Key Points
We identified a high-risk subtype of NK cell–type CAEBV characterized by a high CpG island methylation pattern.
5-Azacytidine is effective against this subtype of NK cell–type CAEBV in vitro and in vivo, suggesting it is a novel potential therapy.
Visual Abstract
Chronic active Epstein-Barr virus (EBV) infection (CAEBV) is an orphan disease characterized by the proliferation and infiltration of EBV-infected T/natural killer (NK) cells into multiple organs. Although CAEBV is a heterogeneous disease with diverse clinical courses, its pathogenesis remains poorly understood. In this study, we explored the molecular mechanisms underlying CAEBV by performing a comprehensive multiomics analysis, including genome, transcriptome, epigenome, and single-cell transcriptome and surface proteome analyses, of 65 patients with CAEBV. Methylation analysis identified 2 distinct subtypes of NK cell–type CAEBV based on the CpG island methylator phenotype (CIMP). In CIMP-positive CAEBV, regions associated with enhancer of zeste homolog 2 binding sites and histone H3 lysine 27 trimethylation exhibited increased DNA hypermethylation, resulting in downregulation of tumor suppressor and antiherpesvirus genes. CIMP-positive CAEBV had a particularly poor prognosis and displayed a “neoplastic” phenotype with a DNA methylation pattern similar to that of extranodal NK/T-cell lymphoma, a higher tumor mutation burden, and frequent copy number alterations. In addition, both in vitro and in vivo functional assays demonstrated that 5-azacytidine, a hypomethylating agent, was a potentially effective agent for high-risk CIMP-positive CAEBV. Finally, we established a method to effectively detect EBV-infected cells in single-cell analysis, suggesting that EBV-infected NK cells have tissue-resident properties and that innate and adaptive immunity to EBV is compromised in patients with CAEBV. The present findings provide insight into the complex molecular features of CAEBV and suggest potential molecular therapies.
Introduction
Epstein-Barr virus (EBV) infects >95% of the world’s population. EBV is the causal agent in various cancers.1 Chronic active EBV infection (CAEBV) is characterized by the proliferation and infiltration of EBV-infected T/natural killer (NK) cells into multiple organs, leading to persistent inflammatory symptoms and multiple organ damage. CAEBV is typically difficult to cure with chemotherapy alone and can progress to overt leukemia or lymphoma of T/NK-cell lineage. Therefore, hematopoietic stem cell transplantation is the only curative therapy for long-term recovery.2-4 However, CAEBV is a heterogeneous disease that can follow different clinical courses, ranging from long-term minimal symptoms that do not require treatment to severe symptoms with hypercytokinemia from the time of presentation. CAEBV with an aggressive phenotype is particularly challenging to control even with transplantation and has an extremely poor prognosis. Consequently, there is an urgent need for novel molecular therapies.5
Although the pathogenesis of CAEBV remains unclear, somatic mutations (such as DDX3X and epigenetic modifiers) and intragenic deletions within the EBV genome have been identified in some patients.6,7 However, the exact contribution of these somatic mutations to the pathogenesis are not fully understood. Studies of other EBV-associated tumors suggest that extensive DNA hypermethylation of the CpG island is involved in the carcinogenesis of EBV-associated gastric cancer, and this phenotype has been termed the CpG island methylator phenotype (CIMP).8,9 However, the epigenetic status in CAEBV remains unclear.
Herein we performed a comprehensive multiomics analysis (genome, transcriptome, epigenome, and single-cell transcriptome and surface proteome) of 65 patients with CAEBV. This multiomics approach highlighted the complex interplay between genetic and epigenetic factors associated with the heterogeneous pathogenesis of CAEBV, which may lead to novel therapeutic strategies.
Methods
Detailed information on the methodology is provided in the supplemental Data, available on the Blood website.
Patients and samples
Peripheral blood mononuclear cells (PBMCs) (n = 44) or bone marrow blood mononuclear cells (n = 1), EBV-infected NK-lymphoblastoid cell lines (NK-LCLs) (n = 6) derived from PBMCs, and/or DNA extracted from whole blood (n = 38) of 65 patients (median age, 12 years; range, 0-60) with NK cell–type or T-cell–type CAEBV were analyzed. The analysis included PBMCs from 4 patients with infectious mononucleosis (IM), 2 patients with IM-like symptoms without primary EBV infection (non-EBV–IM-like), and 4 healthy volunteers as controls. The diagnosis of CAEBV was based on previously described criteria.2,3,10 Detailed clinical information for each patient, the experimental design, and a comparison with previous cohorts are shown in supplemental Tables 1 and 2.
Results
CIMP-positive status silences tumor suppressor and antiherpesvirus genes and is related to poor prognosis
Viable cells were available in 49 cases (NK cell–type, n = 34; T-cell–type, n = 15). These cells were isolated using a cell sorter, and polymerase chain reaction detection of EBV-DNA was used to identify infected and uninfected cell fractions. In the clustering analysis, we focused mainly on NK cell–type CAEBV because the cohort of T-cell–type CAEBV was small and included different subtypes of CD4+, CD8+, and T-cell receptor (TCR) γδ+ T cells.
First, 28 patients with sufficient enrichment of EBV-infected NK cells (defined in principle as EBV-DNA copy number ≥106 copies per μg DNA) were analyzed using RNA sequencing (RNA-seq). However, transcriptome-based unsupervised clustering did not reveal clear subtypes (supplemental Figure 1A).
We performed DNA methylation microarray analysis after adjusting the β-value for each tumor sample using computational estimation of tumor purity in 32 patients, including 4 cases wherein only NK-LCLs were obtained (supplemental Methods). NK cells obtained from 4 patients with EBV-IM, 2 non-EBV–IM-like patients, and 4 healthy volunteers were used as controls.
The methylation data from in-house samples were merged with the deposited data of extranodal NK/T-cell lymphoma (ENKTL) and normal NK cells, and unsupervised clustering was performed (Figure 1A; supplemental Figure 1B). The results showed that some patients with CAEBV formed the same clusters as patients with ENKTL, which are characterized by a high CpG island methylation pattern. The remaining patients formed the same clusters as the controls. We defined the former group as CIMP-positive and the latter group as CIMP-negative. Principal component (PC) analysis further showed that the DNA methylation pattern in CAEBV differed from that observed in controls and exhibited gradations along PC1 according to the degree of DNA methylation (Figure 1B). We focused on PC1 because it accounted for 81.87% of the variance, whereas PC2 accounted for only 3.42%, and thus contained little informative value (supplemental Figure 2A). The estimated tumor purity is shown in supplemental Figure 2B. Although the CIMP-positive group had higher tumor purity than the CIMP-negative group, tumor purity was ≥60% in all cases. This suggests that the methylation profiles of EBV-infected NK cells were accurately evaluated after correction for tumor purity. Additionally, the 4 NK-LCLs, which were composed of pure tumor cells, were classified into 2 CIMP-positive cases and 2 CIMP-negative cases.
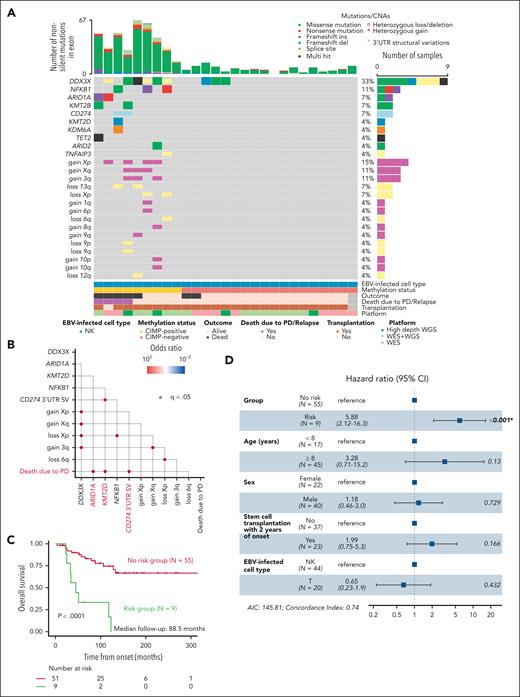
Classification of NK cell–type CAEBV based on the DNA methylation status. (A) Unsupervised hierarchical clustering of promoter-associated CpG island methylation profiles in NK cells from in-house samples (NK cell–type CAEBV, n = 32; IM, n = 4; non-EBV–IM-like, n = 2; healthy volunteers, n = 4) and deposited data (ENKTL, n = 15; normal NK, n = 21). Case/control, disease, and cluster are indicated in different colors (top). DNA methylation heat map of the 3000 most variable probes associated with the promoter (bottom). (B) Principal component analysis of the samples described in panel A using the 3000 most variable probes associated with the promoter. (C) Kaplan-Meier OS curves of 12 CIMP-positive and 20 CIMP-negative CAEBVs. The table below the plot indicates the number of patients at risk at each time point. (D) Forest plots of multivariate analyses of OS performed using the Cox proportional hazard model (hazard ratio ± 95% confidence interval [CI]). CIMP status and known clinical prognostic factors were included in the model. AIC, Akaike information criterion; PC1, principal component 1; PC2, principal component 2.
Classification of NK cell–type CAEBV based on the DNA methylation status. (A) Unsupervised hierarchical clustering of promoter-associated CpG island methylation profiles in NK cells from in-house samples (NK cell–type CAEBV, n = 32; IM, n = 4; non-EBV–IM-like, n = 2; healthy volunteers, n = 4) and deposited data (ENKTL, n = 15; normal NK, n = 21). Case/control, disease, and cluster are indicated in different colors (top). DNA methylation heat map of the 3000 most variable probes associated with the promoter (bottom). (B) Principal component analysis of the samples described in panel A using the 3000 most variable probes associated with the promoter. (C) Kaplan-Meier OS curves of 12 CIMP-positive and 20 CIMP-negative CAEBVs. The table below the plot indicates the number of patients at risk at each time point. (D) Forest plots of multivariate analyses of OS performed using the Cox proportional hazard model (hazard ratio ± 95% confidence interval [CI]). CIMP status and known clinical prognostic factors were included in the model. AIC, Akaike information criterion; PC1, principal component 1; PC2, principal component 2.
All patients who died of disease progression were classified as CIMP-positive, and CIMP-positive patients had significantly lower overall survival (OS) than CIMP-negative patients (log-rank P = 2.3 × 10–3) (Figure 1C). A multivariate Cox regression model with known clinical prognostic factors3,7 showed that methylation status was significantly associated with OS, independent of other prognostic parameters (P = .037) (Figure 1D). Although CIMP-positive patients tended to be older than CIMP-negative patients, both groups included children and adults (age at onset, median [range]: CIMP-positive, 29 [8-47] years; CIMP-negative, 8 [0-36] years; P < 1.0 × 10–3). Notably, CIMP-positive patients had a significantly higher frequency of liver dysfunction than CIMP-negative patients (P = .002) (supplemental Table 3).
Next, we integrated the transcriptome with the methylome and identified genes silenced by DNA methylation compared with controls. The results showed that CIMP-positive patients had a greater number of silenced tumor suppressor genes (such as CDKN1C, TP53I11, and LDOC1) and antiherpesvirus genes (such as MX1 and MX2)11-13 than CIMP-negative patients (Figure 2A; supplemental Figure 3A; supplemental Table 4). Gene enrichment analysis showed that genes involved in the suppression of cell proliferation were enriched among the genes silenced in both CIMP-positive and CIMP-negative patients (Figure 2B; supplemental Figure 3B).
Integrative analysis of the epigenome and transcriptome. (A) Integrative scatterplots contrasting expression differences with DNA methylation differences in CIMP-positive (left) and CIMP-negative (right) CAEBV compared with the normal control. Genes and probes associated with the promoter showing significant differences in gene expression (|log2FC| ≥1.5 and adjusted [adj] P < .05) and DNA methylation (|ΔM| ≥2 and adj P < .05) are highlighted. Tumor suppressor genes and antiherpesvirus genes are highlighted in green and blue, respectively. (B) Pathway analysis of DNA hypermethylated silenced genes in CIMP-positive CAEBV (highlighted in the lower right corner in panel A) performed using Metascape software. (C) LOLA enrichment analysis of 100 differentially hypermethylated regions in promoters. Significantly enriched categories from CISTROME or ENCODE entries of the LOLA Core databases are shown. (D) Expression of EZH2 in NK cells from CIMP-positive, CIMP-negative, EBV-IM, and control cases (healthy volunteers and non-EBV–IM-like). Box plots show median (lines), interquartile ranges (IQRs) (boxes), and ±1.5 × IQR (whiskers). Significance was assessed using the Dunnett T3 test for multiple comparisons against controls. (E) CUT&RUN profile differences in CIMP-positive (S_C_10 NK_LCL) (top) and CIMP-negative (T_H_01 NK_LCL) (bottom) samples compared with the normal control (Control 1 NK cell) for H3K27me3 (left) and H3K4me3 (right) over the average gene body for all genes or hypermethylated and silenced genes in CIMP-positive CAEBV. GO, gene ontology; Log2FC, log2 fold change; TES, transcription end site; TPM, transcripts per million; TSS, transcription start site.
Integrative analysis of the epigenome and transcriptome. (A) Integrative scatterplots contrasting expression differences with DNA methylation differences in CIMP-positive (left) and CIMP-negative (right) CAEBV compared with the normal control. Genes and probes associated with the promoter showing significant differences in gene expression (|log2FC| ≥1.5 and adjusted [adj] P < .05) and DNA methylation (|ΔM| ≥2 and adj P < .05) are highlighted. Tumor suppressor genes and antiherpesvirus genes are highlighted in green and blue, respectively. (B) Pathway analysis of DNA hypermethylated silenced genes in CIMP-positive CAEBV (highlighted in the lower right corner in panel A) performed using Metascape software. (C) LOLA enrichment analysis of 100 differentially hypermethylated regions in promoters. Significantly enriched categories from CISTROME or ENCODE entries of the LOLA Core databases are shown. (D) Expression of EZH2 in NK cells from CIMP-positive, CIMP-negative, EBV-IM, and control cases (healthy volunteers and non-EBV–IM-like). Box plots show median (lines), interquartile ranges (IQRs) (boxes), and ±1.5 × IQR (whiskers). Significance was assessed using the Dunnett T3 test for multiple comparisons against controls. (E) CUT&RUN profile differences in CIMP-positive (S_C_10 NK_LCL) (top) and CIMP-negative (T_H_01 NK_LCL) (bottom) samples compared with the normal control (Control 1 NK cell) for H3K27me3 (left) and H3K4me3 (right) over the average gene body for all genes or hypermethylated and silenced genes in CIMP-positive CAEBV. GO, gene ontology; Log2FC, log2 fold change; TES, transcription end site; TPM, transcripts per million; TSS, transcription start site.
Chromatin modification analysis suggests that DNA methylation is induced by EZH2
The epigenomic functions of the DNA hypermethylation sites were analyzed using a public chromatin immunoprecipitation sequencing database. In CIMP-positive patients, DNA hypermethylation was increased in regions associated with EZH2 (enhancer of zeste homolog 2) binding sites and trimethylation of histone H3 lysine 27 (H3K27me3), which is an inhibitory histone modification (Figure 2C). EZH2 is a functional subunit of PRC2 (polycomb repressive complex 2), which is responsible for the reversible and repressive chromatin modification H3K27me3.14 H3K27me3 is a premarker for DNA hypermethylation. The switch from reversible repression by H3K27me3 to stable silencing by DNA methylation, along with the decrease of H3K27me3 followed by the increase in DNA methylation at the same sites, has been observed previously.15-18
Therefore, we examined the expression of EZH2 in NK cells and found that EZH2 was significantly upregulated among patients with CAEBV and EBV-IM compared with controls, including non-EBV–IM-like patients (Figure 2D). EZH2 was not upregulated in non-EBV–IM-like cases but was in EBV-IM, suggesting that EZH2 was not induced by simple inflammatory responses but by the EBV infection itself, as previously reported.19
To determine whether CpG island hypermethylation is caused by the upregulation of EZH2 in CAEBV, we performed chromatin modification analysis for 3 samples using the Cleavage Under Targets and Release Using Nuclease (CUT&RUN) assay (supplemental Methods).20 We found that the levels of H3K27me3 and EZH2 in promoter regions were lower in CIMP-positive cases than in controls, especially in hypermethylated and silenced genes, whereas they were increased in CIMP-negative cases. In addition, trimethylation of histone H3 lysine 4 (H3K4me3), a marker of transcriptional activation, was decreased to a greater extent in CIMP-positive than in CIMP-negative cases in the same regions (Figure 2E; supplemental Figure 3C).
These results suggest that EZH2 upregulation associated with EBV infection caused the increase in reversible silencing by H3K27me3 (CIMP-negative), followed by a switch to stable silencing with DNA hypermethylation (CIMP-positive) in tumor suppressor and antiherpesvirus genes.
Genome analysis indicates that TMB is higher in CIMP-positive cases
To further characterize the molecular basis, we examined the mutational landscape in 27 patients with NK cell–type CAEBV using whole-exome sequencing or high-depth whole-genome sequencing (Figure 3A; supplemental Tables 5 and 9). Consistent with previous reports,6,7,DDX3X mutations were the most frequent (33%). Epigenetic modifiers such as ARID1A (7%), KMT2B (7%), and KMT2D (4%), as well as structural variants (SVs) involving the 3′-untranslated region (UTR) of CD274 (7%), were also mutated, corroborating previous findings.6,7,21 We newly identified recurrent NFKB1 mutations (11%). Frequent copy number alterations (CNAs) were exclusively observed in CIMP-positive cases (supplemental Table 7). The tumor mutation burden (TMB) was significantly higher in CIMP-positive than in CIMP-negative patients (number of nonsilent mutations in exons per case was 39.78 vs 6.06; P = .0002), which may reflect the tumor-like pathology of CIMP-positive CAEBV. Genotyping of an expanded cohort that included T-cell–type CAEBV using targeted deep sequencing identified similar mutations and CNAs (supplemental Figure 4A-B; supplemental Tables 6-9).
Mutational landscape of CAEBV according to CIMP status. (A) Gene mutations and CNAs in NK cell–type CAEBV with different CIMP status. (B) Pairwise associations among mutated genes found in at least 3 patients with NK cell–type or T-cell–type CAEBV and PD-related death outcome. Only significant correlations (q < .05) are shown together with their odds ratios. ARID1A and KMT2D mutations and CD274 3′-UTR SVs are defined as risk genes and highlighted in red. (C) Kaplan-Meier survival curves for NK cell–type or T-cell–type CAEBV according to risk gene mutations. The table below the plot indicates the number of patients at risk at each time point. (D) Forest plots of multivariate analyses of OS in NK cell–type or T-cell–type CAEBV constructed using the Cox proportional hazard model (hazard ratio ± 95% CI). Risk genes and known clinical prognostic factors were included in the model. AIC, Akaike information criterion; WES, whole-exome sequencing; WGS, whole-genome sequencing.
Mutational landscape of CAEBV according to CIMP status. (A) Gene mutations and CNAs in NK cell–type CAEBV with different CIMP status. (B) Pairwise associations among mutated genes found in at least 3 patients with NK cell–type or T-cell–type CAEBV and PD-related death outcome. Only significant correlations (q < .05) are shown together with their odds ratios. ARID1A and KMT2D mutations and CD274 3′-UTR SVs are defined as risk genes and highlighted in red. (C) Kaplan-Meier survival curves for NK cell–type or T-cell–type CAEBV according to risk gene mutations. The table below the plot indicates the number of patients at risk at each time point. (D) Forest plots of multivariate analyses of OS in NK cell–type or T-cell–type CAEBV constructed using the Cox proportional hazard model (hazard ratio ± 95% CI). Risk genes and known clinical prognostic factors were included in the model. AIC, Akaike information criterion; WES, whole-exome sequencing; WGS, whole-genome sequencing.
Next, we analyzed the patterns of somatic base substitutions in the whole-genome sequencing of 18 patients with CAEBV. The substitution patterns were decomposed into the known mutational signatures from the Catalogue of Somatic Mutations in Cancer version 3, which identified contributions from 3 signatures: Signature 1 (26.4%), Signature 5 (57.7%), and Signature 18 (10.8%). No apparent differences in signature composition according to CIMP status were observed (supplemental Figure 5A-B). Although Signature 1 and Signature 5 are associated with aging, all signatures are present in various cancers and not specific to EBV-associated tumors.22,23
Mutations in epigenetic modifiers and SVs involving the CD274 3′-UTR are associated with poor prognosis
To identify genetic risk factors, survival analysis based on mutation data was performed in the expanded cohort, which included T-cell–type CAEBV. We examined the correlation between mutations present in ≥3 patients with NK cell–type or T-cell–type CAEBV and mortality due to progressive disease (PD) and found a significant correlation between mutations in epigenetic modifier genes, such as ARID1A and KMT2D, or SV involving the CD274 3′-UTR and PD-related death (Figure 3B). All patients with genetic risk had CIMP-positive CAEBV. These patients showed a marked decrease in OS (Figure 3C), and the risk genes were significantly associated with OS, independent of other prognostic parameters in a multivariate Cox regression model (P < .001) (Figure 3D; supplemental Figure 6A-B).
Genome and transcriptome analysis of EBV
We performed phylogenetic analysis using in-house EBV data along with 303 deposited EBV strains categorized by isolated region (inner circle), disease type (middle circle), and CIMP status (outer circle) (supplemental Figure 7A; supplemental Table 10). The inner circle showed a regional clustering of EBV strains. However, in the middle and outer circles, CIMP-positive strains did not cluster distinctly or align closely with EBV strains isolated from ENKTL, suggesting that CIMP status was not associated with specific EBV strains.
Next, we analyzed the EBV transcriptome (supplemental Figure 7B). We found that the RNA expressed in CAEBV was predominantly noncoding (EBERs and BART-microRNAs [miRNAs]), with fewer expressed protein-coding genes than in IM. Although CAEBV exhibits a latency type 2 EBV gene expression pattern with limited gene expression,24,25 supplemental Figure 7B demonstrates that even EBNA-1 and LMP-1, which were previously thought to be expressed in latency type 2, are not expressed in CAEBV. Only LMP-2, BNRF1, and BARF1 were weakly expressed.
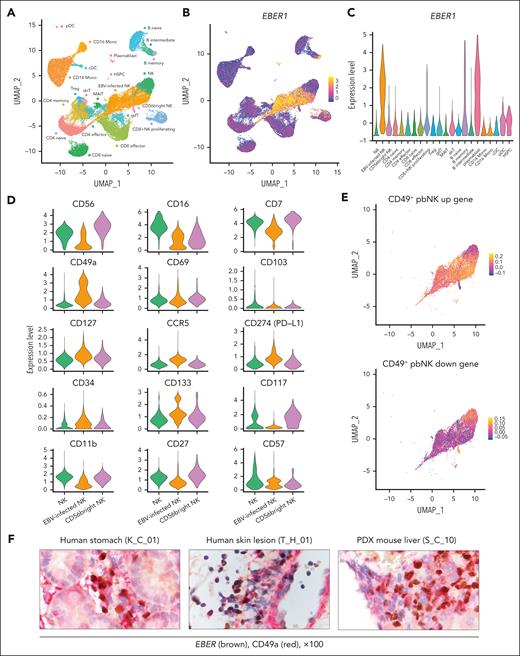
Single-cell transcriptome and surface proteome analysis indicate that EBV-infected NK cells have tissue-resident characteristics
We performed single-cell RNA-seq (scRNA-seq) to analyze the characteristics of EBV-infected cells and the immune response to EBV in CAEBV. According to the unified pipeline (supplemental Methods), scRNA-seq data from 49 878 PBMCs of 6 patients with NK cell–type CAEBV (CIMP-positive, n = 3; CIMP-negative, n = 3), 2 patients with IM, and 2 healthy volunteers were integrated to generate uniform manifold approximation and projection (UMAP). Clustering based on RNA expression was identified in 22 cell types (Figure 4A; supplemental Figure 8A). Annotation was validated using Azimuth (https://azimuth.hubmapconsortium.org/; supplemental Figure 8B). EBV expresses limited genes in CAEBV, and the most universally highly expressed gene, EBER1, is a noncoding RNA without a poly(A) tail, which makes it undetectable by conventional scRNA-seq, which captures RNA via poly(A) tail. Therefore, we applied the Genotyping of Transcriptomes method26 to effectively detect EBER1 and identify EBV-infected cells. A strong peak of EBER1 expression was observed in NK, memory B, and plasma blast cells, consistent with data from NK cell–type CAEBV and IM (Figure 4B-C). NK cells were divided into 3 clusters according to host RNA expression, one of which was defined as “EBV-infected NK” on the basis of the high expression of EBER1. Clustering focused on identifying cell types showed that cells from CIMP-positive and CIMP-negative samples formed the same cluster for each cell type. However, differential expression analysis showed lower expression of the abovementioned silenced genes (CDKN1C, LDOC1, MX1, and MX2) in CIMP-positive “EBV-infected NK” cells compared with CIMP-negative “EBV-infected NK” or control “NK” cells (supplemental Figure 8C), confirming the bulk analysis results.
Enrichment of tissue-resident signatures in EBV-infected NK cells. (A) UMAP embedding of scRNA-seq data for 49 878 cells from PBMCs of 3 patients with CIMP-positive CAEBV, 3 patients with CIMP-negative CAEBV, 2 patients with IM, and 2 healthy volunteers. Twenty-two cell types were defined according to the RNA expression of marker genes (supplemental Figure 8A). (B) UMAP plot colored according to EBER1 expression level. (C) Violin plot showing the expression of EBER1 in each cell type. (D) The expression of NK-lineage-defining surface markers is displayed using violin plots in 3 NK clusters (NK, EBV-infected NK, and CD56bright NK). (E) UMAP embedding of NK cells colored by CD49+ peripheral blood NK (CD49+ pbNK) up (upper) and down (lower) gene score. The score was calculated using a published gene list (supplemental Table 12). (F) Representative images of dual immunostaining for EBER (brown) and CD49a (red) in a human stomach biopsy sample (K_C_01, left), a skin lesion biopsy sample (T_H_01, middle), and a liver sample from a PDX mouse engrafted with NK-LCLs (S_C_10, right). cDC, conventional dendritic cell; dnT, double negative T; gdT, γδ T; HSPC, hematopoietic stem and progenitor cell; MAIT, mucosal associated invariant T; pDC, plasmacytoid dendritic cell; PD-L1, programmed cell death 1 ligand 1; PDX, patient-derived xenograft; Treg, regulatory T.
Enrichment of tissue-resident signatures in EBV-infected NK cells. (A) UMAP embedding of scRNA-seq data for 49 878 cells from PBMCs of 3 patients with CIMP-positive CAEBV, 3 patients with CIMP-negative CAEBV, 2 patients with IM, and 2 healthy volunteers. Twenty-two cell types were defined according to the RNA expression of marker genes (supplemental Figure 8A). (B) UMAP plot colored according to EBER1 expression level. (C) Violin plot showing the expression of EBER1 in each cell type. (D) The expression of NK-lineage-defining surface markers is displayed using violin plots in 3 NK clusters (NK, EBV-infected NK, and CD56bright NK). (E) UMAP embedding of NK cells colored by CD49+ peripheral blood NK (CD49+ pbNK) up (upper) and down (lower) gene score. The score was calculated using a published gene list (supplemental Table 12). (F) Representative images of dual immunostaining for EBER (brown) and CD49a (red) in a human stomach biopsy sample (K_C_01, left), a skin lesion biopsy sample (T_H_01, middle), and a liver sample from a PDX mouse engrafted with NK-LCLs (S_C_10, right). cDC, conventional dendritic cell; dnT, double negative T; gdT, γδ T; HSPC, hematopoietic stem and progenitor cell; MAIT, mucosal associated invariant T; pDC, plasmacytoid dendritic cell; PD-L1, programmed cell death 1 ligand 1; PDX, patient-derived xenograft; Treg, regulatory T.
To characterize EBV-infected NK cells, we performed single-cell surface proteome analysis of 69 surface antigens using droplet-based CITE-seq (cellular indexing of transcriptomes and epitopes by sequencing) (supplemental Table 11).27 CD49a, a tissue-resident marker, showed particularly higher expression in “EBV-infected NK” than in other NK cells, whereas the expression pattern of conventional NK cell developmental markers did not correspond to specific developmental stages (Figure 4D). Tissue-resident NK (trNK) cells, which express canonical markers (including CD49a, CD69, and/or CD103), CCR5, and programmed cell death 1 ligand 1 (PD-L1) among others, are present in various human organs, such as the liver, lung, kidney, uterus, and tonsil.28-34 In addition, CD49a+ CD69– CD103– NK cells with a tissue-resident phenotype have been reported in peripheral blood (CD49+ pbNK).35 We defined a score for CD49+ pbNK upregulated and downregulated genes in each cell according to a published gene list derived from differentially expressed gene analysis of bulk RNA-seq of CD49a+ and CD49a– NK cells in peripheral blood (supplemental Table 12). We observed high levels of CD49+ pbNK upregulated genes in “EBV-infected NK” and high levels of CD49+ pbNK downregulated genes in other NK cells (Figure 4E). Additionally, we confirmed expression of CD49a in EBER-positive cells in patients’ tissues and a patient-derived xenograft mouse model by immunostaining (Figure 4F). These data suggest that EBV-infected NK cells are distinct from conventional NK cells (CD56bright NK or CD56dim/CD16+ NK cells) but share the characteristics of trNK cells.
Single-cell transcriptome and TCR analysis suggest a decreased antivirus response in CAEBV
To elucidate the immune response to EBV in CAEBV, we scored the response to interferon-α (IFN-)α and IFN-β for each cell type on the basis of gene expression in a published gene list. The response to IFN-α and IFN-β was lower in “EBV-infected NK” than in other NK cells (Figure 5A-B; supplemental Figure 8D). This finding is consistent with data showing that MX1 and MX2, which are IFN-stimulated genes with critical antiviral effects in infected cells, particularly against herpesviruses,11-13,36 are silenced by DNA methylation in EBV-infected NK cells.
Innate and EBV-adaptive immune compromise in CAEBV. (A-B) (Top) UMAP embedding of PBMCs colored by IFN-α response score (A) and IFN-β response score range (B). The score was calculated using gene sets termed “GOBP_ GOBP_RESPONSE_TO_INTERFERON_ALPHA (GO:0035455)” and “GOBP_RESPONSE_TO_INTERFERON_BETA (GO:0035456).” (Bottom) Heat maps depicting the average scores in each of 22 cell types. The module score of “EBV-infected NK” was compared with the “NK” score using a 2-sided Welch t test. (C) UMAP embedding of T cells colored by clonal expansion size. Clonal expansion divided into 3 categories (left) and clonal expansion sizes ranging from 0 to 250 (right) are shown. (D) Distribution of the clone status of T cells suspected to be specific to EBV based on the CDR3 amino acid sequence in CAEBV (n = 6), IM (n = 2), or healthy volunteers (n = 2). (E) Rate of EBV-specific T cells with the TCR specific for LMP2 in CAEBV (n = 6), IM (n = 2), or healthy volunteers (n = 2). The difference in the rate of TCR specific to LMP2 between CAEBV and IM was evaluated using the Fisher exact test. cDC, conventional dendritic cell; dnT, double negative T; gdT, γδ T; HSPC, hematopoietic stem and progenitor cell; MAIT, mucosal associated invariant T; pDC, plasmacytoid dendritic cell; Treg, regulatory T.
Innate and EBV-adaptive immune compromise in CAEBV. (A-B) (Top) UMAP embedding of PBMCs colored by IFN-α response score (A) and IFN-β response score range (B). The score was calculated using gene sets termed “GOBP_ GOBP_RESPONSE_TO_INTERFERON_ALPHA (GO:0035455)” and “GOBP_RESPONSE_TO_INTERFERON_BETA (GO:0035456).” (Bottom) Heat maps depicting the average scores in each of 22 cell types. The module score of “EBV-infected NK” was compared with the “NK” score using a 2-sided Welch t test. (C) UMAP embedding of T cells colored by clonal expansion size. Clonal expansion divided into 3 categories (left) and clonal expansion sizes ranging from 0 to 250 (right) are shown. (D) Distribution of the clone status of T cells suspected to be specific to EBV based on the CDR3 amino acid sequence in CAEBV (n = 6), IM (n = 2), or healthy volunteers (n = 2). (E) Rate of EBV-specific T cells with the TCR specific for LMP2 in CAEBV (n = 6), IM (n = 2), or healthy volunteers (n = 2). The difference in the rate of TCR specific to LMP2 between CAEBV and IM was evaluated using the Fisher exact test. cDC, conventional dendritic cell; dnT, double negative T; gdT, γδ T; HSPC, hematopoietic stem and progenitor cell; MAIT, mucosal associated invariant T; pDC, plasmacytoid dendritic cell; Treg, regulatory T.
Next, we performed single-cell TCR sequencing and projected the clonal expansion status onto the UMAP (Figure 5C). We obtained EBV-specific TCR sequence data from a public database, projected it onto the UMAP, and examined the clonal expansion of T cells with EBV-specific TCRs in each disease group. Although we found significant clonal expansion of EBV-specific TCRs in IM, CAEBV did not show the same clonal expansion as IM (Figure 5D). We identified the antigens of EBV that are recognized by clonally expanding T cells with EBV-specific TCRs. The percentage of TCRs specific for LMP2 was significantly lower in CAEBV (Figure 5E).
5-Aza effectively suppresses proliferation of CIMP-positive NK-LCLs both in vitro and in vivo
Finally, we investigated the efficacy of 5-azacytidine (5-Aza), a hypomethylating agent, against CIMP-positive CAEBV. For the in vitro assay, NK-LCLs derived from PBMCs of 3 patients with CAEBV (CIMP-positive, n = 2; CIMP-negative, n = 1) were treated with 5-Aza. Normal NK cells from healthy volunteers (n = 2) were used as controls. In the 2 CIMP-positive NK-LCLs, cell proliferation was inhibited at low concentrations, whereas in the 1 CIMP-negative NK-LCL, inhibition of proliferation was observed only at high concentrations (Figure 6A). Analysis of one of the CIMP-positive NK-LCLs treated with 1× concentration showed a reduction in promoter DNA methylation compared with the dimethyl sulfoxide–treated control, whereas normal NK cells showed no reduction (Figure 6B). RNA-seq identified genes with decreased DNA methylation and increased expression. The expression of the abovementioned silenced genes (MX1 and MX2) and that of genes involved in “negative regulation of cell population proliferation” and “cytokine signaling in immune system” was restored in the CIMP-positive NK-LCLs (Figure 6C-D; supplemental Figure 9A).
Efficacy of 5-Aza in CIMP-positive NK-LCLs. (A) Number of live cells in CIMP-positive NK-LCLs (n = 2), CIMP-negative NK-LCLs (n = 1), and normal NK cells from healthy volunteers (n = 2) treated in vitro with 4 different concentrations of 5-Aza ranging from 8.0 × 10–3 (×1/125) to 1 μg/mL (×1) for 7 days relative to those treated with vehicle (DMSO). Three wells per concentration were used in each experiment (mean ± standard deviation [SD]), and the experiment was repeated twice for validation. The overall P value was calculated using the Kruskal-Wallis test. The P values for pairwise comparisons were calculated using the Mann-Whitney U test. (B) M values were calculated for CIMP-positive NK-LCLs (S_C_10) and NK cells from a healthy volunteer (Control 4) at probes where silencing by DNA methylation was observed in CIMP-positive CAEBV. The ΔM values, representing the difference between the average M values from 3 wells of 5-Aza (1×) treatment and 3 wells of the vehicle control (DMSO), were plotted for each probe (mean ± 95% CI; n = 855 probes). Significance was assessed using the Mann-Whitney U test. (C) Hypomethylated (ΔM < –0.5 and adj P < .05) and upregulated genes in CIMP-positive NK-LCLs (S_C_10) after 7 days of in vitro 5-Aza treatment vs vehicle. Significantly upregulated genes (log2 fold change ≥ 1 and adj P < .05) are highlighted in red. (D) Pathway analysis of DNA hypomethylated and upregulated genes in CIMP-positive NK-LCLs after in vitro 5-Aza treatment was performed using Metascape software. (E) Schematic showing the protocol for the in vivo study of 5-Aza using NOG-IL2 mice injected with CIMP-positive NK-LCLs (S_C_10). (F) Percentage of hCD45+ cells in the peripheral blood of 5-Aza vs vehicle-treated NOG-IL2 mice at 42 days after transplantation of the CIMP-positive NK-LCLs (S_C_10) (mean ± SD; n = 5 mice per group). Differences were evaluated using 2-way analysis of variance. (G-H) Spleen weight (G) and percentage of hCD45+ cells in the spleen (H) on day 42 in mice treated with 5-Aza or vehicle (mean ± SD; n = 5 mice per group). The difference was evaluated using the Mann-Whitney U test. DMSO, dimethyl sulfoxide; dsRNA, double-stranded RNA; GO, gene ontology; R-HSA, reactome-Homo sapiens.
Efficacy of 5-Aza in CIMP-positive NK-LCLs. (A) Number of live cells in CIMP-positive NK-LCLs (n = 2), CIMP-negative NK-LCLs (n = 1), and normal NK cells from healthy volunteers (n = 2) treated in vitro with 4 different concentrations of 5-Aza ranging from 8.0 × 10–3 (×1/125) to 1 μg/mL (×1) for 7 days relative to those treated with vehicle (DMSO). Three wells per concentration were used in each experiment (mean ± standard deviation [SD]), and the experiment was repeated twice for validation. The overall P value was calculated using the Kruskal-Wallis test. The P values for pairwise comparisons were calculated using the Mann-Whitney U test. (B) M values were calculated for CIMP-positive NK-LCLs (S_C_10) and NK cells from a healthy volunteer (Control 4) at probes where silencing by DNA methylation was observed in CIMP-positive CAEBV. The ΔM values, representing the difference between the average M values from 3 wells of 5-Aza (1×) treatment and 3 wells of the vehicle control (DMSO), were plotted for each probe (mean ± 95% CI; n = 855 probes). Significance was assessed using the Mann-Whitney U test. (C) Hypomethylated (ΔM < –0.5 and adj P < .05) and upregulated genes in CIMP-positive NK-LCLs (S_C_10) after 7 days of in vitro 5-Aza treatment vs vehicle. Significantly upregulated genes (log2 fold change ≥ 1 and adj P < .05) are highlighted in red. (D) Pathway analysis of DNA hypomethylated and upregulated genes in CIMP-positive NK-LCLs after in vitro 5-Aza treatment was performed using Metascape software. (E) Schematic showing the protocol for the in vivo study of 5-Aza using NOG-IL2 mice injected with CIMP-positive NK-LCLs (S_C_10). (F) Percentage of hCD45+ cells in the peripheral blood of 5-Aza vs vehicle-treated NOG-IL2 mice at 42 days after transplantation of the CIMP-positive NK-LCLs (S_C_10) (mean ± SD; n = 5 mice per group). Differences were evaluated using 2-way analysis of variance. (G-H) Spleen weight (G) and percentage of hCD45+ cells in the spleen (H) on day 42 in mice treated with 5-Aza or vehicle (mean ± SD; n = 5 mice per group). The difference was evaluated using the Mann-Whitney U test. DMSO, dimethyl sulfoxide; dsRNA, double-stranded RNA; GO, gene ontology; R-HSA, reactome-Homo sapiens.
Next, we used a patient-derived xenograft model of NK cell–type CAEBV using NOD/Shi-scid-IL-2Rγnull mice (NOG-hIL-2 Tg)37 to evaluate the effect of 5-Aza on CIMP-positive CAEBV in vivo. One week after transplantation of the CIMP-positive NK-LCLs (hCD45+), either 5-Aza (n = 5) or vehicle control (n = 5) was administered intraperitoneally, and both groups were euthanized at 42 days (supplemental Methods; Figure 6E). Treatment with 5-Aza significantly decreased the percentage of CIMP-positive NK-LCLs in the peripheral blood (Figure 6F). The weight of the spleen and the percentage of CIMP-positive NK-LCLs in the spleen were significantly lower in the 5-Aza treatment group (Figure 6G-H).
We treated the same NK-LCLs and normal NK cells in vitro with valemetostat, a dual EZH1/2 inhibitor. Valemetostat did not inhibit the proliferation of the 2 CIMP-positive NK-LCLs, whereas it resulted in the suppression of the CIMP-negative NK-LCL (supplemental Figure 9B). Similarly, valemetostat showed no therapeutic effect on the CIMP-positive NK-LCL group in vivo (supplemental Figure 9C).
Discussion
The current study unveiled the epigenetic, genetic, and transcriptomic characteristics of NK cell–type CAEBV. To the best of our knowledge, this study identified, for the first time, 2 distinct subtypes of NK cell–type CAEBV and showed their association with prognosis according to CIMP status. 5-Aza was identified as a promising therapeutic candidate for high-risk CIMP-positive CAEBV. This study highlights novel molecular genetic features for the prediction of diverse clinical courses of CAEBV and suggests possible molecular therapies.
CIMP-positive NK cell–type CAEBV showed a DNA methylation pattern similar to that of ENKTL, a higher TMB, and frequent CNAs. Somatic driver mutations, including DDX3X and epigenetic modifiers, were reported to be associated with poor OS.6,7 To our knowledge, the present study demonstrated for the first time that patients showing accumulation of CNAs in addition to these somatic mutations exhibit DNA hypermethylation that downregulates tumor suppressors, leading to a high-risk “neoplastic” phenotype. This finding is consistent with the fact that CAEBV can clinically progress to ENKTL.3
One possible mechanism of DNA hypermethylation involves H3K27me3 via the upregulation of EZH2 induced by EBV infection given that H3K27me3 is a premarker of DNA hypermethylation.15-18 Similar to our findings, upregulation of EZH2 in both EBV-infected and noninfected cells upon EBV infection has been previously reported.19,38,EZH2 is induced by EBV-encoded proteins and/or NF-κB activation38-40 and represses EBV lytic genes through H3K27me3 modification of EBV genes, suggesting that EZH2 induction is a host innate immune response against EBV.19 These findings suggest that the higher EZH2 levels observed in IM may be due to differences in EBV gene expression patterns.
H3K27me3 levels in promoter regions were increased in CIMP-negative cases compared with controls, whereas they were decreased in CIMP-positive cases. These findings are consistent with the hypothesis that H3K27me3 and DNA methylation are anticorrelated because CpG methylation of nucleosomal DNA inhibits EZH2 binding to H3K27me3-marked nucleosomes.16,41-44 In this study, it was not entirely clear why the shift from H3K27me3 to DNA methylation occurred only in CIMP-positive cases. However, previous studies have reported that ARID1A and KMT2D knockout influences DNA hypermethylation.45-47 These mutations were found only in CIMP-positive cases, and heterozygous loss of these genes is reported to cause haploinsufficiency,46,48 suggesting that these alterations may have contributed to DNA hypermethylation. In addition, these mutations were significantly associated with PD-related death, whereas the most frequent mutation, DDX3X, was not significantly associated with PD-related death. This finding supports that these mutations contribute to malignant transformation through DNA hypermethylation.
Additionally, we found that although expression of many EBV genes was downregulated in CAEBV, LMP-2, BNRF1, BARF1, EBERs, and BART-miRNAs were still detected. LMP2 is reported to induce DNA methylation via DNMT1 in EBV-associated gastric cancer.49 Moreover, BNRF1 contributes to host genome instability and tumorigenesis.50 BARF1, EBERs and BART-miRNAs have been implicated in immune evasion and survival promotion.51-54
On the basis of these results, we propose that the pathogenic mechanism of CAEBV involves H3K27me3 induced by EBV infection, leading to the malignant transformation of EBV-infected NK cells through the accumulation of DNA methylation, driven by persistent EBV infection and/or subsequent genetic mutations due to EBV mutagenicity (see the visual abstract in the online version of this article). Considering the finding that mutational signatures in CAEBV were associated with aging, this hypothesis aligns with the observation that CIMP-positive CAEBV occurs more frequently in older patients, potentially explaining the heterogeneous clinical course of CAEBV. However, further studies using samples from the same patient at different time points are warranted.
The present findings suggest that 5-Aza is a novel therapeutic option for refractory CAEBV. We demonstrated that 5-Aza effectively suppressed proliferation of CIMP-positive NK-LCLs both in vitro and in vivo, whereas valemetostat was not effective. This finding is consistent with the DNA methylation and H3K27me3 status in CIMP-positive CAEBV. Conversely, both 5-Aza and valemetostat suppressed proliferation of CIMP-negative NK-LCLs only at high doses, which is consistent with the findings that CIMP-negative CAEBV also exhibits a different DNA methylation pattern from that of normal NK cells and increased H3K27me3. However, because CIMP-negative NK-LCLs are difficult to maintain in long-term culture, we could not analyze multiple strains. This is consistent with our findings that CIMP-negative cases exhibited a less “neoplastic” phenotype, with reduced DNA hypermethylation and a lower TMB compared with CIMP-positive cases. Further studies are needed to determine the efficacy of 5-Aza and valemetostat against CIMP-negative CAEBV.
We used scRNA-seq and CITE-seq to characterize EBV-infected NK cells and the surrounding noninfected immune cells. Although several studies have performed scRNA-seq of CAEBV, efficient detection of EBV-infected cells has been challenging because of the low expression of the EBV transcriptome in CAEBV.55,56 To address this, we applied the Genotyping of Transcriptomes technique and developed a method for detecting EBV-infected cells effectively. Our data showed that EBV infection does not occur in conventional NK cells, but rather in NK cells with tissue-resident properties. We also demonstrated that CD49a+ NK cells are indeed present in tissues of patients with CAEBV. These findings may explain the organ infiltration of EBV-infected cells in CAEBV. Notably, trNK cells are also present in the tonsils,28 a potential route for EBV infection, and a previous study demonstrated the rare presence of T or NK cells exhibiting EBV infection in human tonsils.57 These findings suggest a mechanism for NK cell infection in CAEBV. Additionally, a recent report showed that ENKTL also has tissue-resident properties,58 which supports the idea that CAEBV can clinically evolve into ENKTL. Although tissue-resident T cells have also been reported,59,60 further single-cell studies focusing on T-cell–type CAEBV are warranted.
Furthermore, we observed a decrease in the type I IFN response score in EBV-infected NK cells. Additionally, single-cell TCR sequencing revealed fewer T cells with EBV-specific TCRs in CAEBV than in IM. Although previous studies reported that EBV and other viruses evade both the innate and adaptive immune systems through viral proteins and miRNAs,1,36 a further functional assay is necessary to confirm our findings.
The major limitation of our study is that it applied bulk analysis using a methylation microarray. However, this limitation is not unique to CAEBV but is a common feature of previous studies of CIMP status in various cancer types.9,61,62 To address this limitation, we used the InfiniumPurify R package to estimate tumor purity and adjusted the β-value for each tumor sample according to the estimated tumor purity.63 Additionally, we incorporated NK-LCLs into our analysis and performed validation using scRNA-seq. Further validation through single-cell methylome analysis is required in future studies.
In summary, multiomics analysis identified a high-risk subtype of NK cell–type CAEBV characterized by tumor-like features, including a high CpG island methylation pattern, a higher TMB, and frequent CNAs. Functional validation demonstrated that 5-Aza effectively suppressed proliferation of such NK cell–type CAEBV, suggesting a potential novel molecular therapeutic approach. Single-cell analysis showed that EBV-infected NK cells have tissue-resident properties, and innate and adaptive immunity to EBV is compromised in patients with CAEBV, although further validation studies are warranted.
Acknowledgments
The authors thank the patients and their families for their cooperation. The authors also thank K. Kodama for technical assistance and M. Inoue for sample collection.
This work was supported by the Japan Society for the Promotion of Science KAKENHI (grants JP18K19467, JP20H00528, JP21K19405, JP23K18264, and JP24H00628 [J.T.], and JP22K07211 [I. Kato]), the Project for Cancer Research and Therapeutic Evolution (grant JP19cm0106509h9904), the Project for Promotion of Cancer Research and Therapeutic Evolution (grants JP22ana221505h0001, 23ama221505h0002, and 24ama221531h0001), Practical Research for Innovative Cancer Control (grant JP19ck0106468h0001) from the Japan Agency for Medical Research and Development, the Princess Takamatsu Cancer Research Fund (J.T.), ISHIZUE 2023 of Kyoto University (J.T.), and Takeda Hosho Grants for Research in Medicine (J.T.).
Authorship
Contribution: R.A., Y.U., N.K., M.M.N., Y.H., H.Y., Hideki Ueno, and S.O. performed sequencing experiments; R.A. performed sequencing data analysis and CUT&RUN experiments, and generated figures and tables; R.A., T.I., Y.S., H.Y., and S.M. developed bioinformatics pipelines; R.A., T.K., K.T., and K. Isobe performed methylation array analysis; R.A., T.M., and H.G. performed functional analysis; Y.I., I. Katano, R.I., and M.I. provided materials and advice for the patient xenograft model mice; M.Y., T.D., Hiroo Ueno, S.N., M. Sawada, H.F., K.K., M. Hiwatari, M.K., M. Sato, A.S., and K.-I.I. provided specimens and collected clinical data; R.A., M.Y., Y.I., and K.-I.I. established cell lines; K.J., M. Hirata, M.F., and H. Haga performed a pathological assessment; R.A. and J.T. wrote the manuscript; T.M., I. Kato, H.K., S.S., T.K., K.T., K. Isobe, K. Izawa, K.U., H. Hiramatsu, and Hiroo Ueno participated in helpful discussions and commented on the research direction; J.T. led the entire project; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Junko Takita, Graduate School of Medicine, Kyoto University, 54 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto 606-8507, Japan; email: jtakita@kuhp.kyoto-u.ac.jp.
References
Author notes
All whole-genome sequencing, whole-exome sequencing, targeted capture sequencing, RNA sequencing, methylation array, CUT&RUN assay, and single-cell RNA sequencing data have been deposited in the Japanese Genotype-phenotype Archive, which is hosted by the DNA Data Bank of Japan (accession number JGAS000735).
All software and bioinformatics tools used in this study are publicly available.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![Classification of NK cell–type CAEBV based on the DNA methylation status. (A) Unsupervised hierarchical clustering of promoter-associated CpG island methylation profiles in NK cells from in-house samples (NK cell–type CAEBV, n = 32; IM, n = 4; non-EBV–IM-like, n = 2; healthy volunteers, n = 4) and deposited data (ENKTL, n = 15; normal NK, n = 21). Case/control, disease, and cluster are indicated in different colors (top). DNA methylation heat map of the 3000 most variable probes associated with the promoter (bottom). (B) Principal component analysis of the samples described in panel A using the 3000 most variable probes associated with the promoter. (C) Kaplan-Meier OS curves of 12 CIMP-positive and 20 CIMP-negative CAEBVs. The table below the plot indicates the number of patients at risk at each time point. (D) Forest plots of multivariate analyses of OS performed using the Cox proportional hazard model (hazard ratio ± 95% confidence interval [CI]). CIMP status and known clinical prognostic factors were included in the model. AIC, Akaike information criterion; PC1, principal component 1; PC2, principal component 2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/146/19/10.1182_blood.2024026805/2/m_blood_bld-2024-026805-gr1.jpeg?Expires=1765494759&Signature=tukgVg1WZHeHRZPfnDa~MMVgQEysa3naeTrR4OGWjY7z70yKeOFoX5bfMC1CJ2fG2gIZ1~u7eQp7ssUYIGRsG73B99MsSnl3OTfnWWD6GPSBQBJWm-zgNQTvTtRWq24QEdIh4Js2HOEZAKCrHciYyb2uZh06iK1Iu4OwfwpkzHyj02zzTzSB-V15LyDk9D~5sZsq1a4ZIh-f2GWo9DAuXZHvWzT5KqpeihShzTmlRD9gE5HZ2JQIBdbixVVDQjQmggzsi1s8Nx066vbw~H4Tawip1sKz9dHZk~2UQp42VBAKW4Ev2iSCPXfkxVml8QSGfoHY86hraNTYeKnpfM8SHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Integrative analysis of the epigenome and transcriptome. (A) Integrative scatterplots contrasting expression differences with DNA methylation differences in CIMP-positive (left) and CIMP-negative (right) CAEBV compared with the normal control. Genes and probes associated with the promoter showing significant differences in gene expression (|log2FC| ≥1.5 and adjusted [adj] P < .05) and DNA methylation (|ΔM| ≥2 and adj P < .05) are highlighted. Tumor suppressor genes and antiherpesvirus genes are highlighted in green and blue, respectively. (B) Pathway analysis of DNA hypermethylated silenced genes in CIMP-positive CAEBV (highlighted in the lower right corner in panel A) performed using Metascape software. (C) LOLA enrichment analysis of 100 differentially hypermethylated regions in promoters. Significantly enriched categories from CISTROME or ENCODE entries of the LOLA Core databases are shown. (D) Expression of EZH2 in NK cells from CIMP-positive, CIMP-negative, EBV-IM, and control cases (healthy volunteers and non-EBV–IM-like). Box plots show median (lines), interquartile ranges (IQRs) (boxes), and ±1.5 × IQR (whiskers). Significance was assessed using the Dunnett T3 test for multiple comparisons against controls. (E) CUT&RUN profile differences in CIMP-positive (S_C_10 NK_LCL) (top) and CIMP-negative (T_H_01 NK_LCL) (bottom) samples compared with the normal control (Control 1 NK cell) for H3K27me3 (left) and H3K4me3 (right) over the average gene body for all genes or hypermethylated and silenced genes in CIMP-positive CAEBV. GO, gene ontology; Log2FC, log2 fold change; TES, transcription end site; TPM, transcripts per million; TSS, transcription start site.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/146/19/10.1182_blood.2024026805/2/m_blood_bld-2024-026805-gr2.jpeg?Expires=1765494759&Signature=lim7fIavM8Wt8tuQ9qn61JcorYx9Fe09IKTaC~dNy-98eSi8ufwpSl~N0Hb94vZOVV4YVoqzGkj6bsvLiZU-PD~CgPZfcJ78k~HVDNF424LV7dCMKpon4MMql7Ad1X~wosMb0u7tfDb8g0lL~Hx7iKc1IJOxaGthIz2WvFOjRI~FiQ7rkxflWUlYGkHuKnN4EM33ZEArWAgD~HUBck-VE82LPjFoTQpcyRuquRLCg7URZSKrC6SCcyF3fIeGk2ypz~vmrq-ZBWxs2cB1GgeZXArClRU698fufB12DUMr22NrBBiX3H777zV~pLN18i5USEvz-jaJpJl5R9Na1C0UfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Efficacy of 5-Aza in CIMP-positive NK-LCLs. (A) Number of live cells in CIMP-positive NK-LCLs (n = 2), CIMP-negative NK-LCLs (n = 1), and normal NK cells from healthy volunteers (n = 2) treated in vitro with 4 different concentrations of 5-Aza ranging from 8.0 × 10–3 (×1/125) to 1 μg/mL (×1) for 7 days relative to those treated with vehicle (DMSO). Three wells per concentration were used in each experiment (mean ± standard deviation [SD]), and the experiment was repeated twice for validation. The overall P value was calculated using the Kruskal-Wallis test. The P values for pairwise comparisons were calculated using the Mann-Whitney U test. (B) M values were calculated for CIMP-positive NK-LCLs (S_C_10) and NK cells from a healthy volunteer (Control 4) at probes where silencing by DNA methylation was observed in CIMP-positive CAEBV. The ΔM values, representing the difference between the average M values from 3 wells of 5-Aza (1×) treatment and 3 wells of the vehicle control (DMSO), were plotted for each probe (mean ± 95% CI; n = 855 probes). Significance was assessed using the Mann-Whitney U test. (C) Hypomethylated (ΔM < –0.5 and adj P < .05) and upregulated genes in CIMP-positive NK-LCLs (S_C_10) after 7 days of in vitro 5-Aza treatment vs vehicle. Significantly upregulated genes (log2 fold change ≥ 1 and adj P < .05) are highlighted in red. (D) Pathway analysis of DNA hypomethylated and upregulated genes in CIMP-positive NK-LCLs after in vitro 5-Aza treatment was performed using Metascape software. (E) Schematic showing the protocol for the in vivo study of 5-Aza using NOG-IL2 mice injected with CIMP-positive NK-LCLs (S_C_10). (F) Percentage of hCD45+ cells in the peripheral blood of 5-Aza vs vehicle-treated NOG-IL2 mice at 42 days after transplantation of the CIMP-positive NK-LCLs (S_C_10) (mean ± SD; n = 5 mice per group). Differences were evaluated using 2-way analysis of variance. (G-H) Spleen weight (G) and percentage of hCD45+ cells in the spleen (H) on day 42 in mice treated with 5-Aza or vehicle (mean ± SD; n = 5 mice per group). The difference was evaluated using the Mann-Whitney U test. DMSO, dimethyl sulfoxide; dsRNA, double-stranded RNA; GO, gene ontology; R-HSA, reactome-Homo sapiens.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/146/19/10.1182_blood.2024026805/2/m_blood_bld-2024-026805-gr6.jpeg?Expires=1765494759&Signature=F34UtzAY24~7~G~9FV4Avf7xnpOB~W46NYUSem91KObIO2I5revteUy88q9X2fgGIBj2PZx8QRla2nV8f8TtmhVNB2M~nHqPMdO3JhTupfb6~8~bfcaVbWA0YCTRwZuK~FjnjY2EOdVVi3pNhLUqyyvAummlyvl6hpigBuBW5u68qF9S6skfb3zUJ5xyGDhbVbEmr3S6ImZM5sPhefdh60a01pweY5nLhyX0KqBgIcjO6lqhVW2Iaaga1wKu8NmNIDUaJSLTdM5Djz0vB1n4Br4p4Ybl4UIdeRxBCnUFh59fzPLU5XpcENthNY4AO2P1ikrIfvA~PcU8RgYEIqq-Gg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal