15.3 (10.0-21.0) | 13.0 (8.0-18.0) | 11.0 (6.0-16.0) | 15.2 (11.0-21.0) | 13.0 (9.0-19.0) | 11.3 (7.0-17.0) |
| Body mass index, kg/m2 | 30 (26-34) | 31 (28-35) | 31 (28-35) | 30 (27-36) | 32 (28-36) | 32 (29-36) |
| SBP, mm Hg | 139 (128-150) | 137 (127-147) | 137 (128-145) | 139 (128-150) | 138 (128-148) | 135 (130-145) |
| DBP, mm Hg | 76 (69-82) | 79 (72-85) | 81 (77-87) | 76 (68-82) | 79 (71-84) | 80 (76-86) |
| Glycated hemoglobin, % | 7.9 (7.3-8.7) | 8.1 (7.4-8.9) | 8.2 (7.5-9.1) | 8.0 (7.4-9.0) | 8.2 (7.6-9.1) | 8.3 (7.6-9.4) |
| eGFR, mL/min per 1.73 m2 | 57 (42-73) | 73 (59-87) | 73 (58-87) | 53 (41-69) | 71 (56-86) | 72 (60-92) |
| UACR, mg/g | 402 (25-1277) | 27 (8-392) | 119 (12-718) | 440 (20-1506) | 17 (8-312) | 37 (9-562) |
| Hemoglobin, g/dL | 121 (113-127) | 144 (137-152) | 166 (161-172) | 110 (104-114) | 132 (125-140) | 159 (153-165) |
| Hematocrit, % | 36 (35-38) | 43 (41-45) | 51 (50-53) | 34 (32-35) | 40 (38-43) | 50 (49-51) |
| WBC count, 109/L | 6.86 (5.71-8.14) | 7.11 (6.05-8.40) | 7.42 (6.32-8.88) | 6.84 (5.77-8.11) | 7.17 (6.04-8.51) | 7.55 (6.39-8.99) |
| Platelet count, 109/L | 232 (194-279) | 226 (190-269) | 215 (181-253) | 273 (220-329) | 258 (217-305) | 223 (194-287) |
| Total cholesterol, mmol/L | 3.95 (3.36-4.71) | 4.07 (3.49-4.86) | 4.48 (3.65-5.40) | 4.46 (3.76-5.23) | 4.68 (3.93-5.59) | 4.82 (4.27-5.59) |
| HDL cholesterol, mmol/L | 1.04 (0.88-1.26) | 1.06 (0.92-1.26) | 1.04 (0.89-1.22) | 1.19 (1.01-1.42) | 1.25 (1.07-1.48) | 1.26 (1.09-1.44) |
| LDL cholesterol, mmol/L | 1.97 (1.51-2.59) | 2.07 (1.58-2.73) | 2.37 (1.73-3.11) | 2.29 (1.73-3.02) | 2.46 (1.83-3.23) | 2.53 (2.02-3.28) |
| Triglycerides, mmol/L | 1.63 (1.14-2.37) | 1.71 (1.22-2.47) | 1.92 (1.37-2.76) | 1.70 (1.25-2.34) | 1.76 (1.32-2.44) | 1.89 (1.35-2.75) |
| Medication, n (%) | ||||||
| ACE inhibitors/ARB | 1490 (90) | 6039 (85) | 452 (86) | 688 (93) | 3601 (85) | 77 (87) |
| Diuretics | 852 (51) | 2976 (42) | 208 (40) | 392 (53) | 1985 (47) | 40 (45) |
| β blocker | 783 (47) | 3678 (52) | 242 (46) | 278 (37) | 2047 (49) | 50 (56) |
| Statin | 1291 (78) | 5431 (77) | 340 (65) | 514 (69) | 2837 (67) | 63 (71) |
| Antithrombotic agents§ | 1173 (71) | 5282 (74) | 377 (72) | 437 (59) | 2607 (62) | 61 (69) |
| Insulin | 1025 (62) | 3762 (53) | 267 (51) | 503 (68) | 2278 (54) | 44 (49) |
| Characteristics . | Male . | Female . | ||||
|---|---|---|---|---|---|---|
| Anemia∗ . | Normal . | Erythrocytosis† . | Anemia∗ . | Normal . | Erythrocytosis† . | |
| N = 1656 . | N = 7095 . | N = 524 . | N = 742 . | N = 4215 . | N = 89 . | |
| Age, y | 64 (57-69) | 63 (56-68) | 60 (53-66) | 62 (56-68) | 64 (56-68) | 60 (56-66) |
| Race, n (%) | ||||||
| White | 1085 (66) | 5431 (77) | 401 (77) | 436 (59) | 3271 (78) | 69 (78) |
| Black or African American | 72 (4) | 193 (3) | 17 (3) | 70 (9) | 196 (5) | 3 (3) |
| Asian | 392 (24) | 1059 (15) | 50 (10) | 188 (25) | 446 (11) | 1 (1) |
| Other‡ | 107 (6) | 412 (6) | 56 (11) | 48 (6) | 302 (7) | 16 (18) |
| Current smoker, n (%) | 213 (13) | 1351 (19) | 160 (31) | 45 (6) | 605 (14) | 27 (30) |
| History of hypertension, n (%) | 1539 (93) | 6452 (91) | 477 (91) | 693 (93) | 3945 (94) | 84 (94) |
| History of CV disease, n (%) | 1028 (62) | 4645 (65) | 341 (65) | 375 (51) | 2276 (54) | 49 (55) |
| History of MI, n (%) | 332 (20) | 1988 (28) | 149 (28) | 99 (13) | 743 (18) | 20 (22) |
| History of cerebrovascular, n (%) | 281 (17) | 1223 (17) | 98 (19) | 129 (17) | 864 (20) | 14 (16) |
| Diabetes duration, y | 15.3 (10.0-21.0) | 13.0 (8.0-18.0) | 11.0 (6.0-16.0) | 15.2 (11.0-21.0) | 13.0 (9.0-19.0) | 11.3 (7.0-17.0) |
| Body mass index, kg/m2 | 30 (26-34) | 31 (28-35) | 31 (28-35) | 30 (27-36) | 32 (28-36) | 32 (29-36) |
| SBP, mm Hg | 139 (128-150) | 137 (127-147) | 137 (128-145) | 139 (128-150) | 138 (128-148) | 135 (130-145) |
| DBP, mm Hg | 76 (69-82) | 79 (72-85) | 81 (77-87) | 76 (68-82) | 79 (71-84) | 80 (76-86) |
| Glycated hemoglobin, % | 7.9 (7.3-8.7) | 8.1 (7.4-8.9) | 8.2 (7.5-9.1) | 8.0 (7.4-9.0) | 8.2 (7.6-9.1) | 8.3 (7.6-9.4) |
| eGFR, mL/min per 1.73 m2 | 57 (42-73) | 73 (59-87) | 73 (58-87) | 53 (41-69) | 71 (56-86) | 72 (60-92) |
| UACR, mg/g | 402 (25-1277) | 27 (8-392) | 119 (12-718) | 440 (20-1506) | 17 (8-312) | 37 (9-562) |
| Hemoglobin, g/dL | 121 (113-127) | 144 (137-152) | 166 (161-172) | 110 (104-114) | 132 (125-140) | 159 (153-165) |
| Hematocrit, % | 36 (35-38) | 43 (41-45) | 51 (50-53) | 34 (32-35) | 40 (38-43) | 50 (49-51) |
| WBC count, 109/L | 6.86 (5.71-8.14) | 7.11 (6.05-8.40) | 7.42 (6.32-8.88) | 6.84 (5.77-8.11) | 7.17 (6.04-8.51) | 7.55 (6.39-8.99) |
| Platelet count, 109/L | 232 (194-279) | 226 (190-269) | 215 (181-253) | 273 (220-329) | 258 (217-305) | 223 (194-287) |
| Total cholesterol, mmol/L | 3.95 (3.36-4.71) | 4.07 (3.49-4.86) | 4.48 (3.65-5.40) | 4.46 (3.76-5.23) | 4.68 (3.93-5.59) | 4.82 (4.27-5.59) |
| HDL cholesterol, mmol/L | 1.04 (0.88-1.26) | 1.06 (0.92-1.26) | 1.04 (0.89-1.22) | 1.19 (1.01-1.42) | 1.25 (1.07-1.48) | 1.26 (1.09-1.44) |
| LDL cholesterol, mmol/L | 1.97 (1.51-2.59) | 2.07 (1.58-2.73) | 2.37 (1.73-3.11) | 2.29 (1.73-3.02) | 2.46 (1.83-3.23) | 2.53 (2.02-3.28) |
| Triglycerides, mmol/L | 1.63 (1.14-2.37) | 1.71 (1.22-2.47) | 1.92 (1.37-2.76) | 1.70 (1.25-2.34) | 1.76 (1.32-2.44) | 1.89 (1.35-2.75) |
| Medication, n (%) | ||||||
| ACE inhibitors/ARB | 1490 (90) | 6039 (85) | 452 (86) | 688 (93) | 3601 (85) | 77 (87) |
| Diuretics | 852 (51) | 2976 (42) | 208 (40) | 392 (53) | 1985 (47) | 40 (45) |
| β blocker | 783 (47) | 3678 (52) | 242 (46) | 278 (37) | 2047 (49) | 50 (56) |
| Statin | 1291 (78) | 5431 (77) | 340 (65) | 514 (69) | 2837 (67) | 63 (71) |
| Antithrombotic agents§ | 1173 (71) | 5282 (74) | 377 (72) | 437 (59) | 2607 (62) | 61 (69) |
| Insulin | 1025 (62) | 3762 (53) | 267 (51) | 503 (68) | 2278 (54) | 44 (49) |
Hematocrit values at baseline were available for 98.5% of the total cohort (14 321/14 543), 98.5% of males (9275/9416), and 98.4% of females (5046/5127). Unless otherwise indicated, values are presented as the median (25th-75th percentile).
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure; UACR, urine albumin-creatinine ratio; WBC, white blood cell.
Anemia was defined as a hematocrit level of <39% in males or <36% in females.
Erythrocytosis was defined as a hematocrit level of >49% in males or >48% in female.
Other include American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, multiple, other, unknown, and not reported.
Antithrombotic agents include antiplatelets and anticoagulants.
Effect of canagliflozin on hematocrit value according to baseline hematocrit levels
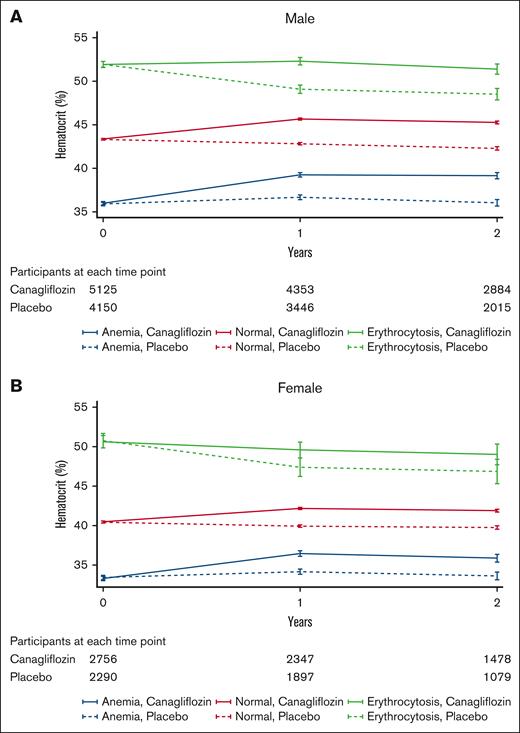
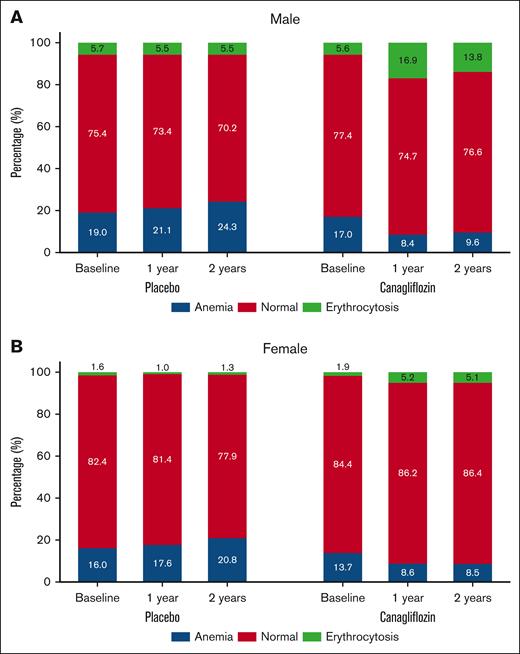
In the CANVAS trial, the treatment effect on hematocrit trajectories over time was comparable between 100 mg and 300 mg canagliflozin groups in both sexes (supplemental Figure 2). Hence, we aggregated the data for participants receiving either dose of canagliflozin for further analysis. Compared with placebo, canagliflozin increased hematocrit levels regardless of baseline hematocrit categories in both sexes (P interaction > .05; Figure 1; supplemental Figure 3). This led to a significant increase in the prevalence of erythrocytosis in the canagliflozin group compared with the placebo in males (16.9% vs 5.5%; odds ratio [OR], 3.51; 95% confidence interval [CI], 2.97-4.15) and females (5.2% vs 1.0%; OR, 5.48; 95% CI, 3.36-8.95) after 1 year. The Medical Dictionary for Regulatory Activities preferred term “Polycythemia” was reported by investigators in 11 males receiving canagliflozin, 1 male in the placebo group, and 1 female receiving canagliflozin. Concurrently, a notable reduction was observed in the prevalence of anemia in the canagliflozin group compared with the placebo in males (8.4% vs 21.1%; OR, 0.36; 95% CI, 0.31-0.41) and females (8.6% vs 17.6%; OR, 0.44; 95% CI, 0.36-0.53) after 1 year after randomization (Figure 2).
Hematocrit changes over time by treatment group and sex. Hematocrit changes over time according to baseline hematocrit categories in males (A) and females (B). Compared with placebo, canagliflozin increased hematocrit levels irrespective of baseline hematocrit status in both sexes. The mean hematocrit values and 95% CIs were calculated using a mixed-effects model for repeated measures. This model incorporated the fixed effects of the treatment arm, trial, trial visit, and treatment-by-visit interaction. The I bars indicate 95% CIs. Anemia was defined as a hematocrit level of <39% in males or <36% in females. Erythrocytosis was defined as a hematocrit level of >49% in males or >48% in females.
Hematocrit changes over time by treatment group and sex. Hematocrit changes over time according to baseline hematocrit categories in males (A) and females (B). Compared with placebo, canagliflozin increased hematocrit levels irrespective of baseline hematocrit status in both sexes. The mean hematocrit values and 95% CIs were calculated using a mixed-effects model for repeated measures. This model incorporated the fixed effects of the treatment arm, trial, trial visit, and treatment-by-visit interaction. The I bars indicate 95% CIs. Anemia was defined as a hematocrit level of <39% in males or <36% in females. Erythrocytosis was defined as a hematocrit level of >49% in males or >48% in females.
Changes in hematocrit categories by treatment and sex. Changes in the proportions of hematocrit categories according to treatment arm in males (A) and females (B). Canagliflozin increased the prevalence of erythrocytosis and decreased the incidence of anemia in both sexes. The odds of erythrocytosis and anemia associated with canagliflozin vs placebo at 1 and 2 years were estimated using logistic regression models with random intercepts for each trial. For males, the ORs for erythrocytosis were 3.51 (95% CI, 2.97-4.15) at 1 year and 2.84 (95% CI, 2.28-3.55) at 2 years and for anemia 0.36 (95% CI, 0.31-0.41) at 1 year and 0.35 (95% CI, 0.30-0.41) at 2 years. For females, the ORs for erythrocytosis were 5.48 (95% CI, 3.36-8.95) at 1 year and 4.23 (95% CI, 2.37-7.58) at 2 years and for anemia 0.44 (95% CI, 0.36-0.53) at 1 year and 0.36 (95% CI, 0.28-0.46) at 2 years. Anemia was defined as a hematocrit level of <39% in males or <36% in females. Erythrocytosis was defined as a hematocrit level of >49% in males or >48% in females.
Changes in hematocrit categories by treatment and sex. Changes in the proportions of hematocrit categories according to treatment arm in males (A) and females (B). Canagliflozin increased the prevalence of erythrocytosis and decreased the incidence of anemia in both sexes. The odds of erythrocytosis and anemia associated with canagliflozin vs placebo at 1 and 2 years were estimated using logistic regression models with random intercepts for each trial. For males, the ORs for erythrocytosis were 3.51 (95% CI, 2.97-4.15) at 1 year and 2.84 (95% CI, 2.28-3.55) at 2 years and for anemia 0.36 (95% CI, 0.31-0.41) at 1 year and 0.35 (95% CI, 0.30-0.41) at 2 years. For females, the ORs for erythrocytosis were 5.48 (95% CI, 3.36-8.95) at 1 year and 4.23 (95% CI, 2.37-7.58) at 2 years and for anemia 0.44 (95% CI, 0.36-0.53) at 1 year and 0.36 (95% CI, 0.28-0.46) at 2 years. Anemia was defined as a hematocrit level of <39% in males or <36% in females. Erythrocytosis was defined as a hematocrit level of >49% in males or >48% in females.
Effect modification by baseline hematocrit levels regarding the primary outcome
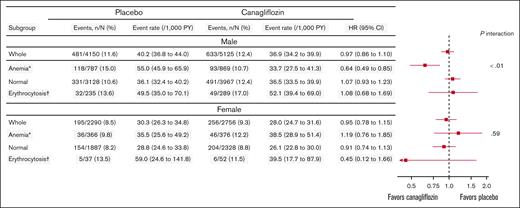
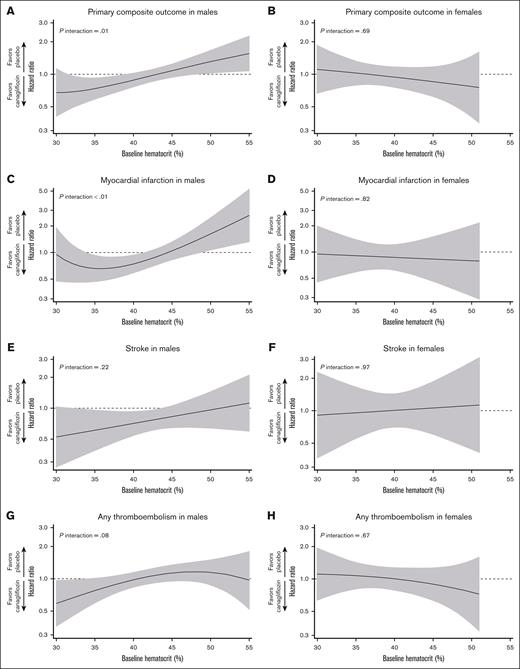
In both sexes, the incidence of the composite primary outcome was numerically higher among patients with anemia or erythrocytosis than in those with normal hematocrit levels in the placebo group (Figure 3). Overall, canagliflozin did not reduce the risk of the primary outcome in males (hazard ratio [HR], 0.97; 95% CI, 0.86-1.10) and females (HR, 0.95; 95% CI, 0.78-1.15; Figure 3; supplemental Figure 4). In males, baseline hematocrit categories modified the treatment effect on the primary outcome (P interaction < .01), with particularly large benefits in anemic patients (HR, 0.64; 95% CI, 0.49-0.85; Figure 3). Consistently, hematocrit as a continuous variable was an effect modifier in males (P interaction = .01), with benefits for those with low hematocrit values (<39%, ie, anemia) but harmful for those with high hematocrit values (>49%, ie, erythrocytosis; Figure 4). Effect modifications by baseline hematocrit levels were not observed in females, regardless of whether treated as categorical or continuous variables (P interaction = .59 and .69, respectively; Figures 3 and 4). Sensitivity analysis adjusting for age, smoking status, history of CV disease and MI, HbA1c, low-density lipoprotein cholesterol, and diastolic blood pressure yielded similar results in both males and females (supplemental Figure 5). Three-way interaction among treatment, baseline hematocrit value (continuous), and sex was significant for the relevant Cox model (P interaction = .02), indicating the effect modification by baseline hematocrit levels in a sex-dependent manner.
Treatment effect on the primary outcome according to baseline hematocrit categories. Canagliflozin did not significantly reduce the risk of the primary outcome in either males (HR, 0.97; 95% CI, 0.86-1.10) or females (HR, 0.95; 95% CI, 0.78-1.15). In males, baseline hematocrit categories significantly modified the treatment effect on the primary outcome (P interaction < .01), with particularly pronounced benefits observed in anemic patients (HR, 0.64; 95% CI, 0.49-0.85). In contrast, no effect modification by baseline hematocrit levels was observed in females (P interaction = .59). The event rate was presented as the number of events per 1000 person-years. ∗Anemia was defined as a hematocrit level of <39% in males or <36% in females. †Erythrocytosis was defined as a hematocrit level of >49% in males or >48% in females.
Treatment effect on the primary outcome according to baseline hematocrit categories. Canagliflozin did not significantly reduce the risk of the primary outcome in either males (HR, 0.97; 95% CI, 0.86-1.10) or females (HR, 0.95; 95% CI, 0.78-1.15). In males, baseline hematocrit categories significantly modified the treatment effect on the primary outcome (P interaction < .01), with particularly pronounced benefits observed in anemic patients (HR, 0.64; 95% CI, 0.49-0.85). In contrast, no effect modification by baseline hematocrit levels was observed in females (P interaction = .59). The event rate was presented as the number of events per 1000 person-years. ∗Anemia was defined as a hematocrit level of <39% in males or <36% in females. †Erythrocytosis was defined as a hematocrit level of >49% in males or >48% in females.
Treatment effects by baseline hematocrit level on the primary outcome and each component. The treatment effect based on baseline hematocrit levels on the primary composite outcome in males (A) and females (B), on MI in males (C) and females (D), on stroke in males (E) and females (F), and on any thromboembolism in males (G) and females (H). In males, the treatment effect on the primary outcome and MI varied according to baseline hematocrit levels (P interaction < .05), showing a benefit in anemic patients but a detrimental effect in those with erythrocytosis. By contrast, no effect modifications were observed in females. In addition, a sex-specific effect modification by hematocrit level was identified for the primary outcome (baseline hematocrit by treatment by sex interaction, P = .02). The black line indicates the point estimate, whereas the gray area represents the 95% CI. The graphs have truncated the lowest and highest 0.5 percentiles.
Treatment effects by baseline hematocrit level on the primary outcome and each component. The treatment effect based on baseline hematocrit levels on the primary composite outcome in males (A) and females (B), on MI in males (C) and females (D), on stroke in males (E) and females (F), and on any thromboembolism in males (G) and females (H). In males, the treatment effect on the primary outcome and MI varied according to baseline hematocrit levels (P interaction < .05), showing a benefit in anemic patients but a detrimental effect in those with erythrocytosis. By contrast, no effect modifications were observed in females. In addition, a sex-specific effect modification by hematocrit level was identified for the primary outcome (baseline hematocrit by treatment by sex interaction, P = .02). The black line indicates the point estimate, whereas the gray area represents the 95% CI. The graphs have truncated the lowest and highest 0.5 percentiles.
Effect modification by baseline hematocrit levels regarding secondary outcomes in males
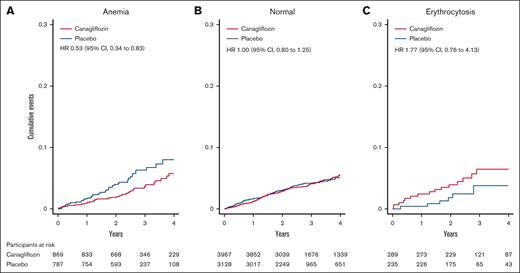
Canagliflozin reduced the risk of stroke (HR, 0.78; 95% CI, 0.62-0.98), MACE (HR, 0.84; 95% CI, 0.74-0.95), CV death (HR, 0.83; 95% CI, 0.69-1.00), all-cause death (HR, 0.84; 95% CI, 0.72-0.97), hospitalization for heart failure (HR, 0.62; 95% CI, 0.49-0.77), and kidney composite outcome (HR, 0.66; 95% CI, 0.52-0.83). Meanwhile, it did not significantly affect the incidence of MI (HR, 0.91; 95% CI, 0.75-1.10), any thromboembolism (HR, 1.00; 95% CI, 0.87-1.15), arterial thromboembolism (HR, 1.06; 95% CI, 0.90-1.23), or venous thromboembolism (HR, 1.14; 95% CI, 0.69-1.89; supplemental Table 4). Except for MI, no effect modification by baseline hematocrit levels was noted (P interaction > .05; Figure 4; supplemental Table 4; supplemental Figure 6). Consistent with the primary outcome analysis, hematocrit as a continuous variable served as an effect modifier in the relationship between treatment and MI (P interaction < .01), showing that canagliflozin reduced the MI risk in anemic patients but increased the risk of MI in patients with erythrocytosis (Figure 4). Similarly, if hematocrit status was treated as categories (anemia, normal, or erythrocytosis), hematocrit levels also altered treatment effects on MI (P interaction = .02), being beneficial for anemic patients (HR, 0.53; 95% CI, 0.34-0.83), neutral for those with normal levels (HR, 1.00; 95% CI, 0.80-1.25), and harmful for patients with erythrocytosis, although not statistically significant (HR, 1.77; 95% CI, 0.76-4.13; supplemental Table 4; Figure 5).
Time to occurrence of MI according to baseline hematocrit status in males. Canagliflozin reduced the risk of MI in patients with anemia (A) (HR, 0.53; 95% CI, 0.34–0.83). In patients with normal hematocrit (B), the treatment effect was neutral (HR, 1.00; 95% CI, 0.80–1.25). In patients with erythrocytosis (C), canagliflozin showed a trend toward increased risk of MI (HR, 1.77; 95% CI, 0.76–4.13). The graphs were truncated at 4 years. Anemia was defined as a hematocrit level of <39%; erythrocytosis was defined as a hematocrit level of >49%.
Time to occurrence of MI according to baseline hematocrit status in males. Canagliflozin reduced the risk of MI in patients with anemia (A) (HR, 0.53; 95% CI, 0.34–0.83). In patients with normal hematocrit (B), the treatment effect was neutral (HR, 1.00; 95% CI, 0.80–1.25). In patients with erythrocytosis (C), canagliflozin showed a trend toward increased risk of MI (HR, 1.77; 95% CI, 0.76–4.13). The graphs were truncated at 4 years. Anemia was defined as a hematocrit level of <39%; erythrocytosis was defined as a hematocrit level of >49%.
Effect modification by baseline hematocrit levels regarding secondary outcomes in females
Canagliflozin reduced the risk of kidney composite outcome (HR, 0.62; 95% CI, 0.44-0.86) among secondary outcomes, with a marginally positive effect on hospitalization for heart failure (HR, 0.72; 95% CI, 0.52-1.02). Treatment effects on secondary outcomes were consistent across baseline hematocrit levels, regardless of whether these were treated as categorical or continuous variables (P interaction > .05; Figure 4; supplemental Table 5).
Discussion
This study demonstrated that canagliflozin increased hematocrit levels regardless of initial values even in patients with erythrocytosis compared with placebo. In addition, canagliflozin increased the prevalence of erythrocytosis from 5.6% to 16.9% and from 1.9% to 5.2% in males and females over 1 year, respectively. Although canagliflozin had a neutral effect on the primary composite outcome (MI, stroke, or any thromboembolism), a significant effect modification by baseline hematocrit levels was observed in men, indicating that canagliflozin provided benefits for anemic male patients but posed potential risks for those with elevated hematocrit levels, whereas no heterogeneity was observed in female patients. The observed heterogeneity in males was primarily driven by MI.
A meta-analysis of 40 randomized clinical trials revealed that SGLT2is significantly increased hematocrit levels by 2.7% compared with placebo.45 Although SGLT2is have been documented to elevate hematocrit in individuals with low and normal levels,41,44 its effect on patients with erythrocytosis has not been verified. Our study extends these findings by demonstrating that SGLT2is significantly increased hematocrit levels in patients with erythrocytosis, regardless of sex. Erythrocytosis predominantly occurs in males, with its prevalence varying depending on the target population and the definition used.26 Although the prevalence of erythrocytosis in individuals with T2DM is not well established, the baseline prevalence in this study was compatible with that of a recent population-based cohort study from the Netherlands (n = 147 167), which reported erythrocytosis in 7.6% of males and 0.4% of females using the same criteria.22 Notably, canagliflozin significantly increased the proportion of erythrocytosis in both sexes. It is important to recognize that SGLT2is are potent hematopoietic agents that can improve anemic conditions, increase the prevalence of erythrocytosis, and exacerbate preexisting erythrocytosis.
The most novel and valuable finding in this study was the effect modification of canagliflozin by baseline hematocrit levels on the primary composite outcome and MI, indicating potential harm in male patients with erythrocytosis. Despite existing concerns that SGLT2is increase hematocrit levels, blood viscosity, and thromboembolism risk,31-34,46,47 no documented reports have validated these concerns in the setting of randomized controlled trials.35 Our study uncovered the potential harms associated with SGLT2i use by focusing on diabetic patients with erythrocytosis, a condition characterized by inherently elevated blood viscosity. This finding is clinically important because diabetic patients with erythrocytosis overlap with those for whom guidelines recommend SGLT2is,9,10 specifically patients with established or high risk of atherosclerotic CV disease. Compared with the rest of the population, patients with erythrocytosis at baseline were more likely to be current smokers, have a history of CV disease or MI, and exhibit poorly controlled lipid and blood pressure levels, consistent with previous reports.22,25,48,49 In diabetic patients with erythrocytosis, glucagon-like peptide-1 receptor agonists, which are equally recommended by guidelines,9,10 should be preferably prescribed over SGLT2is. Meanwhile, we also demonstrated that canagliflozin reduced the risk of the primary outcome and MI in male patients with anemia. We speculated that the increase in hematocrit levels with canagliflozin may help attenuate myocardial ischemia by improving the “supply/demand mismatch.”
Interestingly, the harmful effects of canagliflozin in patients with elevated hematocrit levels were observed in males, but not in females. This observation could align with a recent report from the Mayo Clinic, which documented 100 patients with SGLT2i-associated, JAK2-unmutated erythrocytosis.28 This report showed that 78% of the patients were male, 29% underwent phlebotomy or blood donation as a treatment for erythrocytosis, and 10 patients, all male, experienced thrombotic events, including 4 cases of coronary artery disease. Of note, these results should be interpreted with caution, given that only 17 patients in this report were using canagliflozin and 61 patients had identifiable causes for erythrocytosis. The exact mechanism underlying these sex differences remains unknown. However, this observation can be partially explained by anatomical and physiologic sex differences in coronary arteries. Females have smaller epicardial coronary artery diameters and higher baseline myocardial blood flow, which could result in significantly increased endothelial shear stress, as well as reduced inflammation, platelet activation, and thrombus formation.50 Therefore, females may be less affected by increased blood viscosity in thrombus formation, particularly in the coronary arteries. In addition, males have higher baseline hematocrit levels, and SGLT2is cause more pronounced increases in hematocrit levels in males than females.44 Considering that blood viscosity increases exponentially when hematocrit values are >50%,51 males were more likely to experience higher blood viscosity than females, which may contribute to observed sex differences in the effects of SGLT2i.
Whether to discontinue SGLT2is in patients with erythrocytosis is an important clinical issue for future consideration. Until our current report, there had been no evidence suggesting that SGLT2is increase the risk of thrombosis in patients with erythrocytosis compared with control groups. Therefore, given the robust evidence supporting the benefits of SGLT2is, no clear rationale exists for recommending discontinuation solely based on erythrocytosis. Addressing this issue would require a direct comparison between patients with erythrocytosis who continue and those who discontinue SGLT2is, which is beyond the scope of our study. Nevertheless, we recognize the clinical significance of this question and believe that our findings provide a foundation for future discussion. Further research is warranted to establish evidence-based clinical guidance. The primary strength of this study lies in the utilization of high-quality data from landmark trials, the CANVAS program, and the CREDENCE trial. However, our study has certain limitations. First, this study exclusively included patients with T2DM, limiting the generalizability of the findings to nondiabetic populations. Second, the primary outcome comprised a composite of adjudicated and investigator-reported events. Nonetheless, a high concordance between investigator-reported and adjudicated events has been documented for various ischemic endpoints in double-blind randomized clinical trials.52 Moreover, the primary outcome results were robustly supported by the MI results, as adjudicated by independent committees. Third, this investigation represents an integrated exploratory analysis of 2 clinical trials not originally designed to detect treatment heterogeneity. The limited number of patients and events among those with erythrocytosis raises concerns regarding the statistical power of subgroup analyses, particularly in female subjects, which may hinder the accurate estimation of effect sizes in subpopulations. Finally, the absence of JAK2 genetic testing in our study does not preclude the possibility that some participants had polycythemia vera. Nevertheless, the randomized controlled trial design minimizes the risk of an uneven distribution of such patients that could have biased the results.
In conclusion, our study demonstrated that canagliflozin significantly increased hematocrit levels even in patients with T2DM and erythrocytosis, in both males and females. Safety concerns related to canagliflozin were observed only in males with elevated baseline hematocrit levels, particularly regarding thromboembolic events, including MI. Given the lack of treatment heterogeneity based on hematocrit levels for heart failure hospitalization or kidney outcomes, the use of SGLT2i in patients with erythrocytosis may benefit from a personalized approach that considers sex and the specific outcomes targeted for improvement. These findings warrant further investigation.
Acknowledgments
This study was supported by a research grant program by the Japanese Society on Thrombosis and Haemostasis.
This study, conducted under Yale University Open Data Access (YODA) Project 2023-5367, used data obtained from the YODA Project, which has an agreement with Janssen Research & Development, LLC. The interpretation and reporting of research using this data are solely the responsibility of the authors and do not necessarily represent the official views of the YODA Project or Janssen Research & Development, LLC. The Japanese Society on Thrombosis and Haemostasis was not involved in the design or execution of this study, including data preparation, analysis, interpretation, manuscript preparation, review, or approval, nor in the decision to submit the manuscript for publication.
Authorship
Contribution: Y.D. conceptualized the study; Y.D. and T.H. performed the formal analysis; Y.D. wrote the original draft and the first version of the manuscript; T.H., O.Y., and Y.I. reviewed and edited the draft; and O.Y. and Y.I. supervised the study.
Conflict-of-interest disclosure: Y.D. reports research funding from Nippon Boehringer Ingelheim Co., Ltd. T.H. is on the speakers' bureau at AstraZeneca, Ono Pharmaceutical Co, Ltd, Nippon Boehringer Ingelheim Co, Ltd, Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc, Taisho Pharmaceutical Co, Ltd, and Kowa Company, Ltd, and reports research funding from Astellas Pharma Inc and Kowa Company, Ltd. O.Y. is on the speakers’ bureau at Ono Pharmaceutical Co, Ltd, Nippon Boehringer Ingelheim Co, Ltd, Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc, AstraZeneca, Eli Lilly Japan K.K., Taisho Pharmaceutical Co, Ltd, and Kowa Co, Ltd, and reports research funding from Ono Pharmaceutical Co, Ltd, Nippon Boehringer Ingelheim Co, Ltd, Mitsubishi Tanabe Pharma Corporation, and Astellas Pharma Inc. Y.I. is on the speakers’ bureau at AstraZeneca, Mitsubishi Tanabe Pharma Corporation, Kissei Pharmaceutical Co, Ltd, Astellas Pharma Inc, and Nippon Boehringer Ingelheim Co, Ltd.
Correspondence: Takayuki Hamano, Department of Nephrology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, 467-8601 Nagoya, Japan; email: hamatea@med.nagoya-cu.ac.jp.
References
Author notes
Data from this study will be made available in the public domain via the Yale University Open Data Access Project (https://yoda.yale.edu/).
The full-text version of this article contains a data supplement.