Key Points
Iron overload in HFE-related hemochromatosis and thalassemia leads to reversal of the Vδ2+/Vδ2– ratio in the γδ T-cell compartment.
Iron promotes constant Vδ2+-TCR ligand availability by inhibiting pyrophosphatases, leading to Vδ2+ T-cell exhaustion and cell death.
Visual Abstract
HFE-related hemochromatosis induces systemic iron overload. Although extensive studies indicate a pivotal role for iron homeostasis in αβ T-cell immunity, its effect on γδ T cells is unknown. Here, we found a reversal of the Vδ2+/Vδ2– ratio in the γδ T-cell compartment as a feature of hemochromatosis, which is associated with a Vδ2+ population that cannot be enriched by zoledronic acid (ZOL) stimulation, despite evidence of T-cell receptor (TCR)–ligand formation and strong proliferative behavior. In vivo, reactive oxygen species (ROS) production and exhaustion marker expression are significantly increased on Vδ2+ T cells in hemochromatosis compared with healthy individuals. Ex vivo, hemochromatosis donor–derived Vδ2+ cells are hyporesponsive to TCR stimulation in terms of ROS production but significantly increase their paramount expression of exhaustion markers. Fas-Fas ligand coexpression indicates their high susceptibility to activation-induced cell death. Consistent therewith, FeSO4 alone induces Vδ2+ subset-specific proliferation in healthy peripheral blood mononuclear cells comparable to stimulation by ZOL, and blocking experiments identify FeSO4-induced proliferation as BTN3A1/TCR mediated. Pyrophosphate is key for Vδ2+-TCR ligand formation. Iron, by suppressing pyrophosphatase alkaline phosphatase, promotes their stability. Therefore, our data suggest that the transcriptional repression of pyrophosphatases, as under the conditions of iron overload in hemochromatosis in vivo, leads to the constitutive availability of stress-signaling Vδ2+-TCR ligand and permanent TCR triggering in Vδ2+ T cells even under homeostatic conditions, which ultimately results in their subset-specific, activation-induced cell death. A similar phenotype was observed in patients with iron overload due to inborn hemoglobinopathies, suggesting an inverted Vδ2+/Vδ2– ratio in the γδ T-cell compartment as a hallmark of iron overload.
Introduction
HFE-related hemochromatosis (HH) is an autosomal recessive disorder of iron metabolism characterized by the deficiency of hepcidin, which regulates the only identified cellular iron exporter ferroportin. Hepcidin deficiency is caused by homozygous mutations in HFE, a major histocompatibility complex (MHC) class I–like gene that encodes HFE protein.1 The 2 most common mutations of HFE have been identified; C282Y and H63D. Affected individuals increase absorption of dietary iron and release excessive amounts of iron into the plasma, increasing transferrin saturation. Transferrin saturation levels above the physiological range are associated with the formation of non–transferrin-bound iron, which is rapidly taken up by hepatocytes, pancreatic cells, and cardiac myocytes. Cellular iron overload causes cellular injury by increasing the production of reactive oxygen species (ROS).1 In lymphocytes, ROS support activation-induced cell death (AICD)2 by inducing Fas-L,3,4 assist caspase 3 in inducing ROS-dependent cell death,5 amplify the release of apoptogenic factors from the mitochondria, and increase oligonucleosomal DNA fragmentation.6 Clinical consequences of tissue iron overload include liver cirrhosis, hepatocellular carcinoma (HCC), diabetes, heart failure, arthritis, and hypogonadism. Mainstay therapies are phlebotomy and iron chelation in some patients.7
Iron homeostasis is critical for T-cell immunity, as T cells require iron for proliferation and effector functions. Either iron overload or deficiency adversely affects adaptive immune responses.8,9 Significantly associated with iron overload and severity of disease are low total lymphocyte counts,10-12 abnormal CD4/CD8 ratios, reduced CD8+CD28+ and cytotoxic T lymphocytes,13,14 and increased levels of interleukin-4 (IL-4) and IL-10 produced by CD8+ T cells, promoting TH2 polarization.12
Reduced immune surveillance has been associated with increased cancer risk,15 particularly HCC, and predisposition to infections with otherwise rare bacterial species,16-18 highlighting that T-cell function and iron metabolism are intimately coupled.19
γδ T cells, an important subset of innate lymphocytes, are pivotal for the clearance of infected, dysregulated, and malignant cells. They directly recognize a broad range of stress-related (self-) antigens in the MHC independently. As the first line of defense, they eliminate their targets directly through cytolysis or activation of other immune cells. Their activation entails the production of cytokines, regulates pathogen clearance, and promotes inflammation and antigen presentation, while maintaining tolerance to self and integrity of the tissues in response to stresses.20
According to their Vδ chain, γδ T cells comprise 2 major subsets. Vδ1+ T cells predominate in thymus, mucosa, and epithelial tissues, whereas Vδ2+ T cells are mostly found in blood γδ T cells. The T-cell receptor (TCR) δ chain of Vδ2+ T cells almost exclusively associates with Vγ9 forming Vγ9Vδ2-TCRs, which mainly recognize phosphoantigens (pAgs), that is, phosphorylated nonpeptidic molecules such as (E)-4-hydroxy-3-methylbut-2-enylpyrophosphate synthesized in the bacterial isoprenoid biosynthesis pathway and isopentenyl pyrophosphate (IPP), intermediates of cholesterol biosynthesis in eukaryotic cells. Zoledronic acid (ZOL), by inhibiting farnesyl pyrophosphate synthase of the cholesterol biosynthetic pathway, results in IPP accumulation (supplemental Figure 1, available on the Blood website). For the activation of Vγ9Vδ2-T cells by pAgs, butyrophilins (BTN) are important.21 The composite ligand model assumes BTN2A1 and BTN3A1 extracellular domains being adjacent,22,23 forming a Vγ9Vδ2-TCR ligand to which germ line–encoded regions of TCRVγ9 bind (supplemental Figure 1). pAgs, by binding the intracellular BTN3A1 B30.2 domain, glue BTN3A1 and BTN2A1 together.23,24
Despite the importance of iron homeostasis for overall T-cell immunity and accumulating evidence for its pivotal role in αβ T-cell function, the effect of iron overload on γδ T-cell immunity remains to be elucidated. In this study, to our knowledge, we provide the first and fundamental evidence revealing iron homeostasis and γδ T-cell homeostasis are intimately linked.
Materials and methods
Study approval
This study was in accordance with the ethical standards of the institutional ethic committee of the Medical Faculty of the Eberhard-Karl-University Tübingen; approved reference: 244/2023B02. All participants included gave written informed consent.
Study participants
According to new nomenclature, 33 volunteers diagnosed with having HFE-related hemochromatosis were enrolled: 22 individuals homozygous for C282Y and 11 individuals with compound hemochromatosis (C282Y/H63D). The patients were of European descent, aged 42 to 88 years (70.7 ± 12.6), diagnosed at 48.5 ± 10.8 years, and all presented with clinical signs of iron overload (severe joint pain [29/33], significant heart problems [4/33], [pre]diabetes [15/33], fatigue and/or weakness [33/33], HCC [1/33]). Patients were managed to ensure that serum iron metabolism parameters follow the recommended range of the guidelines (transferrin saturation ≤45%, ferritin levels between 100 and 300 ng/mL in men and 200 ng/mL in women, hepcidin 12-150 ng/mL in women and up to 300 ng/mL in men). All are on maintenance therapy with 2 to 6 phlebotomies per year, and none of the patients received chelation or hepcidin. In addition, none had/needs intensive treatment. Blood samples were taken from freshly phlebotomized blood. Controls were taken from age- and sex-matched healthy donors (HDs).
Non–HFE-related iron overload was evaluated in 23 individuals aged 2 to 17 years, who had thalassemia major (n = 21) or congenital dyserythropoietic anemia (n = 2). All patients were in a chronic transfusion program for several years and, despite chelation, had signs of moderate-to-severe iron overload. Mean ferritin value in this cohort was 2439 ± 1973 μg/L, and T2 iron was 190.8 ± 148.6 μmol/g dry liver weight.
PBMC isolation and cultivation
Peripheral blood mononuclear cells (PBMCs), isolated through density gradient centrifugation (Biocoll Separating Solution; Biochrom Merck, Darmstadt, Germany), were cultured in RPMI-1640 (Sigma-Aldrich, St Louis, MO), 10% fetal bovine serum (Thermo Fisher Scientific, Darmstadt, Germany), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (both from Sigma-Aldrich).
Cell sorting and purification
Vδ2+ T cells were positively isolated with phycoerythrin–anti-Vδ2 monoclonal antibody (supplemental Table 1) and antiphycoerythrin microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) or untouched with a custom-made γδ T-cell isolation kit (StemCell Technologies, Vancouver, Canada) and subsequent Vδ1+ T-cell depletion using fluorescein isothiocyanate (FITC)–anti-Vδ1 monoclonal antibody (supplemental Table 1) and anti-FITC microbeads (Miltenyi Biotec). CD3+ cells were isolated using EasySep Human T-Cell Isolation Kit (StemCell Technologies), CD4+ and CD8+ cells using CD4 Positive Isolation Kit (Thermo Fisher Scientific, Waltham, MA) and CD8 microbeads (Miltenyi Biotec) according to manufacturers’ manuals. Purity of isolated cells was consistently >98%.
Stimulation with ZOL, anti-CD3/CD28 beads, and IMMU510
PBMCs (3 × 106/mL) were cultivated in RPMI-1640 supplemented with IL-2 (30 U/mL; Proleukin; Clinigen, Devault, PA), IL-15 (10 ng/mL; ImmunoTools, Friesoythe, Germany), and ZOL (5 μM, Fresenius, Bad Homburg, Germany). Where indicated, isolated T cells were stimulated with anti-CD3/CD28 beads (Dynabeads, Thermo Fisher Scientific) at a 1:1 bead/cell ratio and proliferation was analyzed on day 7. For anti-TCRCδ stimulation, culture plates were coated with anti-panγδ clone IMMU510 (BD Pharmingen; 1 μg/mL in phosphate-buffered saline with Ca2+/Mg2+, at room temperature, 1 hour), and medium was changed on days 3 and 6.
Cell proliferation measurement
Cell Trace Violet (CTV; Thermo Fisher Scientific)-labeled PBMCs were ZOL stimulated and costained with anti–CD3-PerCP/anti–Vδ2-FITC when analyzed for proliferation on a BD-Canto-II.
Iron chelation treatment
CTV-labeled PBMCs were incubated for 14 hours at 37°C with titrated deferoxamine (DFO, Bio-Techne, Minneapolis, MN), harvested, washed, and reseeded in new plates, and ZOL-stimulated. Proliferation was evaluated on day 7.
Coculture/cross-experiments of Vδ2+ γδ T cells with irradiated autologous or allogeneic PBMCs
Purified, CTV-labeled Vδ2+ T cells were cocultured 1:1 with allogeneic, αβ-depleted PBMCs from irradiated ZOL-pulsed PBMCs (50 Gy), and proliferation was analyzed on day 7.
ROS assay
ROS production was measured with ROS Assay Kit (Thermo Fisher Scientific) following the manufacturer’s protocol.
FeSO₄ spike and dose-response assay
PBMCs were CTV labeled and incubated with ZOL and/or titrated FeSO4, and proliferation of Vδ2+ T cells was assessed by fluorescence-activated cell sorter (FACS) on day 7.
BTN3A1 blockade
Mouse anti-BTN3A1 antibody (clone 103.2; 10 μg/mL) was added to CTV-labeled ZOL-pulsed PBMCs with or without titrated FeSO4, and proliferation of Vδ2+ T cells was assessed on day 7 by FACS.
Flow cytometry
Antibodies are provided in supplemental Table 1. Cells were acquired with BD-FACS-Canto-II, and data were analyzed with FlowJo version 10.8 software (BD Life Sciences), with doublets/dead cells excluded by gating on singlets (forward scatter area/forward scatter height) and viable cells (7-aminoactinomycin D [7-AAD] staining), BD Biosciences, Heidelberg, Germany, or Zombie Aqua Fixable Viability Kit, BioLegend, San Diego, CA).
RNA purification and cDNA synthesis
RNA was extracted with Quick-RNA Miniprep Kit (Zymo-Research, Irvine, CA), and complementary DNA was synthesized with SuperScript III First-Strand Synthesis Super Mix (Life Technologies).
Real-time PCR (quantitative PCR)
Quantitative polymerase chain reaction (PCR) was performed with SYBR Green (Promega, Madison, WI); the primers are provided in supplemental Table 2.
Statistical analysis
Statistical tests were executed with GraphPad Prism. Two groups of normally distributed data were compared with equal variance t test, >2 groups were compared with analysis of variance, and comparisons between 2 groups of nonparametric data were performed with Mann-Whitney U or Kruskal-Wallis test. P < .05 was considered statistically significant.
Results
Altered T-cell compartment in the peripheral blood of patients with HH
As evidenced in previous studies, individuals with HH have significantly higher CD4+/CD8+ αβ T-cell ratios than HDs (Figure 1A).13,14,25 The proportion of γδ T cells relative to αβ T cells among CD3+ cells is not different between HH and HDs (Figure 1B). Vδ2+ T cells are usually the most γδ T cells in the blood of HD; however, we describe, to our knowledge, for the first time a reversal of the Vδ2+/Vδ2– ratio in the γδ T-cell compartment, that is, a significantly higher Vδ2– and a significantly lower Vδ2+ T-cell proportion in the peripheral γδ T-cell pool in individuals with HH compared with HDs (Figure 1C-D). Vδ1 and Vδ3 γδ T cells have a similar effector phenotype and are referred to as Vδ2– T cells. Vδ3+ T cells constitute a minor lymphocyte subset in the blood but are enriched in the liver and in patients with some chronic (viral) infections.26-28
Parameters and T-cell subset compositions in the T-cell compartment of HDs, and individuals with and without HH with chronic iron overload; iron transport molecules are differentially expressed by T-cell subsets. (A) Healthy individuals (n = 30) have significantly less CD4+ and more CD8+ T cells than patients with chronic iron overload (compound n = 11, C282Y n = 22). (B) γδ T-cell absolute number is not significantly different in HFE (n = 33) and HD (n = 30). (C) Patients with HH (n = 33) have significantly more Vδ2– T cells (Vδ2–) than HD (n = 30) and highly significantly fewer Vδ2+ T cells. (D) Vδ2+/Vδ2– ratio is inverted in patients with HH. (E) Expression of molecules involved in Fe import (ZIP8, ZIP1, DMT1) and Fe export (ferroportin) revealed for different T-cell subsets (n = 3 for Vδ1 and Vδ2; n = 5 for CD4+ and CD8+). ∗P < .05; ∗∗∗∗P < .0001; DMT1, divalent metal transporter 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ns, not significant; ZIP, zinc transporter.
Parameters and T-cell subset compositions in the T-cell compartment of HDs, and individuals with and without HH with chronic iron overload; iron transport molecules are differentially expressed by T-cell subsets. (A) Healthy individuals (n = 30) have significantly less CD4+ and more CD8+ T cells than patients with chronic iron overload (compound n = 11, C282Y n = 22). (B) γδ T-cell absolute number is not significantly different in HFE (n = 33) and HD (n = 30). (C) Patients with HH (n = 33) have significantly more Vδ2– T cells (Vδ2–) than HD (n = 30) and highly significantly fewer Vδ2+ T cells. (D) Vδ2+/Vδ2– ratio is inverted in patients with HH. (E) Expression of molecules involved in Fe import (ZIP8, ZIP1, DMT1) and Fe export (ferroportin) revealed for different T-cell subsets (n = 3 for Vδ1 and Vδ2; n = 5 for CD4+ and CD8+). ∗P < .05; ∗∗∗∗P < .0001; DMT1, divalent metal transporter 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ns, not significant; ZIP, zinc transporter.
To determine whether molecules involved in cellular iron metabolism have a subset-specific deleterious effect, Fe2+-importer and -exporter molecules were investigated. Owing to the scarcity of Vδ2+ T cells in patients with HH, T-cell subsets of HDs were investigated. αβ T-cell and γδ T-cell subsets have similar expression of the Fe2+ importers (ZIP8, ZIP14, DMT1) (Figure 1E). The only known Fe2+ exporter, ferroportin, is expressed at low levels by CD4+ T cells, marginally by CD8+ T cells, but not by γδ T cells. The latter makes it unlikely that the absence of the Fe2+ transporter is responsible for the unchanged total number of γδ T cells with a selectively reduced Vδ2+ T-cell subset in patients with HH.
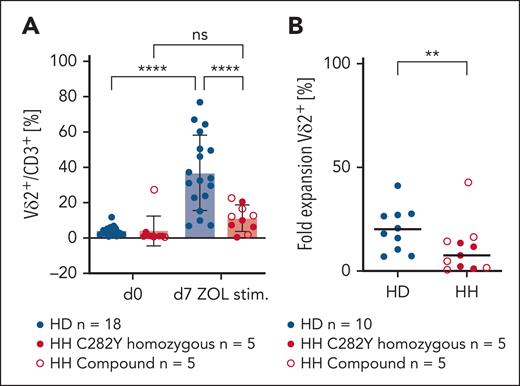
No increase of Vδ2+ T cells after stimulation with ZOL in patients with HH
To investigate functionality of Vδ2+ T cells that are significantly lower in number in individuals with HH compared with HDs, PBMCs from HDs and patients with HH were stimulated in vitro with ZOL. As found in Figure 2A-B, ZOL stimulation did not enrich Vδ2+ T cells in PBMCs from individuals with HH; fold-expansion rate is significantly lower in individuals with HH compared with that in HDs.
Vδ2+ T-cell numbers do not increase in PBMCs of patients with HH in response to ZOL stimulation. (A) The Vδ2+ T-cell pool does not expand after ZOL stimulation in PBMCs of patients with chronic iron overload (C282Y n = 5, compound n = 5) compared with PBMCs of HD (n = 18). Revealed are percentages of Vδ2+ T cells per CD3+ before and after ZOL stimulation (day 7). Vδ2+ proportion/CD3+ is significantly increased on day 7 compared with day 0 in HD PBMCs (analysis of variance, P < .0001) but not in PBMCS of individuals with HFE (P = .6582). (B) Fold-expansion rate of Vδ2+ subset is significantly different in HD (n = 10) and HH (n = 5 C292Y, n = 5 compound) (P = .093). ∗∗P < .01; ∗∗∗∗P < .0001; ns, not significant.
Vδ2+ T-cell numbers do not increase in PBMCs of patients with HH in response to ZOL stimulation. (A) The Vδ2+ T-cell pool does not expand after ZOL stimulation in PBMCs of patients with chronic iron overload (C282Y n = 5, compound n = 5) compared with PBMCs of HD (n = 18). Revealed are percentages of Vδ2+ T cells per CD3+ before and after ZOL stimulation (day 7). Vδ2+ proportion/CD3+ is significantly increased on day 7 compared with day 0 in HD PBMCs (analysis of variance, P < .0001) but not in PBMCS of individuals with HFE (P = .6582). (B) Fold-expansion rate of Vδ2+ subset is significantly different in HD (n = 10) and HH (n = 5 C292Y, n = 5 compound) (P = .093). ∗∗P < .01; ∗∗∗∗P < .0001; ns, not significant.
Individuals with HH are able to produce a functional Vδ2+-TCR ligand
Fe2+ can chelate pyrophosphates (https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-ferrous-pyrophosphate). Thus, one hypothesis could be that Fe2+ prevents IPP from binding to BTN3A1/BTN2A1 cytoplasmic B30.2 domains, hindering ligand formation (supplemental Figure 2). Consequently, poor Vδ2+ expansion might result from impaired/absent activating Vδ2+-TCR ligand in HH. Therefore, we next investigated whether iron-chelating DFO improves Vδ2+ T-cell expansion in patients with HH. Toxicity on T cells by DFO itself was ruled out as found in supplemental Figure 3A-B. Depleting Fe2+ from the medium (100 μM DFO) completely abolished Vδ2+ T-cell proliferation in ZOL-stimulated PBMCs from HDs (Figure 3A). Yet, a physiological range within which T cells can proliferate was identified (Figure 3B, arrow). Graded DFO concentrations had a dose-dependent inhibition of Vδ2+ T-cell proliferation in ZOL-stimulated cultures of HDs and individuals with HH. Vδ2+ T-cell proliferation was inhibited even at lower concentrations in patients with HH than in HDs (Figure 3C). This argues against Fe2+ chelating IPP and preventing TCR-mediated stimulation as a cause of low Vδ2+ accumulation in HH, making hypothesis 1 unlikely.
DFO does not increase the yield of HH Vδ2+ T cells, and HD Vδ2+ cells proliferate vigorously when added to allogeneic TCRαβ-depleted HH PBMCs. (A) 100 μM DFO completely blocks proliferation of Vδ2+ T cells in ZOL-stimulated HD PBMCs (n = 7). (B) Dose titration of DFO to identify a physiological window for T-cell proliferation (n = 1). (C) DFO does not improve the proliferation and yield of Vδ2+ T cells from patients with HH in ZOL-stimulated cultures (n = 3). Compared with ZOL-stimulated PBMCs from HD (n = 2), Vδ2+ T-cell proliferation from individuals with HH (n = 3) is negatively and dose dependently affected at even lower concentrations of DFO than their counterparts from healthy individuals. (D) HH Vδ2+ T cells (n = 3) proliferate when stimulated with ZOL in irradiated PBMCs from HDs, but the increase in number is significantly less than that found in HD Vδ2+ T cells (n = 3), which reveal normal, strong proliferation when stimulated in irradiated PBMCs from patients with HH. Overlay: Superimposed CTV histogram for HD Vδ2+ T cells responding in HH PBMCs (blue) vs HH Vδ2+ T cells responding in autologous PBMCs (red). ∗∗∗∗P < .0001; ns, not significant.
DFO does not increase the yield of HH Vδ2+ T cells, and HD Vδ2+ cells proliferate vigorously when added to allogeneic TCRαβ-depleted HH PBMCs. (A) 100 μM DFO completely blocks proliferation of Vδ2+ T cells in ZOL-stimulated HD PBMCs (n = 7). (B) Dose titration of DFO to identify a physiological window for T-cell proliferation (n = 1). (C) DFO does not improve the proliferation and yield of Vδ2+ T cells from patients with HH in ZOL-stimulated cultures (n = 3). Compared with ZOL-stimulated PBMCs from HD (n = 2), Vδ2+ T-cell proliferation from individuals with HH (n = 3) is negatively and dose dependently affected at even lower concentrations of DFO than their counterparts from healthy individuals. (D) HH Vδ2+ T cells (n = 3) proliferate when stimulated with ZOL in irradiated PBMCs from HDs, but the increase in number is significantly less than that found in HD Vδ2+ T cells (n = 3), which reveal normal, strong proliferation when stimulated in irradiated PBMCs from patients with HH. Overlay: Superimposed CTV histogram for HD Vδ2+ T cells responding in HH PBMCs (blue) vs HH Vδ2+ T cells responding in autologous PBMCs (red). ∗∗∗∗P < .0001; ns, not significant.
To explore whether a functional Vδ2+-TCR ligand is present, we investigated whether Vδ2+ T cells from HDs can proliferate when cocultured with ZOL-pulsed PBMCs from individuals with HH (cross-experiments). As found in Figure 3D, ZOL induced profound proliferation and expansion of Vδ2+ T cells from HDs when PBMCs from individuals with HH (HH1-3) served as antigen-presenting cells, indicating that a functional Vδ2+-TCR ligand is present in patients with HH.
In response to diverse TCR-targeted stimuli, Vδ2+ T cells of patients with HH proliferate and expand, demonstrating that they are functional
Functionality of Vδ2+ cells of patients with HH and HDs was tested with TCR/CD3-driven stimuli. Although a proportion of proliferating Vδ2+ T cells was not significantly different between ZOL-stimulated HH and HDs, fold expansion of Vδ2+ T cells was significantly lower in patients with HH (Figure 4A). Surprisingly, in PBMCs from patients with HH, highly proliferating Vδ2+ T cells with a CTV signal ≤103 were present after ZOL, anti-CD3/CD28, or IMMU510 stimulation (Figure 4B). Following panγδ/TCRCδ-targeting IMMU510 stimulation of PBMCs, a lower proportion of proliferating Vδ2+ T cells were induced in patients with HH compared with HD, but expansion yields were similar; after anti-CD3/CD28 stimulation of PBMCs, proliferation and expansion of Vδ2+ T cells were slightly enhanced in patients with HH compared with HDs (Figure 4A). Thus, although proportion of proliferating Vδ2+ cells was high, expansion was low, suggesting Vδ2+-TCR triggering is possible but survival of these cells might be compromised in patients with HH.
Proliferation and expansion of Vδ2+ T cells in response to graded TCR stimulation. (A) Proliferation and expansion statistics for experiments found in (B). Number of proliferating Vδ2+ cells corresponds to the integral under the curve, highly proliferating cells refers to proliferating Vδ2+ cells with CTV intensity ≤103. The fold expansion of Vδ2+ T cells was calculated as follows: ([Vδ2+ T cells per CD3+ T cells in PBMCs [%] after stimulation])/(Vδ2+ T cells per CD3+ T cells in PBMCs [%] before stimulation). (B) CTV FACS plots of Vδ2+ T cells from HH (n = 3) and HDs (n = 3) after stimulation with ZOL (Vδ2Vγ9-specific stimulation), IMMU510 (pan γδ stimulation), and αCD3/αCD28 (pan T-cell stimulation) in PBMCs ∗P < .05; ∗∗P < .01;. ns, not significant.
Proliferation and expansion of Vδ2+ T cells in response to graded TCR stimulation. (A) Proliferation and expansion statistics for experiments found in (B). Number of proliferating Vδ2+ cells corresponds to the integral under the curve, highly proliferating cells refers to proliferating Vδ2+ cells with CTV intensity ≤103. The fold expansion of Vδ2+ T cells was calculated as follows: ([Vδ2+ T cells per CD3+ T cells in PBMCs [%] after stimulation])/(Vδ2+ T cells per CD3+ T cells in PBMCs [%] before stimulation). (B) CTV FACS plots of Vδ2+ T cells from HH (n = 3) and HDs (n = 3) after stimulation with ZOL (Vδ2Vγ9-specific stimulation), IMMU510 (pan γδ stimulation), and αCD3/αCD28 (pan T-cell stimulation) in PBMCs ∗P < .05; ∗∗P < .01;. ns, not significant.
ROS production and exhaustion marker expression in Vδ2+ T cells from patients with HH
T cells physiologically increase Fe import on TCR triggering29 with increased intracellular Fe, promoting ROS production.29 Although low-to-moderate ROS levels are essential for cell survival and proliferation,30-32 excess production of ROS overwhelms the antioxidant systems, leading to DNA damage,30,31,33 altered metabolism,31,34,35 T-cell exhaustion,36 and AICD2,37 by inducing Fas-L3,4 and strengthening extracellular signal-regulated kinase–mediated pathways.38,39 Therefore, we aimed to identify whether AICD follows Vδ2+ T-cell exhaustion accompanied by overwhelming ROS production in patients with HH.
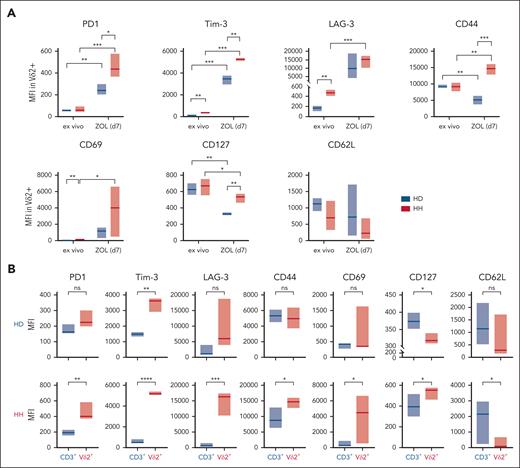
T-cell exhaustion, induced by chronic stimulation,40 is characterized by sustained expression of programmed cell death protein 1 (PD1) in combination with a bundle of markers,41,42 including lymphocyte-activation gene 3 (LAG-3) and T-cell immunoglobulin and mucin-domain containing-3 (TIM-3).43 In contrast to healthy individuals, in vivo Vδ2+ T cells from patients with HH carry these markers and express an early activation antigen CD69 (Figure 5A; supplemental Figure 4). ZOL stimulation further drastically increases the expression of exhaustion markers on HH Vδ2+ T cells, reaching levels above those of ZOL-stimulated Vδ2+ T cells of HDs (Figure 5A; supplemental Figure 4). CD62L shedding from antigen-activated T cells, which prevents their re-entry into the peripheral lymph nodes,44 is a sign of activated T cells,45 as is the downregulation of CD127 because IL-2 secreted by antigen-stimulated T cells downregulates the IL-7 receptor.46 Interestingly, Vδ2+ cells downregulated CD127 significantly weaker in individuals with HH vs individuals with HD, consistent with lower cytokine production in exhausted T cells47 (Figure 5A; supplemental Figure 4).
Exhaustion markers expressed in Vδ2+ cells and CD3+ cells before and after ZOL stimulation. (A) MFIs of the respective exhaustion markers reveal that ZOL-stimulated HH Vδ2+ T cells upregulate the exhaustion marker (HH n = 4) highly significantly compared with healthy controls (HD n = 3). (B) ZOL induces the significant and subset-specific expression of exhaustion markers in Vδ2+ cells but not in the CD3+ compartment of individuals with HH (n = 4) and, besides the activation marker Tim-3, neither in Vδ2+ cells nor in CD3+ cells of HDs (n = 3). The population designated as CD3+ includes all T cells except Vδ2+ cells, that is, αβ+, Vδ1+, and Vδ1–/Vδ2– T cells. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; MFI, mean fluorescence intensity; ns, not significant.
Exhaustion markers expressed in Vδ2+ cells and CD3+ cells before and after ZOL stimulation. (A) MFIs of the respective exhaustion markers reveal that ZOL-stimulated HH Vδ2+ T cells upregulate the exhaustion marker (HH n = 4) highly significantly compared with healthy controls (HD n = 3). (B) ZOL induces the significant and subset-specific expression of exhaustion markers in Vδ2+ cells but not in the CD3+ compartment of individuals with HH (n = 4) and, besides the activation marker Tim-3, neither in Vδ2+ cells nor in CD3+ cells of HDs (n = 3). The population designated as CD3+ includes all T cells except Vδ2+ cells, that is, αβ+, Vδ1+, and Vδ1–/Vδ2– T cells. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; MFI, mean fluorescence intensity; ns, not significant.
Importantly, the expression of exhaustion markers is subset specific. In both patients with HH and HDs, ZOL stimulation exclusively and significantly induces upregulation of PD1, TIM-3, LAG-3, CD69, and CD44 on Vδ2+ T cells, but not on other T-cell subsets, including αβ, Vδ1, and Vδ1–/Vδ2– γδ T cells (Figure 5B). Next, ROS expression after T-cell activation29 was monitored at days 0, 3, 6, and 9 in Vδ2+ T cells in response to the following 3 different activating signals: (1) IL-2/15, which regulates TCR-independent T-cell activation/proliferation; (2) IL-2/15 + phorbol 12-myristate 13-acetate (PMA), a potent pan T-cell stimulation by activating protein kinase C and extracellular signal-regulated kinase pathways; and (3) IL-2/IL-15 + ZOL, that is, Vδ2+ subset-specific, TCR-dependent stimulation. In contrast to significantly higher ROS levels ex vivo on day 0, stimulation with cytokines with or without ZOL resulted in discrete but significantly lower ROS production on days 3, 6, and 9 in Vδ2+ T cells from patients with HH compared with HDs (Figure 6A). The finding that Vδ2+ T cells of individuals with HH are unable to produce ROS above baseline in response to subset-specific TCR stimulation (Figure 6A) is in line with selective autophagic degradation of ROS-producing mitochondria, termed mitophagy, to protect the cell.48-51 Whereas supraphysiological TCR stimulation likely reaches the critical threshold for mitophagy in chronically stimulated Vδ2+ T cells from patients with HH, Vδ2+ T cells from HDs generate ROS on stimulation with ZOL with an oscillatory curve that peaks at day 6 (Figure 6A).
ROS production in Vδ2+ T cells in differentially stimulated cultures and expression of Fas/Fas-L in ZOL-stimulated Vδ2+ vs CD3+ cells. (A) ROS produced in Vδ2+ T cells on days 0, 3, 6, and 9 in IL-2/IL-15, IL-2/IL-15/PMA, and IL-2/IL-15/ZOL-stimulated PBMCs (n = 3), respectively. Half of the medium was changed on days 3 and 6 and supplemented with IL-2/IL-15, as indicated by arrows. (B) Expression of AICD-mediating death receptor Fas (CD95) and its ligand Fas-L (CD95L) are specifically and significantly higher expressed in ZOL-activated Vδ2+ cells from patients with HH (n = 3) than Vδ2+ cells from HDs (n = 3) and the respective CD3+ compartments in HH and HD. The population designated as CD3+ includes all T cells except Vδ2+ cells, that is, αβ+, Vδ1+, and Vδ1–/Vδ2– T cells. (C) 7-AAD was used to distinguish viable from apoptotic/dead cells (for gating strategy, see supplemental Figure 5). Fas is expressed by all ZOL-stimulated Vδ2+ cells that is, in live and apoptotic condition in HH and HD; Fas-L is only detected in apoptotic gates and significantly higher expressed by ZOL-stimulated Vδ2+ cells from individuals with HH than HDs. MFI of Fas and Fas-L are comparably high for living Vδ2+ cells but significantly higher for apoptotic Vδ2+ cells of HH vs HD PBMCs. (D) Percentage of apoptotic Vδ2+ cells is significantly higher in ZOL-stimulated PBMCs HH (n = 4) compared with HDs (n = 3). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; MFI, mean fluorescence intensity; ns, not significant; PMA, phorbol 12-myristate 13-acetate.
ROS production in Vδ2+ T cells in differentially stimulated cultures and expression of Fas/Fas-L in ZOL-stimulated Vδ2+ vs CD3+ cells. (A) ROS produced in Vδ2+ T cells on days 0, 3, 6, and 9 in IL-2/IL-15, IL-2/IL-15/PMA, and IL-2/IL-15/ZOL-stimulated PBMCs (n = 3), respectively. Half of the medium was changed on days 3 and 6 and supplemented with IL-2/IL-15, as indicated by arrows. (B) Expression of AICD-mediating death receptor Fas (CD95) and its ligand Fas-L (CD95L) are specifically and significantly higher expressed in ZOL-activated Vδ2+ cells from patients with HH (n = 3) than Vδ2+ cells from HDs (n = 3) and the respective CD3+ compartments in HH and HD. The population designated as CD3+ includes all T cells except Vδ2+ cells, that is, αβ+, Vδ1+, and Vδ1–/Vδ2– T cells. (C) 7-AAD was used to distinguish viable from apoptotic/dead cells (for gating strategy, see supplemental Figure 5). Fas is expressed by all ZOL-stimulated Vδ2+ cells that is, in live and apoptotic condition in HH and HD; Fas-L is only detected in apoptotic gates and significantly higher expressed by ZOL-stimulated Vδ2+ cells from individuals with HH than HDs. MFI of Fas and Fas-L are comparably high for living Vδ2+ cells but significantly higher for apoptotic Vδ2+ cells of HH vs HD PBMCs. (D) Percentage of apoptotic Vδ2+ cells is significantly higher in ZOL-stimulated PBMCs HH (n = 4) compared with HDs (n = 3). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; MFI, mean fluorescence intensity; ns, not significant; PMA, phorbol 12-myristate 13-acetate.
ROS regulates AICD by inducing death receptor Fas.34 Although ZOL stimulation induced comparable level of Fas on αβ T cells and Vδ1 and Vδ1–/Vδ2– γδ T cells in individuals with HH and HDs, Vδ2+ T cells from patients with HH express Fas significantly higher than αβ T cells and Vδ1 and Vδ1–/Vδ2– γδ T cells from HDs (Figure 6B). Fas and Fas-L are comparably high expressed in live gates but significantly higher by apoptotic Vδ2+ T cells of HH vs HD PBMCs (Figure 6C). The same expression pattern applies to Fas-L (Figure 6C). Fas/Fas-L coexpression enables fratricide by cis and trans signaling and killing in trans by bystander cells.52 Selective Fas/Fas-L coexpression on Vδ2+ T cells from individuals with HH demonstrates their high susceptibility to AICD (Figure 6D) and is evidenced by 7-AAD viability staining, which reveals a significantly higher proportion of apoptotic, Fas/Fas-L coexpressing Vδ2+ cells in ZOL-stimulated Vδ2+ cells from HH compared with HD samples (supplemental Figure 5).
Iron selectively induces TCR-mediated proliferation of Vδ2+ T cells in HDs
To confirm mechanistically that iron leads to selective proliferation of Vδ2+ T cells, we reveal in spike experiments that titrated administration of exogenous Fe2+ at concentrations observed in patients with HH (10-100 μM) dose dependently and selectively stimulates proliferation of Vδ2+ T cells in HDs (Figure 7A; supplemental Figure 6) and the expression of diverse activation markers (supplemental Figure 7). The proliferation induced by 25 μM FeSO4 is as strong as that induced by maximal stimulation with ZOL (Figure 7A). Blocking BTN3A1 using a BTN3A1-targeting antibody 103.2 canceled this effect (Figure 7B). This demonstrates that FeSO4-induced proliferation of Vδ2+ T cells in HD is BTN3A1 mediated, thus TCR mediated. In contrast, Vδ2+ T cells in individuals with HH exhibited significantly lower expansion (Figure 7B). They proliferate only at higher Fe concentrations and do not reach ZOL-induced proliferation levels (Figure 7A-B; supplemental Figure 6).
Iron selectively induces proliferation of Vδ2+ T cells in HDs, represses ALP transcription in primary tissues and cell lines, and iron overload is associated with the same phenotype in thalassemia (Th) as in individuals with HH. (A) Fe2+ spiked into HD PBMCs at concentrations observed in HH (10-100 μM) dose dependently and selectively stimulates Vδ2+ T-cell proliferation (supplemental Figure 3). HH Vδ2+ T cells have a significant lower replication index (fold expansion of responding Vδ2+ T cells) and a significantly lower expansion index (fold expansion of whole Vδ2+ cell population) than HD Vδ2+ T cells. (B) Fe2+-induced proliferation (n = 5) can be abrogated by BTN3A1-blocking antibody 103.2 in HD (open bars) (n = 5). (C) Fe2+ potently represses ALP transcription in primary tissues and derived cell lines. RNA was isolated 3 days after exposure to titrated Fe2+, allowing a meaningful chronological timeframe for transcriptional repression and measuring resulting proliferation of Vδ2Vg9 (analyzed at day 7). (D) Patients with Th major (HFE-independent iron overload n = 26) exhibit same phenotypic abnormalities in the γδ T-cell compartment as individuals with HH (C282Y n = 22, compound n = 11) compared with healthy individuals (n = 30). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; ns, not significant.
Iron selectively induces proliferation of Vδ2+ T cells in HDs, represses ALP transcription in primary tissues and cell lines, and iron overload is associated with the same phenotype in thalassemia (Th) as in individuals with HH. (A) Fe2+ spiked into HD PBMCs at concentrations observed in HH (10-100 μM) dose dependently and selectively stimulates Vδ2+ T-cell proliferation (supplemental Figure 3). HH Vδ2+ T cells have a significant lower replication index (fold expansion of responding Vδ2+ T cells) and a significantly lower expansion index (fold expansion of whole Vδ2+ cell population) than HD Vδ2+ T cells. (B) Fe2+-induced proliferation (n = 5) can be abrogated by BTN3A1-blocking antibody 103.2 in HD (open bars) (n = 5). (C) Fe2+ potently represses ALP transcription in primary tissues and derived cell lines. RNA was isolated 3 days after exposure to titrated Fe2+, allowing a meaningful chronological timeframe for transcriptional repression and measuring resulting proliferation of Vδ2Vg9 (analyzed at day 7). (D) Patients with Th major (HFE-independent iron overload n = 26) exhibit same phenotypic abnormalities in the γδ T-cell compartment as individuals with HH (C282Y n = 22, compound n = 11) compared with healthy individuals (n = 30). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001; ns, not significant.
Iron-mediated Vδ2+ proliferation/enrichment was also observed in vivo: Increasing iron level in iron deficient (ID) uninflamed patients through a single-dose iron IV (1 g Fe/70 kg body weight = ∼250 μM) leads to the activation/proliferation/enrichment of Vδ2+ cells, but the deletion of this already exhausted Vδ2+ subset in patients with chronic low-grade inflammation (supplemental Figure 8).
Fe2+ significantly represses ALP transcription in primary tissue and cell lines
Iron promotes pyrophosphate stability, which is key for Vδ2+-TCR ligand formation, by inhibiting pyrophosphatases. Multiple studies reveal decreased phosphatase activity as a result of iron treatment,53-58 and synovial iron sequestration and inhibition of pyrophosphatases by iron have been suggested as pathophysiological post-transcriptionally regulated mechanisms leading to joint damage, a pathophysiology shared by individuals with HH and osteoarthritis.59,60 TCR-γδ stimulatory capacity by pyrophosphates is sensitive to alkaline phosphatase (ALP) treatment.61 Phosphatases degrade pyrophosphates and biphosphates. As phosphates do not bind appreciably to the B30.2 domain, phosphatase activity suppresses pyrophosphate-mediated γδ-TCR ligand formation.
Evaluation of the effect of Fe2+ on ALP expression in 7 cell lines from 4 different tissue types reveals that Fe2+ significantly represses ALP transcription, demonstrated here after 3 days of exposure (Figure 7C). Liver, the organ most affected by hemochromatosis damage, has the strongest repression with almost 100%, >80% in muscle, and bone 20% to 30% (Figure 7C). These results support hypothesis 2 (supplemental Figure 2): Iron overload confers permanent availability of the Vδ2+-TCR ligand by inhibiting pyrophosphatases.
To investigate whether iron overload in general rather than the specific HH phenotype/genotype causes selective reduction of peripheral Vδ2+ T cells, we also studied a group of individuals with iron overload independent from hemochromatosis: patients with thalassemia major. All individuals in this cohort exhibited severe iron accumulation caused by repeated blood transfusions and the disease-associated iron utilization disorder.62 They compellingly have the same phenotypic abnormalities as individuals with HH, characterized by normal percentages of TCRγδ+/CD3+ but an inverted Vδ2+/Vδ2– ratio (Figure 7D). These data highlight that the observed effects of Fe2+ on Vδ2+ T cells are not HH specific but potentially apply to all conditions characterized by chronic iron overload.
Discussion
In this study, we identify a numerical anomaly in the γδ-T-cell compartment as a feature of hemochromatosis. Vδ2+ T cells, normally the most abundant population in the peripheral γδ T-cell pool, are significantly reduced. The simultaneous significant increase in Vδ2– T cells reverses the Vδ2+/Vδ2– ratio in the peripheral γδ T-cell compartment, whereas the total share of γδ T cells in CD3+ lymphocytes is unchanged.
To demonstrate whether a functional Vγ9Vδ2-TCR ligand is present, we tested whether hemochromatosis-related increased free intracellular Fe2+ potentially forms Fe2+ IPP chelates, preventing IPP from binding to the BTN3A1 B30.2 domain, which is essential for Vδ2+-TCR ligand formation (supplemental Figure 1). However, DFO failed to increase Vδ2+ cells in stimulated hemochromatosis PBMCs and cross-experiments demonstrated vigorous proliferation of HD-derived Vδ2+ T cells when cocultured with ZOL-pulsed PBMCs from patients with HH, indicating that a functional ligand is formed in patients with HH. Vδ2+ T cells from patients with HH also demonstrated impeccable functionality by distinct response pattern to specificity-graded TCR/CDR3 stimuli.
Interestingly, the more subset-specific Vδ2+ activation was applied, the lower was the yield of Vδ2+ T cells from patients with HH. Fas-L–mediated AICD preventing lymphoproliferation is a potential mechanism for this.63 We found significantly increased ROS production, strong expression of exhaustion markers in vivo on Vδ2+ T cells from individuals with HH compared with HDs. Ex vivo, HH donor–derived Vδ2+ T cells were also hyporesponsive to supraphysiological TCR stimulation in terms of ROS production, reflecting abnormal ROS fluxes associated with mitophagy contributing to T-cell hyporesponsiveness.64 At the same time, Vδ2+ cells significantly increased their paramount expression of exhaustion markers further and have a subset-specific coexpression of Fas/Fas-L, indicating high susceptibility to AICD (Figure 6B,D). A significantly higher proportion of apoptotic Fas/Fas-L coexpressing Vδ2+ T cells in HD vs HH PBMCs after ZOL stimulation supports this hypothesis, providing direct evidence for lack of enrichment despite strong proliferation (Figure 6C-D; supplemental Figure 5).
Possible explanations for the observed overstimulation relate to a common clinical pathology in HH, the manifestation of arthritis. HH is one of a few conditions linked to calcium pyrophosphate deposition in joints58,65 derived from the inhibitory action of iron on pyrophosphatases.58,65,66 Treatment of iron overload decreases disease activity.53-56,67 With regard to γδ T-cell homeostasis, this suggests that high intracellular iron levels and an expanded labile iron pool in the tissues promote the stability and accumulation of intracellular IPP and its prolonged or even permanent binding to BTN3A1 B30.2 domain. In line with this, we demonstrate, to our knowledge, for the first time that Fe2+ is a potent repressor of phosphatase transcription.
In a key experiment, we then reveal that spiked Fe2+ alone, at concentrations prevalent in hemochromatosis (10-100 μM), selectively activates and stimulates the proliferation of Vδ2+ T cells in healthy PBMCs, with 25 μM FeSO4 stimulating proliferation as strongly as supraphysiological ZOL. These findings were reproduced in vivo in ID patients where a single-dose iron IV leads to a significant increase in Vδ2+/CD3+ and the Vδ2+/γδ T-cell compartment (supplemental Figure 8A).
The possibility that in vivo liver damage caused by iron accumulation further contributes to altered homeostasis of Vδ2+ T cells is highly suggestive because stress-induced MHC class I polypeptide-related sequence A and B and UL16 binding protein, ligands for Vδ2+ T cell’s activating killing receptor NKG2D, and iron-induced ferroptosis, promoting IL-18 expression,68 a potent Vδ2+ activator particularly in conjunction with IL-12,69 further increase the activating potential of tissues for Vδ2+ cells.
The in vivo observations suggest that this is likely. Although a single dose of IV iron, leading to a significant increase of iron levels, results in strong activation (proliferation) of Vδ2+ in anemic ID patients (supplemental Figure 8A), the already exhausted/overstimulated Vδ2+ subset in patients with chronic low-grade inflammation is deleted (supplemental Figure 8B). This suggests a fatal synergy between chronic low-grade inflammation and iron overload regarding Vδ2+ homeostasis, with both factors being present in hemochromatosis.
Exhausted T cells retain some antipathogen effector function, resulting in a pathogen/host “stalemate.”70 There is general consensus that exhausted T cells, compared with effector or memory T cells, feature altered, sometimes reduced, effector functions, such as decreased (but not absent) cytokine production, increased chemokine expression, persistently high levels of multiple inhibitory receptors, reduced proliferative capacity, and a biased epigenetic signature.70
How would losing the Vγ9Vδ2 subset affect an individual? Activated by bacterial pAgs, Vγ9Vδ2 kill infected cells and pathogens early in infection. With ROS, interferon gamma/tumor necrosis factor alpha/IL-17 and IL-2271 Vγ9Vδ2 disrupt iron uptake and bacterial enzymes, limit iron availability and bacterial growth by increasing ferritin72,73 and hepcidin expression,74 essential for combating siderophiles, activate immune cells, promote adaptive immunity,75 support memory formation, and orchestrate immune surveillance. Consequently, their loss could lead to chronic/recurrent infections, impaired defense against (siderophilic) bacteria, weakened memory function, and disrupted immune coordination.
MHC-unrestricted antigen recognition and independence from cancer neoantigens76,77 allow Vγ9Vδ2 to recognize tumors early in development through their TCR and natural killer receptors. Prevalence of γδ T cells in the epithelia, such as the lung, liver, intestine, and skin, organs in which malignancies are most difficult to treat, is the basis for their critical role in immune surveillance and explains why tumor-infiltrating γδ T cells are a favorable prognostic marker across many human cancer types.78-81
Thus, the increased cancer susceptibility demonstrated in γδ T-cell–deficient mice82,83 most likely also exists in humans.
γδ T cells take center stage in the maintenance of tissue integrity.84 Individuals with HH have iron-related organ damage and an increased risk for HCC. Our study reveals a severely depleted Vγ9Vδ2 subset in hemochromatosis. Accordingly, a recent study extends our finding by demonstrating a causal link between selective depletion of the Vγ9Vδ2-T-cell subset and HCC and that, conversely, a higher abundance of intratumoral γδ T cells is associated with improved survival in HCC.85 Although Zakeri et al85 recommend induction/expansion of tissue-resident γδ T cells by systemic/intratumoral delivery of amino-bisphosphonates, our data rise caution for this approach in patients with HH, because tissue toxicity arising from the quantity of toxic tissue reactive iron species86 remains, and continued overstimulation of existing Vδ2+ T cells might further deteriorate immune surveillance.
Promising cell-based alternatives may be Vδ1+ T cells, predominant in these patients. Localized in peripheral tissues, they act in MHC independently, respond to tumors with proinflammatory cytokines, inhibit growth-promoting factors,87 resist AICD, and can persist in the circulation for years.88,89 Vδ1+ T cells attack diverse cancers, including lymphoid and myeloid malignancies,90-94 neuroblastoma,87 and lung, colon, and pancreatic cancers.95-97 Recent research highlights a Vδ1+ compartment in healthy breast tissue, geared toward cytolysis and interferon gamma production.98 Clinical-grade Vδ1+ T cells are available99 and may be enhanced with CARs100 for immunotherapy in patients with HH (with HCC).
In conclusion, although the proportion of γδ T cells in CD3+ lymphocytes is unchanged, we identify an inverted Vδ2+/Vδ2– ratio in the γδ T-cell compartment of patients with hemochromatosis. A similar phenotype has been observed in patients with iron overload due to inborn hemoglobinopathies, suggesting an inverted Vδ2+/Vδ2– ratio in the γδ T-cell compartment as a hallmark of iron overload.
Acknowledgments
The authors thank the participating patients and healthy volunteers. The authors also thank Lena Oevermann, Charite Berlin, for providing EDTA blood samples from 8 patients with thalassemia. Graphical abstract and supplemental Figures 1 and 2 were created with BioRender.com.
Funding for this work was provided by the intramural promotional program of the Faculty of Medicine, University of Tuebingen, the Interdisziplinäres Zentrum für Klinische Forschung Promotionskolleg (I.B., V.H.). D.E. was supported by a grant of the Jürgen Manchot Foundation, Düsseldorf, Germany. H.H. was supported by the Stefan Morsch Stiftung, Birkenfeld, Germany. K.S. was supported by the Reinhold Beitlich Stiftung Tübingen, Germany, and the Hector Stiftung Weinheim, Germany (grant M2111).
Authorship
Contribution: D.E., I.B., V.H., Y.W., and M.E. performed the experiments and statistical analysis, processed the laboratory samples, and prepared the figures; M.D., H.H., and J.S. provided research support and study coordination; K.S. was principal investigator, provided the initial observation, formed the working hypotheses, conceptualized the study, designed the research, interpreted the data, and wrote the manuscript; all data were collected, stored, and analyzed by the investigators; K.S. has full access to the data and human samples collected from the study and has final responsibility for the content of the manuscript; and all authors reviewed the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karin Schilbach, Department of Pediatric Hematology and Oncology, University Children's Hospital Tübingen, Hoppe-Seyler St 1, 72076 Tübingen, Germany; email: karin.schilbach@med.uni-tuebingen.de.
References
Author notes
D.E. and I.B. contributed equally to this study.
Original data are available on request from the author, Derya Erdogdu (derya.guengoer@med.uni-tuebingen.de or derya.erdogdu@student.uni-tuebingen.de).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.




![Proliferation and expansion of Vδ2+ T cells in response to graded TCR stimulation. (A) Proliferation and expansion statistics for experiments found in (B). Number of proliferating Vδ2+ cells corresponds to the integral under the curve, highly proliferating cells refers to proliferating Vδ2+ cells with CTV intensity ≤103. The fold expansion of Vδ2+ T cells was calculated as follows: ([Vδ2+ T cells per CD3+ T cells in PBMCs [%] after stimulation])/(Vδ2+ T cells per CD3+ T cells in PBMCs [%] before stimulation). (B) CTV FACS plots of Vδ2+ T cells from HH (n = 3) and HDs (n = 3) after stimulation with ZOL (Vδ2Vγ9-specific stimulation), IMMU510 (pan γδ stimulation), and αCD3/αCD28 (pan T-cell stimulation) in PBMCs ∗P < .05; ∗∗P < .01;. ns, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/146/2/10.1182_blood.2024025345/3/m_blood_bld-2024-025345-gr4.jpeg?Expires=1765882087&Signature=Up16ikd4mJHv-7PyHYlN7SYGYWUrW2dQAAJ1J0W3~cjrX~8rcp-CsymZ1iQSo-9F85TW34TbwcCMUclvWjR~FPp7U8rz1osyv0AyA99eCMdxuwfg5dy9WEAezHimzMAwtsefII2oPFr-kb6cNoWgpX8GA5Rlipl9vGdl~qW73~jDk~Wk67gsHoE31V7CFqAzTGj0eMWEU32CZaTXRONnG-9eBJHA7FKwKdoLJcIbNPAHbh5QXpRk-iooKofcKAX5sye4wRnJIaUy0aByJ4xJKTEm0Nj~VeIFhxUTQVCVXE2rTmuVastzxZWDMUe7kyklCcPmsWa3Vppdyoe4d5tFJA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal