In this issue of Blood, an international group of experts coordinated by the European Research Initiative on CLL (ERIC) reports consensus recommendations on the diagnosis, workup, and evaluation of treatment responses in Richter transformation of small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL).1
Richter transformation in CLL is defined as development of an aggressive lymphoma that is clonally related to the CLL cells in 80% of cases. This is an uncommon event (2%-10% of CLL transform at a rate of 0.5% of CLLs per year) and is associated with dismal outcome unless allogenic stem cell transplantation can be performed. Risk factors for transformation of CLL include certain B-cell receptor stereotypes, unmutated immunoglobulin heavy chain variable region gene, NOTCH1 mutation, TP53 aberrations (ie, del(17p) and/or TP53 mutations), and complex karyotype.2-4
Richter transformation to Hodgkin lymphoma or plasmablastic lymphoma can occur but the most common subtype of Richter transformation is to diffuse large B-cell lymphoma (DLCBL), and the article by Kittai et al1 focuses on this subtype (see figure). The tumors often retain the genetic aberrations of the underlying CLL and are characterized by alterations in cell cycle regulators, chromatin regulators, NF-κB signaling, and NOTCH signaling. Unfortunately, transformation is also associated with short overall survival even in the era of targeted therapies.
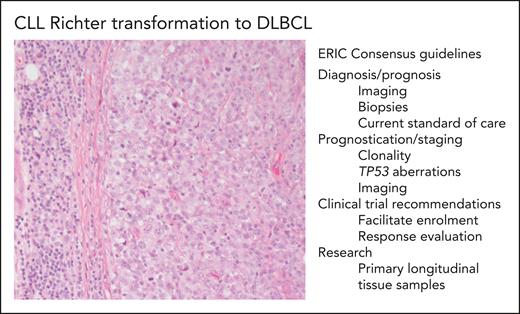
CLL Richter transformation to DLBCL and ERIC consensus guidelines. The photomicrograph (original magnification ×200; hematoxylin and eosin stain) depicts an area of SLL/CLL to the left and an infiltrate of large transformed cells in sheets, which corresponds to Richter transformation to DLBCL, in the right part of the photo. Some of the important points from the international consensus recommendations on diagnosis, evaluation, and research of Richter transformation are listed in the figure.
CLL Richter transformation to DLBCL and ERIC consensus guidelines. The photomicrograph (original magnification ×200; hematoxylin and eosin stain) depicts an area of SLL/CLL to the left and an infiltrate of large transformed cells in sheets, which corresponds to Richter transformation to DLBCL, in the right part of the photo. Some of the important points from the international consensus recommendations on diagnosis, evaluation, and research of Richter transformation are listed in the figure.
Several recommendations on optimal diagnostic workup, regarding both imaging and biopsies, are given. As an example, it might be difficult for the pathologist to distinguish Richter transformation from CLL with enlarged proliferation centers or from “pseudo-Richter transformation” in patients withdrawing from Bruton tyrosine kinase inhibitors (reviewed by Sander et al5), and the recommendations provide advice on how to avoid such pitfalls. The outcome of Richter transformation is much more dismal in patients with a DLBCL clonally related to the underlying CLL than in cases with a “de novo” DLBCL. It is therefore essential to investigate clonal relationship, and established (immunoglobulin gene rearrangement) as well as potential new methods are discussed.
Patients with Richter transformation are often excluded from clinical trials of novel agents for CLL and DLBCL. There are several explanations for this, not least of which is the aggressiveness of the disease, with rapidly declining physical status. Kittai et al strongly recommend that “every effort should be made” to include these patients in clinical trials and provide recommendations on how this goal can be achieved by altering and expanding eligibility criteria and end points.
There are still many unanswered questions regarding the pathogenesis of Richter transformation. Interestingly, small subclones, so-called Richter seeds, carrying genetic and transcriptome profiles of the Richter transformation can be identified already at CLL diagnosis 6 to 19 years before transformation.2 Studies on mechanisms underlying the expansion of these subclones and on how the tissue microenvironment regulates the development of Richter transformation could potentially lead to new therapeutic options. Needless to say, such studies require adequate amounts of biologically banked tissue, preferably also including viable frozen cells, and prospective sample collection at CLL diagnosis, progression, and Richter transformation is recommended.
In summary, Richter transformation of CLL is a rare event, and collaborative studies as well as standardized workup procedures are needed to improve our understanding of disease mechanisms, ultimately leading to better patient outcome. These unifying consensus recommendations for clinicians, pathologists, and researchers are welcome and timely.
Conflict-of-interest disclosure: B.S. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal