Sickle cell trait (SCT) may increase the risk of chronic kidney disease (CKD). We aimed to determine the pooled statistics of the association between SCT and CKD. Studies published up to May 2024 that were available on PubMed, Embase, Global Health Library, and Web of Science were screened. We included studies that reported odds ratios or hazard ratios (HRs) of CKD and/or end-stage renal disease (ESRD) and that compared adults with SCT to those without SCT. The risk of bias was evaluated using the Risk Of Bias In Nonrandomized Studies-of Exposures tool. The pooled SCT prevalence was calculated among patients with CKD/ESRD. A random-effects analysis was performed. Only studies with low or some concerns of bias were included, corresponding to 18 847 participants with SCT and 1 060 818 without SCT. Participants with SCT had higher odds of having an estimated glomerular filtration rate (eGFR) of ≤60 mL/min per 1.73 m2, proteinuria, and eGFR ≤60 mL/min per 1.73 m2 and/or proteinuria. The pooled prevalence of SCT among African American individuals with ESRD was 10%; however, the heterogeneity was very high (I2, 85.6%). There was a higher HR for ESRD in the studies that included both males and females than in the study that included only females, suggesting that males have a higher risk of ESRD. Controversial results were observed for the association of CKD with hypertension and diabetes. SCT increases the risk of developing CKD and ESRD. PROSPERO registration: CRD42021275274.
Introduction
Sickle cell anemia (SCA) is caused by a single nucleotide mutation in the β-globin gene (HBB gene), which leads to the substitution of valine for glutamic acid.1 This leads to the polymerization of mutated hemoglobin and the sickling of red blood cells under low oxygen conditions, causing hemolysis, vaso-occlusion, and organ damage.1 SCA occurs in individuals with the homozygous hemoglobin S (HbSS) mutation. Sickle cell trait (SCT) is a heterozygous condition with a mutation in a single allele of the HBB gene, which leads to the production of both normal hemoglobin (hemoglobin A [HbA], 55%-65% of circulating concentration) and abnormal hemoglobin (HbS, 30%-40%).1 The mutation affects >300 million individuals worldwide with most cases in Africa, the Middle East, and India.2 In the United States, SCT has an incidence of 7.3% in African American (AA) newborns, 0.7% in Hispanic people, and 0.3% in White people.3
Unlike in SCA in which the high concentration of HbS (80%-90.5%) causes red blood cells to sickle, the higher concentrations of HbA in SCT usually prevent the development of serious pathologic conditions, such as red blood cell hemolysis and vaso-occlusive episodes.4 Consequently, SCT has generally been considered a benign condition with affected individuals typically having a normal life expectancy and quality of life. However, the Centers for Disease Control and Prevention (CDC) recently acknowledged that individuals with SCT can, on rare occasions, develop complications, such as hematuria, splenic infarction, and retinopathy.5
In SCA, chronic kidney disease (CKD) is among the most common chronic organ disorders that affects 28% to 68% of patients and contributes to early mortality in 16% to 18% of cases.6 The physiological characteristics of the kidney, such as the presence of a hypoxic environment, acidosis, and hypertonicity, favor the polymerization of HbS, which leads to hemolysis, vascular damage, and medullary infarction.7 These normal renal conditions may also promote the polymerization of HbS in SCT. Both SCT and CKD are more prevalent in African and Hispanic populations, suggesting that SCT may be a genetic risk factor for CKD in these groups.
According to the CDC, CKD is not listed as a complication that individuals with SCT can develop.5 Some studies have found that individuals with SCT are at a higher risk of CKD,8-22 whereas others have not been able to replicate this association.23-28 Misdiagnosis between SCT and SCA, differences in the study design and populations, the presence of confounders, and small effects and sample sizes may explain this discrepancy.
We aimed to calculate the pooled odds ratio (OR) of CKD outcomes in individuals with SCT in comparison with participants without the mutation. This meta-analysis updates and expands on the previously published meta-analysis of 5 US population–based studies of AA individuals8 by incorporating more recent studies conducted both within and outside the United States. This study also included additional populations with a high proportion of individuals of African origin, such as people from the Caribbean, and examined potential risk factors for CKD. Because SCT screening is currently performed in the United States, these results could provide evidence that supports the implementation of preventive measures, early detection, and treatment interventions for CKD in individuals with SCT.
Methods
The protocol has been registered with the International Prospective Register of Systematic Reviews (PROSPERO, CRD42021275274) and is available on the PROSPERO website. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (supplemental Tables 1 and 2).
Search strategy
The PubMed, Embase, Global Health Library, and Web of Science databases were searched until 4 May 2024 without language restrictions. A combination of free-text terms and medical subject headings were used with the operators “AND” and “OR” (supplemental Methods 1). Only articles with English titles and abstracts were evaluated. The reference lists of eligible articles were screened.
Study eligibility
We included randomized, cross-sectional, and cohort (retrospective and prospective) studies that presented data on the odds ratios (ORs) or hazard ratios (HRs) of CKD and/or end-stage renal disease (ESRD) in adults with and without SCT. We excluded case reports and case series. The included studies required a clear diagnosis of SCT and CKD. We included studies that enrolled individuals from the general population, inpatient or outpatient clinics, or populations with identified comorbidities such as diabetes or high blood pressure. Studies that primarily enrolled pregnant women or individuals with specific conditions, such as HIV infection, were excluded. Studies from any geographic region and published in any language were considered.
Study selection
Articles were stored in Rayyan software, and duplicate entries were removed. Abstracts and titles were screened by 2 reviewers (D.J.A.-G. and Y.E.C.-S.). If the abstract did not provide sufficient information, the article was considered for full review. Studies selected for full review were analyzed by 2 reviewers (A.R.A. and Y.E.C.-S.) to determine final eligibility. Reference lists of selected articles were manually evaluated. Discrepancies between reviewers were resolved through discussion with a third reviewer (D.J.A.-G.). Authors with only abstract publications or missing information in the published article were contacted to provide additional unpublished data.
Data extraction
Data extraction was performed using online forms in Google Forms. We extracted information on the study design, enrollment criteria, methods for determining SCT and CKD status, patient characteristics (race, age, and sex), adjusted ORs or HRs for the outcomes of interest with their 95% confidence intervals (CIs), and the confounders used for adjustment. The CKD outcomes that were extracted included an estimated glomerular filtration rate (eGFR) below 60 mL/min per 1.73 m2 (using any method), proteinuria or albuminuria (using any method), CKD composite outcomes (eGFR below 60 mL/min per 1.73 m2 and/or proteinuria), and ESRD. Two reviewers (Y.E.C.-S. and D.J.A.-G.) independently extracted the data, and discrepancies were resolved through discussion between the reviewers.
Risk-of-bias assessment
The Risk Of Bias In Nonrandomized Studies-of Exposures tool, adapted for our study, was used to assess the risk of bias.29 Briefly, the following criteria were applied in the preliminary considerations for risk-of-bias assessment: age and sex were regarded as the minimum necessary confounding factors, and self-reported diagnoses of SCT and CKD were deemed inappropriate methods of diagnosis.
In domain 1 (risk of bias owing to confounding), age, sex, diabetes, and hypertension were considered essential confounding factors (signaling question 1.1). “Weak no” was assigned to studies that adjusted only for age and sex.
In domain 2 (risk of bias arising from measurement of the exposure), the question regarding methods that could lead to error or misclassification (question 2.2) was answered with a weak yes if any of the following methods were used: differential solubility of HbS or solubility tests. Methods assigned a strong yes included International Classification of Diseases codes, insurance records, and hospital charts. Reliable laboratory methods (assigned no) included isoelectric focusing, polymerase chain reaction, high-performance liquid chromatography, electrophoresis, sequencing, genotyping, and genetic imputation. Genotype imputation is a biostatistical method that uses a reference set of haplotypes from a large number of individuals to compare and predict genetic variants in the unknown sample in which only a subset of shared haplotype had been genotyped. It is a precise methodology for a wide range of single-gene Mendelian disorders, including SCT.30 The imputation of the rs334 variant demonstrated a high-quality score, with an r2 ranging from 0.83 to 0.86,8,19 and a high κ index when the reference and imputed panels were compared (0.91; 95% CI, 0.87-0.95).8 The use of hemoglobin A1c (HbA1C) tests to detect SCT was considered inappropriate.31
In domain 6 (risk of bias arising from measurement of the outcome), we added a signaling question to classify the methods used for outcome detection. Methods with low risk of bias for CKD were eGFR below 60 mL/min per 1.73 m2, measured using serum creatinine or cystatin C with any validated equation (Cockcroft-Gault, Chronic Kidney Disease Epidemiology Collaboration equation, Modification of Diet in Renal Disease) and CKD composite, defined as the presence of abnormal proteinuria or albuminuria (>30 mg/g or >30 mg per 24 hours) and/or eGFR below 60 mL/min per 1.73 m2. Proteinuria was defined as a 24-hour or timed proteinuria of >30 mg per 24 hours, and albuminuria was defined as a urinary albumin-to-creatinine ratio of >30 mg/g. ESRD was defined as stage 5 CKD (eGFR <15 mL/min per 1.73 m2 sustained for at least 3 months), patients on dialysis, and patients listed in the US Renal Data System. For all outcomes, direct review of hospital records and International Classification of Diseases codes were classified as having some concerns of bias, whereas indirect review of hospital or insurance records was classified as high risk of bias. For proteinuria, a strip test and random urine sample testing were considered as methods with some concerns.
No major adaptations were made in domains 3 (risk of bias in selection of participants for the study or the analysis) and 5 (risk of bias owing to missing data).
Domain 4 (risk of bias owing to postexposure interventions) was not evaluated, because SCT individuals do not receive any special clinical care.
Two independent researchers (Y.E.C.-S. and D.J.A.-G.) evaluated the risk of bias. Any discordant results were resolved through discussion between the researchers.
Statistical methods
The interrater agreement and the κ index were calculated to measure the consistency between the reviewers. The robvis (Risk-of-bias VISualization) tool32 was used to generate a traffic-light plot and a summary plot weighted by the effective sample size.33
We conducted a random effects meta-analysis to estimate the adjusted pooled ORs or HRs and 95% confidence intervals (CIs) for the comparison of the odds of CKD between participants with SCT and those without SCT. Heterogeneity was evaluated using Higgins I2. Because fewer than 10 publications were available for each outcome, we conducted a visual evaluation of the funnel plot asymmetry to detect publication bias in outcomes with no substantial heterogeneity. A sensitivity analysis was performed using the leave-one-out approach. All analyses were conducted using Stata version 18.0 (StataCorp).
Results
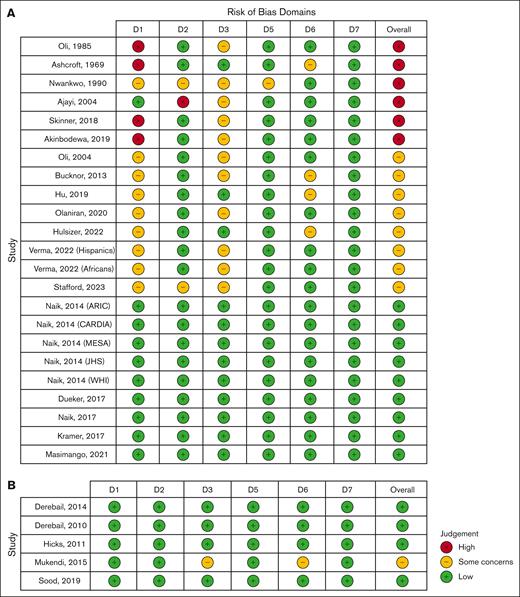
In total, 2480 articles were identified of which 74 were selected for full revision. The κ index between both reviewers was 0.91 (95% CI, 0.81-1.00). A total of 28 studies were included for assessment of the risk of bias (Figure 1).
Pooled effect sizes of the association of SCT and CKD
Of the 28 selected studies, 23 were used to calculate the pooled effect size of the association between SCT and CKD. The preliminary assessment of the risk of bias identified 4 studies with a very high risk of bias. The reasons for this classification were the use of HbA1C tests to diagnose SCT25; a lack of clear information about the diagnosis of SCT and the use of self-reported diagnoses of SCT15; no adjustment for the minimum confounders with incomparable groups15,23; and a high probability of inclusion of patients with sickle cell disease.11 After a complete assessment, the final classification, weighted by the effective sample size, showed that 87% of the studies had some concerns (n = 8 cohorts), 11% had low risk (n = 9 cohorts), and 2% had high risk of bias (n = 6 cohorts; supplemental Figure 1). The primary reasons for the high risk and some concerns of bias were unadjusted results for at least age and sex, and unadjusted results for diabetes and/or hypertension, respectively (Figure 2A).
Traffic-light plots showing the assessment of risk of bias using the Risk Of Bias In Nonrandomized Studies-of Exposures tool. (A) Studies considered for the evaluation of the association between SCT and CKD. (B) Studies considered for the determination of the pooled prevalence of SCT in patients with CKD/ESRD. Domains: D1, bias owing to confounding; D2, bias arising from measurement of the exposure; D3, bias in the selection of participants for the study (or the analysis); D5, bias owing to missing data; D6, bias arising from measurement of the outcome; and D7, bias in the selection of the reported result. D4 was not evaluated because patients with SCT do not receive any clinical care.
Traffic-light plots showing the assessment of risk of bias using the Risk Of Bias In Nonrandomized Studies-of Exposures tool. (A) Studies considered for the evaluation of the association between SCT and CKD. (B) Studies considered for the determination of the pooled prevalence of SCT in patients with CKD/ESRD. Domains: D1, bias owing to confounding; D2, bias arising from measurement of the exposure; D3, bias in the selection of participants for the study (or the analysis); D5, bias owing to missing data; D6, bias arising from measurement of the outcome; and D7, bias in the selection of the reported result. D4 was not evaluated because patients with SCT do not receive any clinical care.
Only studies with some concerns and low risk of bias were included to determine the OR or HR of CKD/ESRD in SCT, resulting in 13 publications (or 17 cohorts because 5 cohorts were presented in a single publication8). These cohorts included 18 847 participants with SCT and 1 060 818 participants without SCT. The characteristics of the included cohorts are shown in Table 1. The predominant race was African (13 cohorts).8-10,13,16-18,20,22 The most common sampling method was population based (10 cohorts),8-10,16,19,21 the most frequent method of SCT detection was genotyping with or without genotype imputation (10 cohorts),8-10,17,19,21 and the most prevalent method of CKD outcome was eGFR below 60 mL/min per 1.73 m2 with or without proteinuria (10 cohorts).8-10,18,19,21 Five of the included studies were also adjusted for acute kidney injury, smoking, and obesity,9 and an individual study was additionally adjusted for the presence of APOL1 high-risk genotypes.10
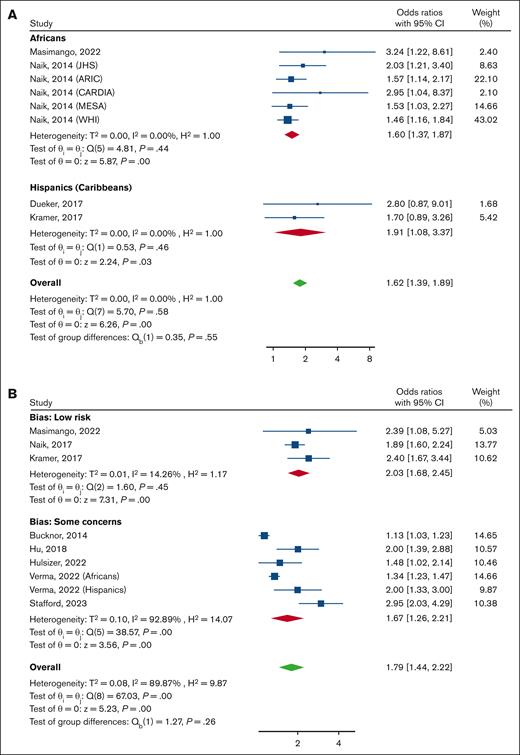
All studies that evaluated the outcome of an eGFR below 60 mL/min per 1.73 m2 were classified as having a low risk of bias. The evidence from these studies indicated a 62% increase in the odds of CKD for participants with SCT when compared with participants without an HbS mutation with an OR of 1.62 (95% CI, 1.39-1.89), as shown in Figure 3A. The subgroup analysis demonstrated that the results were consistent across different races, with no significant difference between AA individuals (OR, 1.60; 95% CI, 1.37-1.87), Hispanic individuals or individuals from the Caribbean (OR, 1.91; 95% CI, 1.08-3.37).
Forest plot showing the pooled ORs of CKD and the 95% CIs for the comparison of participants with SCT and those without. (A) CKD defined as having an eGFR <60 mL/min per 1.73 m2, subgrouped by race. (B) CKD composite (having an eGFR <60 mL/min per 1.73 m2 and/or proteinuria) subgrouped by risk of bias. A random-effects model was used. ARIC, Atherosclerosis Risk in Communities Study; CARDIA, Coronary Artery Risk Development in Young Adults; JHS, Jackson Heart Study; MESA, Multi-Ethnic Study of Atherosclerosis; WHI, Women’s Health Initiative.
Forest plot showing the pooled ORs of CKD and the 95% CIs for the comparison of participants with SCT and those without. (A) CKD defined as having an eGFR <60 mL/min per 1.73 m2, subgrouped by race. (B) CKD composite (having an eGFR <60 mL/min per 1.73 m2 and/or proteinuria) subgrouped by risk of bias. A random-effects model was used. ARIC, Atherosclerosis Risk in Communities Study; CARDIA, Coronary Artery Risk Development in Young Adults; JHS, Jackson Heart Study; MESA, Multi-Ethnic Study of Atherosclerosis; WHI, Women’s Health Initiative.
Nine studies evaluated the probability of an increased risk of CKD composite. Among them, 3 studies had a low risk of bias and 6 had some concerns. This meta-analysis included the results of 1629 participants with SCT and 211 343 without SCT. Overall, participants with SCT had a higher risk of having the CKD composite (OR, 1.79; 95% CI, 1.44-2.22). The risk was higher among the studies with a low risk of bias (OR, 2.03; 95% CI, 1.68-2.45; I2, 14.3%) and slightly lower in the studies with some concerns of bias (OR, 1.67; 95% CI, 1.26-2.21). There was very high heterogeneity among the studies with some concerns of bias (I2 = 92.9%; Figure 3B).
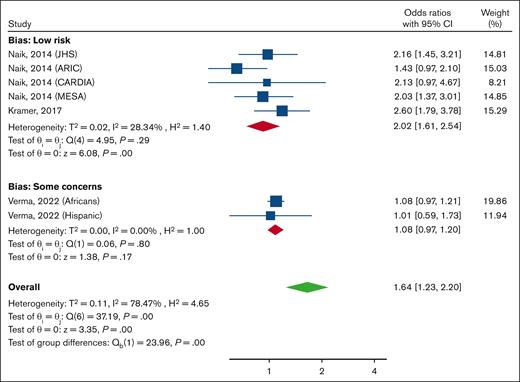
When evaluating the likelihood of having proteinuria, a significant difference was observed in the subgroup analysis based on the risk of bias (P ≤ .01), and this may explain the overall high heterogeneity. Among studies with a low risk of bias (n = 5; involving 713 individuals with SCT and 12 720 with normal HbA), having SCT increased the odds of having proteinuria (OR, 2.02; 95% CI, 1.61-2.54; I2, 28.3%). However, no significant association was observed among the studies with some concerns (7176 participants with SCT and 135 972 without SCT; OR, 1.08; 95% CI, 0.97-1.20; I2 ≤ 0.1%; Figure 4).
Forest plot showing pooled ORs and 95% CIs for proteinuria for the comparison of participants with SCT and those without, subgrouped by risk of bias. A random-effects model was used. ARIC, Atherosclerosis Risk in Communities Study; CARDIA, Coronary Artery Risk Development in Young Adults; JHS, Jackson Heart Study; MESA, Multi-Ethnic Study of Atherosclerosis; WHI, Women’s Health Initiative.
Forest plot showing pooled ORs and 95% CIs for proteinuria for the comparison of participants with SCT and those without, subgrouped by risk of bias. A random-effects model was used. ARIC, Atherosclerosis Risk in Communities Study; CARDIA, Coronary Artery Risk Development in Young Adults; JHS, Jackson Heart Study; MESA, Multi-Ethnic Study of Atherosclerosis; WHI, Women’s Health Initiative.
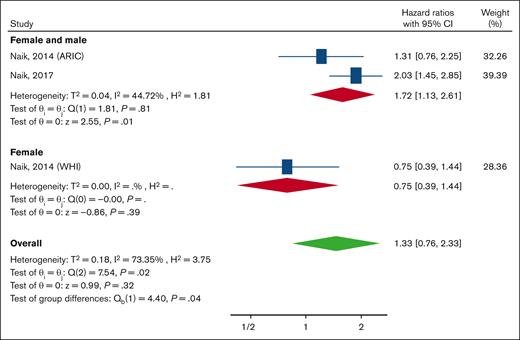
Three studies determined the HR of ESRD in SCT (n = 1600) in comparison with participants without the condition (n = 19 468), and all these studies were classified as having a low risk of bias. Overall, participants with SCT had a higher but not statistically significant incidence of ESRD (HR, 1.33; 95% CI, 0.76-2.33) with very high heterogeneity (I2 = 73.45). The subgroup analysis by sex revealed that there was a significant difference in the HR between studies that included both females and males and the study that included only females (P = .04). The HR in studies that included both females and males was 1.72 (95% CI, 1.13-2.61), and this group showed a lower heterogeneity (I2 = 44.7%; Figure 5).
Forest plot showing the pooled HRs and 95% CIs for the incidence of ESRD for the comparison of individuals with SCT and those without. A random-effects model was used. ARIC, Atherosclerosis Risk in Communities Study; WHI, Women’s Health Initiative.
Forest plot showing the pooled HRs and 95% CIs for the incidence of ESRD for the comparison of individuals with SCT and those without. A random-effects model was used. ARIC, Atherosclerosis Risk in Communities Study; WHI, Women’s Health Initiative.
Participants with SCT had a significant decrease in the mean eGFR change per year (mean, −0.29; 95% CI, −0.45 to −0.13) when compared with those without SCT. However, the heterogeneity of this estimation was high (I2, 67.7%), and the result may be altered by intra- and interindividual variations in the eGFR values (supplemental Figure 2).
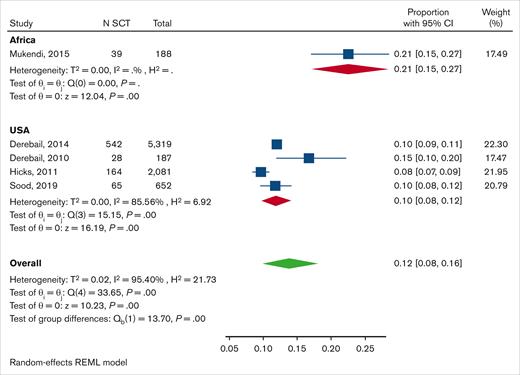
Pooled prevalence of SCT in patients with CKD or ESRD
Five publications were used to calculate the pooled prevalence of SCT among patients with CKD/ESRD. Four of these studies were classified as having a low risk of bias,27,34-36 whereas a study raised some concerns about bias in terms of participant selection (convenience sampling and an unclear recruitment place for patients diagnosed with CKD) and CKD assessment (which was based on a single determination of serum creatinine26; Figure 2B). General characteristics of these studies are included in Table 2; 4 of these were conducted in the United States and the other in Africa.
The pooled prevalence of SCT among AA individuals with CKD/ESRD was 10% (95% CI, 8-12) with very high heterogeneity (I2, 85.6%). There were no noticeable differences between the cohorts in terms of age and sex that could account for the observed heterogeneity (Table 2). A single publication was conducted in Africa and reported a prevalence of 21% (95% CI, 15-27%; Figure 6).
Forest plot showing pooled prevalence of SCT among patients with CKD/ESRD subgrouped by country. A random-effects model was used. REML, restricted maximum likelihood.
Forest plot showing pooled prevalence of SCT among patients with CKD/ESRD subgrouped by country. A random-effects model was used. REML, restricted maximum likelihood.
Risk factors for CKD in SCT
Only 4 studies evaluated common CKD risk factors, such as diabetes and hypertension.8,10,18,28 However, they used different metrics for CKD outcomes, which prevented us from calculating the pooled statistics. One early publication of 5 population-based cohorts of AA individuals found no association between diabetes or hypertension and CKD in SCT.8 However, the authors reported a nonsignificantly higher adjusted incidence of CKD among participants with SCT without hypertension (OR, 2.26; 95% CI, 1.65-3.09) than among those with hypertension (OR, 1.50; 95% CI, 1.14-1.98).8 A later publication by the same authors in a population-based cohort of AA individuals also reported a lack of association with diabetes but found a significantly higher prevalence of CKD among participants with SCT without hypertension (adjusted OR [aOR], 2.94; 95% CI, 2.09-4.12) than among those with hypertension (aOR, 1.63; 95% CI, 1.34-1.98).10 The study of 181 Afro-Caribbean patients with type 2 diabetes mellitus found nonsignificantly higher adjusted odds of albuminuria among patients with diabetes with SCT than among those without SCT (aOR, 1.19; 95% CI, 0.53-2.68).28 A large retrospective study of inpatient and outpatient clinical data of AA individuals found that patients with SCT with diabetes (mean, −0.80; 95% CI, −0.88 to −0.72) and hypertension (mean, −0.58; 95% CI, −0.66 to −0.51) experienced a faster decline in eGFR per year than participants with SCT without diabetes (mean, 0.13; 95% CI, 0.06-0.20) and hypertension (mean, 0.04; 95% CI, −0.04 to 0.12). This study also found that a baseline eGFR of >90 mL/min per 1.73 m2, low HbSS, and elevated HbA were associated with a faster eGFR decline, whereas elevated levels of HbF and HbA2 prevented eGFR decline.18
Results from a pair of AA cohorts found no association of APOL1 and SCT with CKD outcomes.8,10 However, a cross-sectional study in a sub-Saharan African population found that the APOL1 high-risk genotype and SCT significantly interacted in the outcome of lower eGFR (P value for interaction = .012).9 In addition, a population-based cohort evaluated the coinheritance of α-thalassemia and SCT and found that each additional copy of the −α3.7 deletion in the α-globin gene significantly lowered the risk of CKD in participants with SCT (P value for genotype-genotype interaction = .019).18
Publication bias
Publication bias was evaluated for the outcome of having an eGFR <60 mL/min per 1.73 m2, and all 8 included studies had a low risk of bias and low heterogeneity (I2 < 0.01%). The funnel plot indicated that smaller studies reported higher effect sizes, whereas larger studies presented more conservative effect sizes (supplemental Figure 3). We were unable to calculate statistics to determine the probability of publication bias because fewer than 10 studies were included for all outcomes. Funnel plots were not constructed for other evaluated outcomes because of the smaller number of studies or the presence of high heterogeneity.
Sensitivity analysis
Results from the leave-one-out meta-analysis did not identify any significantly influential studies in the evaluated associations, although some trends were observed. Specifically, omitting the study that was conducted exclusively among AA females increased the effect size for having an eGFR below 60 mL/min per 1.73 m2 (supplemental Figure 4A).8 In addition, excluding certain studies with some concerns of bias increased the effect size for proteinuria17 and the CKD composite17,20,22 (supplemental Figure 4B-C). Finally, omitting the study with the smallest sample size, which was conducted in 4 dialysis units in North Carolina,35 decreased the overall prevalence of SCT in patients with CKD/ESRD (supplemental Figure 4D).
Discussion
SCT is no longer considered to be a benign condition, because it recently has been recognized that participants with SCT can, on rare occasions, develop some complications.5 We found that SCT increased the risk of developing CKD and ESRD. Adjusting the analyses for sex, age, diabetes, and hypertension showed that participants with SCT were more likely to have an eGFR <60 mL/min per 1.73 m2 and proteinuria than those with a normal hemoglobin genotype. Participants with SCT also exhibited a more rapid decline in eGFR with a mean decrease of −0.29 mL/min per 1.73 m2 per year.
Similar to our results, a previous meta-analysis of individual data from 5 population-based studies of AA individuals found that SCT carriers have an increased risk of CKD, a decline in eGFR, and albuminuria when compared with noncarriers.8 Our meta-analysis confirmed and expanded these results to Hispanic populations, primarily those of Caribbean origin among which African ancestry is more predominant, and to African descendants living in other countries, such as the United Kingdom. In addition, we included more recently published studies among AA individuals.
In contrast with our results, the previously mentioned meta-analysis found no association with the incidence of ESRD.8 In that study, a single cohort included only female participants, whereas the other cohort included both sexes. We included an additional cohort of AA individuals (including both males and females) that was published later by the same authors10 and found that SCT was associated with a higher incidence of ESRD when considering only the cohorts that include both sexes. We hypothesized that male sex may be associated with a higher risk of progression to ESRD than female sex. Supporting this hypothesis, a longitudinal study in AA individuals found that male sex was associated with a faster decline in eGFR (differences in mean change in eGFR per year: male, −1.22; 95% CI, −1.33 to −1.10 vs female, −0.07; 95% CI, −0.13 to −0.01).18 We conducted a meta-regression analysis to examine sex differences in the outcomes of CKD and proteinuria; however, no significant differences were found (data not shown). The results of this meta-regression analysis should be taken with caution because not all the included studies reported the number of males and females in each group (SCT vs non-SCT).
The pooled prevalence of SCT among AA individuals with CKD/ESRD was slightly higher than the prevalence of SCT reported in 5 US population–based cohorts (range, 6.4%-9.3%).8 However, regional- and population-specific data may be needed when interpreting these findings, because the incidence of SCT among newborns in the United States varies significantly by geographic location, even within the same racial group.37
Controversial results were found for the association of diabetes and hypertension with CKD between population-based cohorts8 and a large retrospective study.18 These discrepancies may be attributed to differences in the study design and/or populations. Most studies did not account for confounders or interaction factors common in African individuals, such as the coinheritance of α-thalassemia, as recommended by the CDC.38 Results from a population-based study suggest that the coinheritance of α-thalassemia protects participant with SCT from developing CKD.39
Many of the publications did not assess the coexistence of APOL1 high-risk genotypes that have been associated with an increased risk of CKD among AA individuals. However, a pair of publications on large AA cohorts found that the coinheritance of the APOL1 high-risk genotype did not increase the risk of CKD in SCT.8,10
One limitation of the data that we used in this review is that most of the publications reported aORs, which may overestimate the true effect size because of the high prevalence of CKD in AA individuals (around 16%).40,41 Only a single study among those included reported an adjusted risk ratio with a lower effect size (risk ratio, 1.13; 95% CI, 1.03-1.23) than the pooled estimate calculated in this study.20 Another limitation is that most of the studies were conducted in AA individuals, which limits the generalizability of these findings because socioeconomic, environmental, and behavioral factors also contribute to the development of CKD. An additional limitation of this meta-analysis is the lack of regional prevalence data on SCT in the general adult AA population, which would allow for more accurate comparisons with the prevalence of SCT in patients with CKD/ESRD.
Larger population-based and longitudinal studies in both pediatric and adult populations are needed to determine the progression and onset of the disease, as well as the specific risk factors for CKD.2,7,8,10,42 Although not fully evaluated, some similarities in CKD development have been observed between patients with SCT and those with sickle cell disease, such as hematuria, impaired urinary concentration, renal papillary necrosis, and proteinuria.1,7,35,42,43 If future studies confirm similar pathologic mechanisms, early biomarkers and treatment interventions for CKD in SCA may also be beneficial for SCT. A recent genetic study has shown that individuals with SCT experienced a statistically significant decline in kidney function after COVID-19 than individuals without SCT who also had COVID-19 (OR, 1.77; 95% CI, 1.16-2.67).17 The impact of other infections, such as influenza A, has only been documented in an individual case.44 Raising awareness of CKD in SCT is crucial for health professionals and patients to prevent and treat CKD and manage complications, such as those arising from infections like COVID-19 or dehydration.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health grant SC1HL150685 (M.J.). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
Contribution: Y.E.C.-S. and M.J. provided supervision, had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis; Y.E.C.-S. conceptualized and designed the study, collected data, performed the statistical analyses, and drafted the manuscript; G.B.J. provided administrative and technical support; D.J.A.-G., A.R.A., P.L.A., and R.D.M. contributed to data collection; and J.K., V.K.D., M.N., and M.J. provided intellectual guidance; and all authors contributed to the concept and design of the study, analysis or interpretation of data, and critical revision of the manuscript for important intellectual content.
Conflict-of-interest disclosure: V.K.D. reports being an author of one of the studies included in this meta-analysis. The remaining authors declare no competing financial interests.
Correspondence: Marina Jerebtsova, College of Medicine, Howard University, 520 W St NW, Room 311, Washington, DC 20059; email: marina.jerebtsova@howard.edu.
References
Author notes
Original data are available on request from the corresponding author, Marina Jerebtsova (marina.jerebtsova@howard.edu).
The full-text version of this article contains a data supplement.