Key Points
The fitness status of patients with previously untreated CLL does not impair safety and efficacy of Ven-Obi.
Venetoclax dose reductions are more common in unfit patients, but only reduced dose intensities of <70% are associated with shorter PFS.
Visual Abstract
Venetoclax-obinutuzumab (Ven-Obi) is a standard first-line therapy for chronic lymphocytic leukemia (CLL). The impact of age, fitness, and dose reductions remains unclear. We analyzed patients treated with Ven-Obi in the CLL13 and CLL14 trials, excluding patients with TP53 aberrations. Fitness was assessed using the cumulative illness rating scale (CIRS, >6) and creatinine clearance (≤70 mL/min). Among 410 patients (median age, 67 years), 55.7% were unfit (median age, 72 years) and 44.3% were fit (median age, 58 years). Overall response rate (ORR) was 89.5% in unfit and 96.1% in fit patients. Rates of undetectable minimal residual disease (uMRD) at <10–4 were 80.3% in unfit and 85.1% in fit patients. Progression-free survival (PFS) at 3 years was 86.4% vs 87.5% (hazard ratio, [HR], 1.12; 95% confidence interval [CI], 0.70-1.81; P = .63). Overall survival at 3 years was 91.8% vs 96.9% (HR, 2.02; 95% CI, 0.90-4.55; P = .088). Adverse events included neutropenia (62.7% unfit, 56.9% fit), infusion-related-reactions (44.3% unfit, 56.9% fit), fatigue (15.8% unfit, 35.9% fit), and infections (57.5% unfit, 69.6% fit). Venetoclax dose reductions of <80% occurred in 39.6% of unfit and 17.6% of fit patients, with lower ORR (83.3% vs 98.2%) and uMRD rates (74.2% vs 87.9%) in those with reduced dose intensities, but similar PFS. Dose reductions of <70% were associated with shorter PFS. Overall, this study shows comparable efficacy and toxicity of Ven-Obi in fit and unfit patients with CLL.
Introduction
National and international treatment guidelines on chronic lymphocytic leukemia (CLL) use age and fitness as major stratification parameters to guide treatment choice, particularly in the first-line setting.1 These recommendations were derived from studies on chemotherapy and chemoimmunotherapy in CLL, for which regimens such as fludarabine, cyclophosphamide, and rituximab (FCR) were shown to be more toxic in older or unfit patients than bendamustine rituximab (BR; CLL10).2 Based on explorative data, the randomized CLL11 trial used a creatinine clearance cutoff of <70 mL/min and a cumulative illness rating scale (CIRS) score of >6 points as indicators of unfit patients. It demonstrated the feasibility and efficacy of chlorambucil-obinutuzumab in these patient populations.3 These parameters were subsequently considered in multiple other randomized studies to investigate therapies for older and unfit patients with CLL, such as the iLLUMINATE, ELEVATE-TN, and GLOW studies.4-6 However, after the results of 2 randomized phase 3 studies, the CLL14 study, recruiting older and unfit (“slow go”) patients with CLL, and the CLL13 study, recruiting younger and fit (“go go”) patients, venetoclax-obinutuzumab (Ven-Obi) became a standard first-line treatment for all patients with treatment-naive CLL.7,8 Given the favorable efficacy and safety outcomes reported in those studies in both fit and unfit patients, the question arises whether fitness status; as defined by CIRS; age, and/or creatinine clearance, should still be considered in the context of Ven-Obi or as a stratification parameter for future trials or in treatment guidelines of CLL.
In addition to efficacy and safety outcomes with Ven-Obi in fit and unfit patients, dose adjustments and permanent treatment discontinuations are of high clinical relevance. With continuous Bruton tyrosine kinase (BTK) inhibitor treatment and, in some instances, also with chemotherapy and chemoimmunotherapy, early treatment discontinuation and relevant dose reductions have been reported to affect survival adversely.9 For venetoclax, similar analyses in the first-line setting are missing. However, a recent post hoc analysis from the MURANO study suggested inferior progression-free survival (PFS) in patients with premature treatment discontinuation before 2 years, whereas venetoclax dose modifications were not associated with outcomes.10
Given these current uncertainties around the role of fitness and dose intensity in the context of Ven-Obi treatment, this analysis explores the role of age and fitness in patients treated with Ven-Obi in CLL, and studies the impact of early permanent venetoclax discontinuation and dose modifications on clinical outcomes.
Methods
Clinical studies and treatment schedule
Analyses were performed on data from the Ven-Obi arms of the CLL13 and CLL14 protocols. In CLL13, which enrolled from 2016 to 2019, patients with previously untreated CLL, a CIRS score of ≤6, and creatinine clearance of >70 mL/min, which were considered as cutoffs for defining fit patient populations, and without TP53 deletion or mutation were considered eligible.7,11 In the CLL14 trial, which enrolled from 2015 to 2016, patients with untreated CLL, a CIRS score of >6, and/or a creatinine clearance of <70 mL/min were considered unfit and eligible, regardless of TP53 deletion/mutation.8,12 For this analysis, patients with TP53 deletion/mutation were excluded to ensure balanced features across the CLL13 and CLL14 populations.
The treatment schedule of the Ven-Obi arm in both CLL13 and CLL14 was identical and consisted of 12 cycles lasting 28 days each. Obinutuzumab was administered IV for 6 cycles, starting with 100 mg on day 1 and 900 mg on day 2 (or 1000 mg on day 1), 1000 mg on day 8 and 1000 mg on day 15 of cycle 1, and subsequently 1000 mg on day 1 of cycles 2 through 6. Daily oral venetoclax was initiated on day 22 of cycle 1, starting with a 5-week dose ramp-up (1 week each of 20, 50, 100, and 200 mg, then 400 mg daily for 1 week), and then continuing at 400 mg daily until completion of cycle 12.
Statistical analyses
Patients with ≥1 dose of the study drug were considered and categorized as fit or unfit (CIRS score of >6 and/or creatinine clearance of <70 mL/min). Correlations regarding minimal residual disease (MRD) in the peripheral blood and response (both assessed at end of treatment [EOT]) were assessed by the χ2 test. PFS and overall survival (OS) were analyzed using the Kaplan-Meier methodology and Cox proportional hazards regression modeling. Independent prognostic variables for PFS were identified by multivariable analyses using Cox proportional hazards regression modeling with stepwise forward and backward selection procedures; only those factors were considered that showed an univariable significant difference (P < .05). Venetoclax dose intensity was calculated as the ratio of the given and planned total dose according to the treatment schedule of the 12 treatment cycles as defined in the study protocol (excluding patients with treatment discontinuation due to progressive disease [PD]/death). Obinutuzumab dose intensity was assessed analogously based on the protocol-defined total dose of 8 g obinutuzumab. Adverse events (AEs) were analyzed up to 28 days after EOT with a focus on events with a frequency ≥10% and AEs of particular interest (neutropenia, anemia, thrombocytopenia, febrile neutropenia, infections, and tumor lysis syndrome). All statistical tests were 2-sided, with the significance level at 0.05. P values were considered descriptive without adjustments for multiple testing. Analyses were performed using SPSS version 28 (SPSS, Chicago, IL).
The trial was approved by the institutional review board or independent health authorities at each participating institution and was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation Guidelines for good clinical practice.
Results
Patient characteristics
In total, 410 patients were included for this analysis, 228 from the CLL13 trial and 182 from the CLL14 trial. The median observation time was 49.2 months (interquartile range [IQR], 37.0-65.8); for CLL13, the median observation time was 38.9 months (IQR, 33.8-46.3), and 66.7 months in CLL14 (IQR, 64.4-70.8). Median age at enrollment was 67 years (IQR, 58-73); 55.7% were grouped as unfit, including 46 patients from CLL13 who were classified as unfit according to CIRS and creatinine clearance (median age, 72 years; median CIRS score, 8; median creatinine clearance, 63.5 mL/min), 44.3% as fit (median age, 58 years; median CIRS score, 2; median creatinine clearance, 91.9 mL/min). In the unfit cohort, 40.4% of patients were female, whereas only 17.1% of the fit patients were female. The median time between CLL diagnosis and study entry was 36.4 months (IQR, 15.9-67.5) in the unfit cohort and 24 months (IQR, 6.1-50.1) in the fit cohort. Constitutional symptoms were more common in unfit than in fit patients (46.9% vs 35.6%). Baseline genetic characteristics, including deletion 11q, deletion 13q, and immunoglobulin heavy chain variable region (IGHV) status, were balanced between fit and unfit patients (Table 1).
Patient characteristics
| Characteristic . | Unfit . | Fit . | Total . | P value . | |||
|---|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | ||
| All patients, N | 228 | 181 | 409 | ||||
| CLL13 | 46 | 20.2 | 181 | 100 | 227 | 55.5 | <.001 |
| CLL14 | 182 | 79.8 | 0 | 0 | 182 | 44.5 | |
| Age, y | 228 | 181 | 409 | ||||
| Median | 72 | 58 | 67 | <.001 | |||
| IQR | 66-76 | 53-66 | 58-73 | ||||
| ≤65 | 48 | 21.1 | 136 | 75.1 | 184 | 45.0 | <.001 |
| >65 and ≤70 | 57 | 25.0 | 24 | 13.3 | 81 | 19.8 | |
| >70 and ≤75 | 60 | 26.3 | 16 | 8.8 | 76 | 18.6 | |
| >75 and ≤80 | 39 | 17.1 | 5 | 2.8 | 44 | 10.8 | |
| >80 | 24 | 10.5 | 0 | 0 | 24 | 5.9 | |
| Sex | 228 | 181 | 409 | ||||
| Female | 92 | 40.4 | 31 | 17.1 | 123 | 30.1 | <.001 |
| Male | 136 | 59.6 | 150 | 82.9 | 286 | 69.9 | |
| ECOG status | 228 | 181 | 409 | ||||
| Median | 1 | 0 | 0 | <.001 | |||
| IQR | 0-1 | 0-1 | 0-1 | ||||
| Total CIRS score | 228 | 181 | 409 | ||||
| Median | 8 | 2 | 5 | <.001 | |||
| IQR | 5-10 | 1-3 | 2-8 | ||||
| >3 | 197 | 86.4 | 43 | 23.8 | 240 | 58.7 | <.001 |
| >4 | 188 | 82.5 | 24 | 13.3 | 212 | 51.8 | <.001 |
| >5 | 171 | 75.0 | 16 | 8.8 | 187 | 45.7 | <.001 |
| >6 | 158 | 69.3 | 0 | 0 | 158 | 38.6 | <.001 |
| Binet stage | 228 | 181 | 409 | ||||
| A | 56 | 24.6 | 43 | 23.8 | 99 | 24.2 | .452 |
| B | 80 | 35.1 | 74 | 40.9 | 154 | 37.7 | |
| C | 92 | 40.4 | 64 | 35.4 | 156 | 38.1 | |
| Time from diagnosis to study entry, mo | 228 | 181 | 409 | ||||
| Median | 36.4 | 24 | 31.9 | <.001 | |||
| IQR | 15.9-67.5 | 6.1-50.1 | 10.4-64.2 | ||||
| Constitutional symptoms | 228 | 180 | 408 | ||||
| No | 121 | 53.1 | 116 | 64.4 | 237 | 58.1 | .026 |
| Yes | 107 | 46.9 | 64 | 35.6 | 171 | 41.9 | |
| Missing information | 0 | 0 | 1 | 0.6 | 1 | 0.2 | |
| Creatinine clearance, mL/min | 227 | 181 | 408 | ||||
| Median | 63.5 | 91.9 | 78.5 | <.001 | |||
| IQR | 53.0-77.2 | 81.6-114.5 | 60.9-96.0 | ||||
| <70 | 155 | 68.3 | 0 | 0 | 155 | 38.0 | <.001 |
| <60 | 98 | 43.2 | 0 | 0 | 98 | 24.0 | <.001 |
| <50 | 38 | 16.7 | 0 | 0 | 38 | 9.3 | <.001 |
| del(17p) | 228 | 181 | 409 | ||||
| Yes | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| del(11q) | 228 | 181 | 409 | ||||
| Yes | 37 | 16.2 | 39 | 21.5 | 76 | 18.6 | .201 |
| Trisomy 12 | 228 | 181 | 409 | ||||
| Yes | 52 | 22.8 | 38 | 21 | 90 | 22 | .719 |
| del(13q) | 228 | 181 | 409 | ||||
| Yes | 123 | 53.9 | 100 | 55.2 | 223 | 54.5 | .842 |
| IGHV mutational status | 217 | 172 | 389 | ||||
| Unmutated | 122 | 56.2 | 108 | 62.8 | 230 | 59.1 | .213 |
| Mutated | 95 | 43.8 | 64 | 37.2 | 159 | 40.9 | |
| Missing information | 11 | 4.8 | 9 | 5 | 20 | 4.9 | |
| TP53 status | 228 | 181 | 409 | ||||
| None | 228 | 100 | 181 | 100 | 409 | 100 | NA |
| Mutation and/or deletion | 0 | 0 | 0 | 0 | 0 | 0 | |
| Serum β2-microglobulin, mg/L | 219 | 179 | 398 | ||||
| Median | 4.1 | 3.9 | 3.9 | .069 | |||
| IQR | 3.0-5.7 | 3.1-4.7 | 3.1-5.2 | ||||
| Serum thymidine kinase, U/L | 212 | 176 | 388 | ||||
| Median | 27.6 | 39.2 | 31.5 | <.001 | |||
| IQR | 14.6-46.6 | 20.6-76.7 | 16.9-62.4 | ||||
| Platelets, ×109/L | 228 | 181 | 409 | ||||
| Median | 139 | 146 | 143 | .306 | |||
| IQR | 100.0-195.5 | 108.0-197.0 | 105.0-196.5 | ||||
| Leukocytes, ×109/L | 228 | 181 | 409 | ||||
| Median | 72.8 | 88.2 | 76.5 | .092 | |||
| IQR | 33.7-141.7 | 35.7-200.0 | 35.3-164.3 | ||||
| Lymphocytes, ×109/L | 227 | 180 | 407 | ||||
| Median | 60.4 | 75.9 | 64.1 | .139 | |||
| IQR | 24.0-124.3 | 31.0-170.3 | 25.2-146.7 | ||||
| Neutrophils, ×109/L | 227 | 180 | 407 | ||||
| Median | 4 | 4.4 | 4.2 | .830 | |||
| IQR | 2.5-6.3 | 2.5-6.6 | 2.5-6.5 | ||||
| LDH, U/L | 228 | 177 | 405 | ||||
| Median | 264.5 | 261 | 262 | .439 | |||
| IQR | 200.5-360.3 | 213.0-320.0 | 205.0-344.0 | ||||
| Characteristic . | Unfit . | Fit . | Total . | P value . | |||
|---|---|---|---|---|---|---|---|
| n . | % . | n . | % . | n . | % . | ||
| All patients, N | 228 | 181 | 409 | ||||
| CLL13 | 46 | 20.2 | 181 | 100 | 227 | 55.5 | <.001 |
| CLL14 | 182 | 79.8 | 0 | 0 | 182 | 44.5 | |
| Age, y | 228 | 181 | 409 | ||||
| Median | 72 | 58 | 67 | <.001 | |||
| IQR | 66-76 | 53-66 | 58-73 | ||||
| ≤65 | 48 | 21.1 | 136 | 75.1 | 184 | 45.0 | <.001 |
| >65 and ≤70 | 57 | 25.0 | 24 | 13.3 | 81 | 19.8 | |
| >70 and ≤75 | 60 | 26.3 | 16 | 8.8 | 76 | 18.6 | |
| >75 and ≤80 | 39 | 17.1 | 5 | 2.8 | 44 | 10.8 | |
| >80 | 24 | 10.5 | 0 | 0 | 24 | 5.9 | |
| Sex | 228 | 181 | 409 | ||||
| Female | 92 | 40.4 | 31 | 17.1 | 123 | 30.1 | <.001 |
| Male | 136 | 59.6 | 150 | 82.9 | 286 | 69.9 | |
| ECOG status | 228 | 181 | 409 | ||||
| Median | 1 | 0 | 0 | <.001 | |||
| IQR | 0-1 | 0-1 | 0-1 | ||||
| Total CIRS score | 228 | 181 | 409 | ||||
| Median | 8 | 2 | 5 | <.001 | |||
| IQR | 5-10 | 1-3 | 2-8 | ||||
| >3 | 197 | 86.4 | 43 | 23.8 | 240 | 58.7 | <.001 |
| >4 | 188 | 82.5 | 24 | 13.3 | 212 | 51.8 | <.001 |
| >5 | 171 | 75.0 | 16 | 8.8 | 187 | 45.7 | <.001 |
| >6 | 158 | 69.3 | 0 | 0 | 158 | 38.6 | <.001 |
| Binet stage | 228 | 181 | 409 | ||||
| A | 56 | 24.6 | 43 | 23.8 | 99 | 24.2 | .452 |
| B | 80 | 35.1 | 74 | 40.9 | 154 | 37.7 | |
| C | 92 | 40.4 | 64 | 35.4 | 156 | 38.1 | |
| Time from diagnosis to study entry, mo | 228 | 181 | 409 | ||||
| Median | 36.4 | 24 | 31.9 | <.001 | |||
| IQR | 15.9-67.5 | 6.1-50.1 | 10.4-64.2 | ||||
| Constitutional symptoms | 228 | 180 | 408 | ||||
| No | 121 | 53.1 | 116 | 64.4 | 237 | 58.1 | .026 |
| Yes | 107 | 46.9 | 64 | 35.6 | 171 | 41.9 | |
| Missing information | 0 | 0 | 1 | 0.6 | 1 | 0.2 | |
| Creatinine clearance, mL/min | 227 | 181 | 408 | ||||
| Median | 63.5 | 91.9 | 78.5 | <.001 | |||
| IQR | 53.0-77.2 | 81.6-114.5 | 60.9-96.0 | ||||
| <70 | 155 | 68.3 | 0 | 0 | 155 | 38.0 | <.001 |
| <60 | 98 | 43.2 | 0 | 0 | 98 | 24.0 | <.001 |
| <50 | 38 | 16.7 | 0 | 0 | 38 | 9.3 | <.001 |
| del(17p) | 228 | 181 | 409 | ||||
| Yes | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| del(11q) | 228 | 181 | 409 | ||||
| Yes | 37 | 16.2 | 39 | 21.5 | 76 | 18.6 | .201 |
| Trisomy 12 | 228 | 181 | 409 | ||||
| Yes | 52 | 22.8 | 38 | 21 | 90 | 22 | .719 |
| del(13q) | 228 | 181 | 409 | ||||
| Yes | 123 | 53.9 | 100 | 55.2 | 223 | 54.5 | .842 |
| IGHV mutational status | 217 | 172 | 389 | ||||
| Unmutated | 122 | 56.2 | 108 | 62.8 | 230 | 59.1 | .213 |
| Mutated | 95 | 43.8 | 64 | 37.2 | 159 | 40.9 | |
| Missing information | 11 | 4.8 | 9 | 5 | 20 | 4.9 | |
| TP53 status | 228 | 181 | 409 | ||||
| None | 228 | 100 | 181 | 100 | 409 | 100 | NA |
| Mutation and/or deletion | 0 | 0 | 0 | 0 | 0 | 0 | |
| Serum β2-microglobulin, mg/L | 219 | 179 | 398 | ||||
| Median | 4.1 | 3.9 | 3.9 | .069 | |||
| IQR | 3.0-5.7 | 3.1-4.7 | 3.1-5.2 | ||||
| Serum thymidine kinase, U/L | 212 | 176 | 388 | ||||
| Median | 27.6 | 39.2 | 31.5 | <.001 | |||
| IQR | 14.6-46.6 | 20.6-76.7 | 16.9-62.4 | ||||
| Platelets, ×109/L | 228 | 181 | 409 | ||||
| Median | 139 | 146 | 143 | .306 | |||
| IQR | 100.0-195.5 | 108.0-197.0 | 105.0-196.5 | ||||
| Leukocytes, ×109/L | 228 | 181 | 409 | ||||
| Median | 72.8 | 88.2 | 76.5 | .092 | |||
| IQR | 33.7-141.7 | 35.7-200.0 | 35.3-164.3 | ||||
| Lymphocytes, ×109/L | 227 | 180 | 407 | ||||
| Median | 60.4 | 75.9 | 64.1 | .139 | |||
| IQR | 24.0-124.3 | 31.0-170.3 | 25.2-146.7 | ||||
| Neutrophils, ×109/L | 227 | 180 | 407 | ||||
| Median | 4 | 4.4 | 4.2 | .830 | |||
| IQR | 2.5-6.3 | 2.5-6.6 | 2.5-6.5 | ||||
| LDH, U/L | 228 | 177 | 405 | ||||
| Median | 264.5 | 261 | 262 | .439 | |||
| IQR | 200.5-360.3 | 213.0-320.0 | 205.0-344.0 | ||||
Patient characteristics for total study population and according to unfit or fit status. For comparisons of metric variables, Mann Whitney U test was used; for comparisons of categorical variables, χ2 test was used.
IGHV, immunoglobulin heavy chain region; LDH, lactate dehydrogenase; NA, not applicable; y, years.
Efficacy outcomes according to fitness
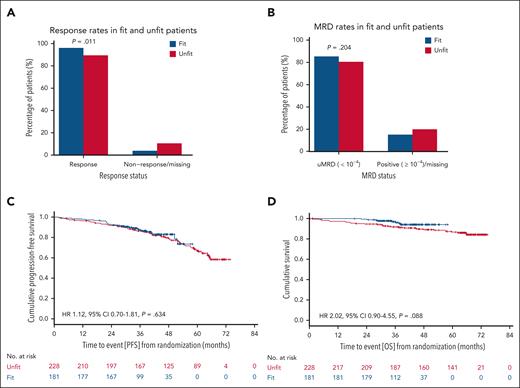
The overall response rate (ORR) at EOT was 89.5% in unfit (204/228 patients) and 96.1% in fit patients (174/181 patients) (P = .011), and complete remission rates (CRRs) were 51.8% (118/228 patients) and 54.1% (98/181 patients; P = .631), respectively (Figure 1A). The undetectable MRD (uMRD; <10–4) rates were 80.3% in unfit (183/228 patients) and 85.1% in fit patients (154/181 patients; P = .204; Figure 1B). At 3 years, 86.4% of unfit vs 87.5% of fit patients were alive and in remission (hazard ratio [HR], 1.12; 95% confidence interval [CI], 0.70-1.81; P = .634; Figure 1C). The 3-year OS rates were 91.8% in unfit vs 96.9% in fit patients (HR, 2.02; 95% CI, 0.90-4.55; P = .088; Figure 1D). Overall, 30 patients passed away in the unfit cohort and 8 in the fit cohort. Infections and second cancers were the most common causes of death and investigators mostly considered these events unrelated to study treatment (supplemental Table 1, available on the Blood website).
Response and survival outcomes according to fitness status. Blue bars and curves represent fit patients; red bars and curves represent unfit patients. (A) ORRs in fit and unfit patients. (B) MRD rates in fit and unfit patients. (C) PFS in fit and unfit patients. (D) OS in fit and unfit patients.
Response and survival outcomes according to fitness status. Blue bars and curves represent fit patients; red bars and curves represent unfit patients. (A) ORRs in fit and unfit patients. (B) MRD rates in fit and unfit patients. (C) PFS in fit and unfit patients. (D) OS in fit and unfit patients.
A CIRS score of >6 was not associated with shorter PFS and OS in univariable testing (HR, 1.21; 95% CI, 0.78-1.88; P = .391 and HR, 1.52; 95% CI, 0.76-3.04; P = .233); likewise, other CIRS cutoffs were not associated with PFS. A CIRS cutoff of >3, >4, and >5 was associated with shorter OS (HR, 2.64; 95% CI, 1.08-6.46; P = .033; HR, 2.37; 95% CI, 1.06-5.30; P = .037; and HR, 2.23; 95% C, 1.05-4.72; P = .037; respectively). Likewise, although a creatinine clearance of <70 mL/min was not associated with PFS and OS in univariable testing (HR, 1.32; 95% CI, 0.88-1.97; P = .185; and HR, 1.85; 95% CI, 0.96-3.54; P = .064; respectively), a creatinine clearance of <50 mL/min was associated with shorter OS (HR, 2.55; 95% CI, 1.20-5.43; P = .015). CIRS and creatinine clearance were not independently associated with PFS in multivariable testing (supplemental Table 3).
Safety outcomes according to fitness
AEs (any grade) considered of interest included neutropenia, which occurred in 62.7% of unfit and 56.9% of fit patients (febrile neutropenia, 4.4% in each group). The median duration of neutropenia was 8 days (IQR, 7-15) in unfit patients and 11 days (IQR, 6-28) in fit patients. Granulocyte colony-stimulating factor was administered in 48.8% of patients (46.5% unfit patients, 51.9% fit patients). The proportion of patients with neutropenia ranged between 53.3% and 72.7% across all age groups, with higher rates observed in patients aged >75 years (supplemental Figure 2). Infusion-related reactions occurred in 44.3% of unfit and 56.9% of fit patients. Fatigue was reported in 15.8% of unfit and 35.9% of fit patients; and headaches in 8.8% of unfit and 18.2% of fit patients. Infections occurred in 57.5% of unfit and 69.6% of fit patients; in particular, nasopharyngitis was reported for 10.5% of unfit and 24.3% of fit patients. COVID-19 occurred in 3 unfit (2 fatal) and 5 fit (2 fatal) patients. Other common AEs were similar in fit and unfit patients (Figure 2). Similar patterns were also observed when comparing young vs older patients according to exploratory age cutoffs for ages <65, 65 to 70, 70 to 75, 75 to 80, and >80 years (supplemental Figure 1).
Frequency of AEs according to fitness status. Blue bars and curves represent fit patients; red bars and curves represent unfit patients. AEs are categorized by preferred terms according to Medical Dictionary for Regulatory Activities (MedDRA) coding.
Frequency of AEs according to fitness status. Blue bars and curves represent fit patients; red bars and curves represent unfit patients. AEs are categorized by preferred terms according to Medical Dictionary for Regulatory Activities (MedDRA) coding.
Dose intensity and treatment discontinuation
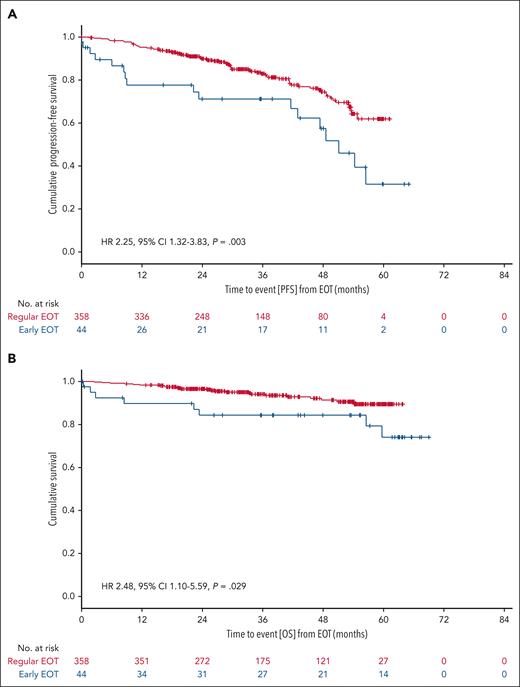
Venetoclax dose intensity was calculated as the ratio of the administered cumulative dose and the planned cumulative dose according to the protocol-specified 12 cycles of venetoclax. Treatment discontinuation was defined as any permanent discontinuation of venetoclax for reasons other than PD or death. Early venetoclax discontinuations were more common in unfit than in fit patients (15.8% vs 5.0%), primarily due to AEs (88.9% in fit patients, and 75.0% in unfit patients who discontinued) or patient withdrawal (0% in fit patients, 22.2% in unfit patients). The median time to early venetoclax discontinuation was 6.3 months (IQR, 3.1-8.7); in unfit patients, the median time to early venetoclax discontinuation was 4.7 months (IQR, 1.9-8.7), in fit patients 7.8 months (IQR, 6.7-10.0). Patients with early venetoclax discontinuation had a 3-year PFS rate from EOT of 71.2%, compared with 83.0% in patients who completed venetoclax as planned (HR, 2.25; 95% CI, 1.32-3.83; P = .003; Figure 3A). The 3-year OS rate from the EOT was 84.3% and 94.0% (HR, 2.48; 95% CI, 1.10-5.59; P = .029), respectively (Figure 3B).
Landmark survival analyses according to treatment completion status. Blue curves represent patients with early EOT; red curves represent patients with regular EOT. (A) Landmark PFS from EOT. (B) Landmark OS from EOT.
Landmark survival analyses according to treatment completion status. Blue curves represent patients with early EOT; red curves represent patients with regular EOT. (A) Landmark PFS from EOT. (B) Landmark OS from EOT.
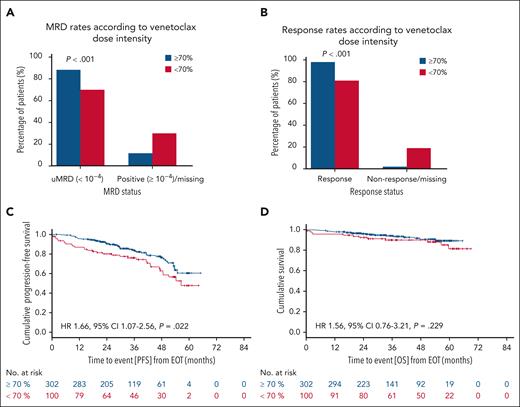
Older patients more commonly experienced venetoclax dose reductions (excluding patients with treatment discontinuation due to PD or death), with lower dose intensities in patients aged between 75 and 80 years (median dose intensity, 80.0% [IQR, 55.1-100]) or in patients aged >80 years (median dose intensity, 67.3% [IQR, 28.3-99.5]) than in patients aged ≤65 years (median dose intensity, 98.6% [IQR, 90.0-100]; supplemental Table 2). Venetoclax dose intensity reductions of <80% occurred in 29.9% of all patients (39.6% unfit, 17.6% fit). In total, interruptions of venetoclax intake occurred in 46.2% of patients (45.1% unfit, 47.4% fit) and lasted for a median of 16 days (IQR, 7-35). Venetoclax dose modifications below the regular daily 400 mg occurred in 66.7% of patients (71.1% unfit, 60.8% fit); these dose adjustments were maintained for a median of 84 (IQR, 15-252) days (191 [IQR, 28-280] days in unfit patients, and 30 [IQR, 8-138] days in fit patients). ORR in patients with venetoclax dose intensity of <80% was 83.3%, and 98.2% in patients with venetoclax dose intensity of ≥80% (P < .001). CRRs were 46.7% and 56.4% (P = .074), uMRD <10–4 rates were 74.2% and 87.9% (P < .001). The 3-year PFS rate from the EOT for patients with and without venetoclax dose intensity of <80% was 79.3% and 82.8%, respectively (HR, 1.40; 95% CI, 0.91-2.15; P = .123). The 3-year OS rate from EOT was 90.8% in patients with reduced venetoclax dose intensity of <80% and 94.1% in patients with venetoclax dose intensity of ≥80% (HR, 1.41; 95% CI, 0.69-2.88; P = .34). Venetoclax dose intensity of <80% with dose reductions maintained until the EOT were not associated with a worse PFS or OS compared with intermittent dose reductions (supplemental Figures 3 and 4). Venetoclax dose intensity reductions of <70% occurred in 24.9% (34.7% unfit patients and 12.5% fit patients), reductions of <60% in 19.2% (28.0% unfit patients and 8.0% fit patients), and reductions of <50% in 14.4% (21.8% unfit patients and 5.1% fit patients; Table 2). For venetoclax dose intensity of <70% vs ≥70%, ORR was 81.0% vs 98.0% (P < .001), CRR was 46.0% vs 56.0% (P = .083), and uMRD <10–4 rate was 70.0% vs 88.4% (P < .001; Figure 4A-B). The 3-year PFS rate from the EOT for patients with and without venetoclax dose intensity of <70% was 76.1% vs 83.6% (HR, 1.66; 95% CI, 1.07-2.56; P = .022), and the 3-year OS rate was 90.0% vs 94.2% (HR, 1.56; 95% CI, 0.76-3.21; P = .229; Figure 4C-D). In patients with venetoclax dose intensity of <70%, the most frequent AEs leading to early treatment discontinuation and/or treatment adjustments included blood and lymphatic disorders (59.1% of all AEs leading to treatment discontinuation, mostly neutropenia), infections (8.3%), investigations (8.3%), and gastrointestinal disorders (5.3%). More pronounced dose reductions of <60% and <50% were associated with a significantly lower ORR and uMRD rate and a shorter PFS (Table 2).
Frequencies and associations of different venetoclax dose intensities and clinical outcomes
| Dose intensity, % . | Frequency in unfit vs fit patients, % . | ORR, % . | CRR, % . | uMRD <10–4, % . | 3-Year PFS from EOT, % . | 3-Year OS from EOT, % . |
|---|---|---|---|---|---|---|
| <80 vs ≥80 | 39.6 vs 17.6 | 83.3 vs 98.2 (P < .001) | 46.7 vs 56.4 (P = .074) | 74.2 vs 87.9 (P < .001) | 82.8 vs 79.3 (HR,1.40; 95% CI, 0.91-2.15; P = .123) | 94.1 vs 90.8 (HR, 1.41; 95% CI 0.69-2.88; P = .340) |
| <70 vs ≥70 | 34.7 vs 12.5 | 81.0 vs 98.0 (P < .001) | 46.0 vs 56.0 (P = .083) | 70.0 vs 88.4 (P < .001) | 76.1 vs 83.6 (HR, 1.66; 95% CI, 1.07-2.56; P = .022) | 90.0 vs 94.2 (HR, 1.56; 95% CI, 0.77-3.21; P = .229) |
| <60 vs ≥60 | 28.0 vs 8.0 | 75.3 vs 98.2 (P < .001) | 44.2 vs 55.7 (P = .068) | 63.6 vs 88.6 (P < .001) | 73.1 vs 83.7 (HR, 1.80; 95% CI, 1.14-2.84; P = .011) | 89.8 vs 93.9 (HR, 1.42; 95% CI, 0.65-3.10; P = .379) |
| <50 vs ≥50 | 21.8 vs 5.1 | 70.7 vs 97.7 (P < .001) | 43.1 vs 55.2 (P = .087) | 58.6 vs 88.1 (P < .001) | 69.2 vs 83.8 (HR, 2.25; 95% CI, 1.40-3.62; P < .001) | 90.1 vs 93.7 (HR, 1.39; 95% CI, 0.59-3.26; P = .449) |
| Dose intensity, % . | Frequency in unfit vs fit patients, % . | ORR, % . | CRR, % . | uMRD <10–4, % . | 3-Year PFS from EOT, % . | 3-Year OS from EOT, % . |
|---|---|---|---|---|---|---|
| <80 vs ≥80 | 39.6 vs 17.6 | 83.3 vs 98.2 (P < .001) | 46.7 vs 56.4 (P = .074) | 74.2 vs 87.9 (P < .001) | 82.8 vs 79.3 (HR,1.40; 95% CI, 0.91-2.15; P = .123) | 94.1 vs 90.8 (HR, 1.41; 95% CI 0.69-2.88; P = .340) |
| <70 vs ≥70 | 34.7 vs 12.5 | 81.0 vs 98.0 (P < .001) | 46.0 vs 56.0 (P = .083) | 70.0 vs 88.4 (P < .001) | 76.1 vs 83.6 (HR, 1.66; 95% CI, 1.07-2.56; P = .022) | 90.0 vs 94.2 (HR, 1.56; 95% CI, 0.77-3.21; P = .229) |
| <60 vs ≥60 | 28.0 vs 8.0 | 75.3 vs 98.2 (P < .001) | 44.2 vs 55.7 (P = .068) | 63.6 vs 88.6 (P < .001) | 73.1 vs 83.7 (HR, 1.80; 95% CI, 1.14-2.84; P = .011) | 89.8 vs 93.9 (HR, 1.42; 95% CI, 0.65-3.10; P = .379) |
| <50 vs ≥50 | 21.8 vs 5.1 | 70.7 vs 97.7 (P < .001) | 43.1 vs 55.2 (P = .087) | 58.6 vs 88.1 (P < .001) | 69.2 vs 83.8 (HR, 2.25; 95% CI, 1.40-3.62; P < .001) | 90.1 vs 93.7 (HR, 1.39; 95% CI, 0.59-3.26; P = .449) |
Response and landmark survival outcomes according to dose intensity. Blue bars and curves represent patients with a venetoclax dose intensity of ≥70%; red bars and curves represent patients with a venetoclax dose intensity of <70%. (A) ORRs. (B) MRD rates. (C) Landmark PFS from EOT. (D) Landmark OS from EOT.
Response and landmark survival outcomes according to dose intensity. Blue bars and curves represent patients with a venetoclax dose intensity of ≥70%; red bars and curves represent patients with a venetoclax dose intensity of <70%. (A) ORRs. (B) MRD rates. (C) Landmark PFS from EOT. (D) Landmark OS from EOT.
Per protocol, the dose of an obinutuzumab infusion could not be altered, and, in total, 8 administrations of 1000 mg obinutuzumab (ie, cumulative dose of 8000 mg obinutuzumab) were planned. Obinutuzumab doses could be skipped or delayed when clinically indicated. In total, 13.9% of patients received a dose intensity of <8 g (16.0% unfit, 11.4% fit). Patients with reduced obinutuzumab dose intensity had a lower ORR (78.6% vs 96.2%, P < .001), CRR (37.5% vs 56.1%, P = .014), and uMRD rate (60.7% vs 87.6%, P < .001). PFS and OS did not differ between patients with and without obinutuzumab dose reductions (supplemental Figures 5 and 6).
Discussion
The burden of comorbidities and/or fitness of patients with CLL has influenced treatment recommendations over the last 2 decades.1,13-15 Several prospective and retrospective studies have shown the high prognostic relevance of the fitness status on posttreatment outcomes.16,17 Various scoring systems such as CIRS and Eastern Cooperative Oncology Group or Karnofsky performance status, which describe fitness or comorbidity, were correlated with treatment tolerability and safety. Most of this evidence was generated in the era of chemotherapy and chemoimmunotherapy and later extended in studies of targeted treatment.4,6 However, it has remained uncertain whether the parameters assessing the fitness or comorbidity of patients retained their relevance in the era of targeted agents.
The Ven-Obi regimen was the first fixed-duration targeted treatment strategy introduced for first-line CLL treatment.8 It marked a paradigm shift from the previous continuous treatment strategy with BTK inhibitor monotherapy. In the context of BTK inhibitor treatment, pivotal randomized studies were run separately for unfit patients, for example, the iLLUMINATE or ELEVATE-TN studies,4,6 or for fit patients, for example, the Eastern Cooperative Oncology Group 1912 studies or SEQUOIA study.18,19 This situation was primarily due to the chemoimmunotherapy comparator arms, which either used chlorambucil-obinutuzumab (Clb-Obi) for unfit patients, commonly defined by the CIRS score and creatinine clearance, or BR or FCR for fit patients. Similarly, the first registrational Ven-Obi study, the CLL14 protocol,8 focused on unfit patients, whereas the later CLL13 protocol focused exclusively on fit patients,7 due to the comparator arms of either Clb-Obi or FCR/BR, respectively. Because both CLL14 and CLL13 demonstrated a high efficacy of fixed-duration Ven-Obi, the clinical relevance of the fitness distinction has become uncertain when using this regimen. This analysis indicates that fitness, defined by CIRS score of >6 and/or creatinine clearance of <70 mL/min, was not associated with different efficacy outcomes and with common side effects. Highly relevant side effects such as neutropenia and infections were not more frequent in unfit compared with fit patients. Surprisingly, infusion-related reactions and fatigue were more common in fit than unfit patients. This finding could be explained by a higher immune fitness facilitating cytokine-mediated reactions, as described for cytokine-release syndrome or autoimmune side effects of other cancer therapies.20 Also, other studies have reported a higher incidence of cancer-related fatigue in younger patients, possibly due to differences in host factors, such as increased inflammatory cytokine responses, and symptom perception.21,22 These data demonstrate that the fitness assessed by the conventional CIRS score and creatinine clearance–based definition is of limited clinical relevance in fixed-duration Ven-Obi treatment. This finding is clinically relevant because treatment-related AEs, such as tumor lysis syndrome (TLS) occurring during venetoclax therapy, were expected to occur more frequently in patients with reduced renal function. The results shown here demonstrate that measures implemented in the CLL13 and CLL14 protocols, such as TLS risk stratification, laboratory monitoring, 5-weekly dose ramp-up, and administration of hydration and uricostatics, can effectively mitigate TLS risk in both fit and unfit patients with CLL.23 Similarly, higher levels of comorbidity, as indicated by higher CIRS scores or lower creatinine clearance values, did not show any association with PFS. This result suggests that older patients with CLL and coexisting conditions can be safely and effectively treated with the Ven-Obi regimen. However, it remains possible that the functional impact of the fitness status, as captured by established geriatric assessments,24 could have a clinically relevant role in the management of the Ven-Obi regimen because neither CIRS scores nor creatinine clearance capture sufficient information on functional impairment. These could include muscle strength, performance, body composition, as well as more comprehensive evaluations of daily functioning. Data from a phase 2 study recently suggest that multidimensional geriatric assessment could allow the identification of patients at increased risk of AEs,25 but further studies with dedicated geriatric evaluation are required to explore the impact of frailty and impaired physical resilience in the context of targeted CLL therapy.
Treatment dose intensity and early discontinuations have particular prognostic relevance in continuous BTK treatment because patients usually require multiple years of treatment to maintain effective disease control. For ibrutinib, studies have demonstrated an association between higher dose intensity and longer PFS, with dose interruptions beyond 7 to 14 days and discontinuations reportedly associated with shorter PFS.26 In this analysis, venetoclax dose reductions as well as early discontinuations were generally more common in unfit patients than in fit patients. Although dose reductions to 80% did not significantly alter survival outcomes, reduced venetoclax dose intensity of <70% was associated with a shorter PFS. Likewise, early treatment discontinuations were associated with shorter PFS and OS. This observation could be attributed to a reduced degree of long-term disease control due to early discontinuation of first-line therapy. However, most PFS and OS events occurred after permanent treatment discontinuation, indicating that AEs or coexisting conditions likely contributed to both the discontinuation and the eventual PFS or OS event. Therefore, the exact prognostic impact of early treatment discontinuation is difficult to dissect in this analysis.
In summary, this analysis demonstrates that a reduced fitness status, as defined by CIRS score and creatinine clearance, is not associated with lower tolerability or efficacy of fixed-duration Ven-Obi therapy in patients with CLL. Therefore, fitness status as trained and tested during the era of chemoimmunotherapy for CLL, has lost its relevance for treatment decisions in CLL with regard to treatment with Ven-Obi. It is necessary to define new thresholds with a focus on specific comorbidities (such as cardiovascular or renal disease) for the current era of targeted agents such as inhibitors of B-cell lymphoma 2 or BTK. Most importantly, the combined data set from the prospective CLL13 and CLL14 trials presented here proves that the Ven-Obi regimen is comparably safe and efficient for patients with CLL with reduced fitness or comorbidity.
Acknowledgments
The authors thank patients, their families, nurses, and physicians for participating in the trials.
The CLL14 trial was supported by F. Hoffmann-La Roche and AbbVie. The CLL13 trial was funded by F. Hoffmann-La Roche, AbbVie, and Janssen. O.A.-S. received support from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), Projektnummer 524342988. K.F. received support from DFG SFB 1530 project C04.
Authorship
Contribution: O.A.-S., M.F., A.G., S.R., K.F., B.E., and M.H. conducted the analyses, interpreted the data, and wrote the manuscript; E.T., C.S., and S.S. provided the fluorescence in situ hybridization, TP53, and IGHV data; M.R. supervised the minimal residual disease analyses in CLL14; K.-A.K. supervised the flow cytometry analysis; and J.v.T., A.-M.F., F.S., E.T., C.S., L.S., A.S., J.L., R.W., D.S., A.K., G.J., C.d.C.-B., T.I., M.G., P.T., A.J., T.T., V.L., P.S., M.-D.L., C.-M.W., A.P.K., and C.N. reviewed the data and revised the manuscript.
Conflict-of-interest disclosure: O.A.-S. reports consultancy and speakers bureau role with, and honoraria and research funding from, AbbVie, AstraZeneca, Janssen, and Roche; reports membership on an entity’s board of directors or advisory committees with AbbVie, AstraZeneca, Ascentage, Gilead, Janssen, and Roche; and reports speakers bureau role with Adaptive, BeiGene, Eli Lilly, and Gilead. M.F. reports honoraria and research funding from AbbVie; and reports research funding from Roche, Janssen, AstraZeneca, and BeiGene. S.R. reports honoraria from Merck Sharpe & Dohme (MSD). J.v.T. reports consultancy with, honoraria and travel grant from, and speakers bureau role with, AbbVie, Roche, AstraZeneca, and Janssen. A.-M.F. reports honoraria and research funding from AstraZeneca; reports travel support from AbbVie; and received research funding from Celgene. F.S. reports research funding from AstraZeneca; and received travel support from Lilly Pharma. E.T. reports consultancy, honoraria, travel support, and research funding from, and speakers bureau role with, AbbVie and Roche; reports consultancy with, honoraria and travel support from, and speakers bureau role with Janssen-Cilag and AstraZeneca; and reports consultancy with, travel support from, and speakers bureau role with, BeiGene. C.S. reports honoraria from, and speakers bureau role with, AbbVie; reports consultancy with, honoraria from, and speakers bureau role with, AstraZeneca; reports travel support from BeiGene; and reports consultancy with Janssen-Cilag. R.W. reports honoraria and research funding from Janssen; received honoraria from AbbVie; and reports research funding from BioOra. G.J. reports honoraria from AbbVie, Jazz, Novartis, and Servier; and reports research cooperation with Laboratoire Delbert. C.d.C.-B. reports consultancy with, honoraria from, and membership on an entity’s board of directors or advisory committees for AbbVie, AstraZeneca, BeiGene, Janssen, and Octapharma. T.I. reports consultancy with, and membership on an entity’s board of directors or advisory committees for Novartis, AstraZeneca, and AbbVie. M.G. reports consultancy with, and travel support from, AbbVie, AstraZeneca, BeiGene, and Janssen; and received travel support from F. Hoffmann-La Roche. A.J. reports consultancy and speakers bureau role with Novartis, Eli Lilly, Amgen, Sanofi, AbbVie, Gilead, Roche, Janssen-Cilag, Takeda, MSD, AstraZeneca, BeiGene, and Argenx. T.T. reports consultancy with, and honoraria from AbbVie, Roche, Janssen, AstraZeneca, and Takeda; and received research funding from Janssen. C.-M.W. reports consultancy with, honoraria from, membership on an entity’s board of directors or advisory committees for, travel support from, and speakers bureau role with, Hoffmann-La Roche, AbbVie, Janssen, Gilead, AstraZeneca, Lilly Pharma, GlaxoSmithKline (GSK), and BeiGene. K.-A.K. reports consultancy with, research funding from, and speakers bureau role with, Roche, Janssen, and AbbVie. M.R. reports consultancy with, and research funding from, AbbVie; reports consultancy with, and honoraria and travel support from, AstraZeneca; reports consultancy with, and honoraria and research funding from, Roche; and reports consultancy with, and honoraria from, Janssen. S.S. reports consultancy with, and honoraria, travel support, and research funding from, AbbVie, Amgen, AstraZeneca, Celgene, Gilead, GSK, Roche, Janssen, Novartis, and Sunesis. A.P.K. reports consultancy with, and honoraria and research funding from, Lava, AstraZeneca, Bristol Myers Squibb, Genentech, Janssen, and AbbVie. C.N. reports consultancy with, and research funding from, AstraZeneca, Janssen, AbbVie, BeiGene, Genmab, CSL Behring, Octapharma, Takeda, and Novo Nordisk Foundation. K.F. reports honoraria and travel support from AbbVie; reports consultancy with AstraZeneca; and received honoraria and travel support from Roche. B.E. reports consultancy with, and research funding from, Gilead; reports consultancy with, and research funding from, and speakers bureau role with, Janssen; reports consultancy and speakers bureau role with Lilly; reports consultancy with, honoraria from, and speakers bureau role with, MSD; reports honoraria and research funding from, and speakers bureau role with, F. Hoffmann-La Roche; and reports consultancy with, honoraria and research funding from, and speakers bureau role with, AbbVie, BeiGene, and AstraZeneca. M.H. reports consultancy with, and honoraria and research funding from, Janssen, Roche, AstraZeneca, Gilead, BeiGene, and AbbVie. The remaining authors declare no competing financial interests.
Correspondence: Othman Al-Sawaf, Department I of Internal Medicine, Faculty of Medicine and University Hospital Cologne, University of Cologne, Gleueler Str 176, 50937 Cologne, Germany; email: othman.al-sawaf@uk-koeln.de; and Michael Hallek, Department I of Internal Medicine, Faculty of Medicine and University Hospital Cologne, University of Cologne, Gleueler Str 176, 50937 Cologne, Germany; email: michael.hallek@uk-koeln.de.
References
Author notes
Presented, in part, at the annual meeting of the American Society of Hematology, San Diego, CA, 6 December 2023.
The data used in this study are available on request from the corresponding authors, Othman Al-Sawaf (othman.al-sawaf@uk-koeln.de) and Michael Hallek (michael.hallek@uk-koeln.de), for academic noncommercial purposes. Project proposals with the intent-to-achieve aims will be evaluated by the German CLL Study Group and its partners.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal