Key Points
In the VenR arm, the 7-year PFS rate was 23%, and the OS rate was 70%; the median time from PD after MRD conversion to next therapy was 5 months.
In a substudy, 25 patients were retreated with VenR; median PFS was 23 months, ORR was 72%, and 32% achieved uMRD at retreatment EOCT.
Visual Abstract
Fixed-duration venetoclax-rituximab (VenR) in patients with relapsed/refractory chronic lymphocytic leukemia (CLL) in the phase 3 MURANO trial resulted in superior progression-free survival (PFS) and overall survival (OS) vs bendamustine-rituximab (BR). We report the final analyses of MURANO (median follow-up, 7 years). Patients were randomized to VenR (venetoclax 400 mg daily for 2 years plus monthly rituximab for 6 months; n = 194) or BR (6 months; n = 195). In a substudy, patients with progressive disease (PD) received VenR as retreatment or crossover from BR. At the final data cut (3 August 2022), the median PFS with VenR was 54.7 months vs 17.0 months with BR. The 7-year PFS with VenR was 23.0%. The 7-year OS was 69.6% and 51.0%, respectively. Among VenR-treated patients with undetectable minimal residual disease (MRD; uMRD) and no PD at end of treatment (EOT; n = 83), the median PFS from EOT was 52.5 vs 18.0 months in patients with MRD at EOT (n = 35; P < .0001). Fourteen patients had enduring uMRD. Three distinct mutations in BCL2 in 4 patients were identified. In the substudy, 25 patients were retreated with VenR, and 9 patients crossed over to VenR; the median PFS was 23 and 27 months, and the best overall response rate was 72% and 89%, respectively. At the end of combination treatment (EOCT), after retreatment or crossover, 8 and 6 patients achieved uMRD, respectively. No new safety findings were observed. Overall, these final MURANO analyses support consideration of fixed-duration VenR therapy for patients with relapsed/refractory CLL. This trial was registered at www.clinicaltrials.gov as #NCT02005471.
Introduction
Relapsed/refractory chronic lymphocytic leukemia (CLL) remains largely incurable despite developments in targeted therapy, including Bruton tyrosine kinase inhibitors (BTKis),1-6 phosphatidylinositol 3-kinase delta inhibitors,7-9 and the B-cell lymphoma 2 inhibitor venetoclax.10,11 Patients continue to experience progressive disease (PD), and resistance becomes more prevalent over time. Venetoclax induces high response rates in CLL,12,13 including patients with adverse biologic features, such as unmutated immunoglobulin heavy chain gene (IGHV),10,14,15 deletion in chromosome 17p (del(17p)),16,17 and genomic complexity (GC)18-20; although tumor protein P53 (TP53) aberrations and high GC retain an adverse impact on progression-free survival (PFS) with venetoclax.13,21 Because combinations of targeted therapies are being used in earlier lines of treatment,22,23 subsequent treatment options for patients with relapsed/refractory CLL should be considered.
Minimal residual disease (MRD) is used in clinical trials as an end point, with MRD status at end of treatment (EOT) often predicting long-term clinical outcomes24-28; patients with undetectable MRD (uMRD) generally have better PFS and overall survival (OS) than those with residual detectable disease.25,29 Serial MRD assessment identifies patients with increasing subclinical disease burden months before recurrence30 and is increasingly integrated into trials, with the aim of establishing its role in future clinical practice.
The phase 3 MURANO trial (ClinicalTrials.gov identifier: NCT02005471) investigated the efficacy and safety of fixed-duration venetoclax-rituximab (VenR) vs bendamustine-rituximab (BR) in patients with relapsed/refractory CLL.26,31,32 The primary analysis reported significantly longer PFS with VenR vs BR, with benefits observed in all subgroups analyzed, including unmutated IGHV or del(17p)/TP53 mutation.32 PFS and OS benefits of VenR were sustained at 3, 4, and 5 years.26,31,33 At 5 years, the median PFS was 53.6 vs 17.0 months (P < .0001), and the 5-year OS rates were 82.1% vs 62.2% (P < .0001) with VenR vs BR.33 More VenR-treated patients had uMRD in peripheral blood (PB) at end of combination treatment (EOCT) vs BR-treated patients.31 The genetic risk factors TP53 and GC (≥3 copy number alterations [CNAs]) negatively affected MRD rates and PFS in both arms.26
We report the final analyses of MURANO, with a median follow-up of 7 years and 5 years after completion of VenR, specifically, updated PFS and OS, MRD evaluation, and next-line therapy outcomes. Furthermore, we report outcomes from a substudy in which patients were retreated with or crossed over to VenR.
Methods
Study design
Eligibility criteria for MURANO have been published elsewhere.32 Patients with relapsed/refractory CLL were randomized to receive VenR (venetoclax 400 mg daily for 2 years plus monthly rituximab for the first 6 months) or BR (for 6 months). Patients with PD were followed for disease response to any subsequent anti-CLL therapy and were assessed for time to second PFS event and OS. In the substudy (2018 onward), patients initially randomized in the main study, with confirmed PD and in need of therapy (per International Workshop on CLL [iwCLL] 2008 criteria34), who had not received any new anti-CLL therapy, were eligible. A minimum time off therapy was not required. Patients received VenR on the same schedule as the main study, either as retreatment or as crossover from BR (see supplemental Methods, available on the Blood website). Assessments of next-line therapy, except those conducted in the substudy, were performed outside of the study. Patients who initiated new anti-CLL therapy without an investigator-assessed response were unevaluable.
The trial was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation of Good Clinical Practice guidelines. The protocol was approved by the ethics committees at each participating institution; all patients provided written informed consent. The clinical cutoff date for this analysis was 3 August 2022. Eligibility criteria, dosing, prophylactic measures, and monitoring were as previously published.32
End points and clinical assessments
The primary efficacy end point of the main study was investigator-assessed PFS, defined as the time from randomization to PD, relapse, or death. MRD status in PB was a secondary end point of the main study, assessed at cycle 4, 2 to 3 months after EOCT, and every 3 to 6 months thereafter. MRD was centrally measured in PB using allele-specific oligonucleotide polymerase chain reaction (PCR) and/or flow cytometry.21,22 Patients were categorized by MRD status: uMRD (<1 CLL cell per 10 000 leukocytes; MRD value <0.0001 [10–4]); MRD+ (≥10–4); low MRD+ (10–4 to <10–2); and high MRD+ (≥10–2).25 MRD conversion (considered to have occurred at the first MRD+ assessment) was defined as 2 consecutive assays detecting MRD+ or PD by iwCLL criteria34 in patients previously achieving uMRD.
Other end points in the main study (defined in the supplemental Methods) included OS, event-free survival, complete response (CR), and partial response (PR) rates (by iwCLL 2008 criteria34), duration of response (DOR), time to next treatment (TTNT), and safety. Safety data collected in the posttreatment period only (not including adverse events [AEs] occurring during treatment) included: prespecified AEs of concern, serious AEs related to study treatment, and development of a second primary malignancy (SPM). Further details of safety monitoring have been described previously.31,32
The main substudy objective was to report outcomes among patients retreated with VenR or crossed over from BR to VenR and determine whether VenR retreatment or crossover is a viable option in pretreated patients. Substudy outcomes were also compared among patients categorized by genetic profile (eg, del(17p) and/or TP53 mutations, unmutated IGHV, or GC).
Molecular assessments
Molecular assessments for analysis of GC were conducted using high-density array comparative genomic hybridization (aCGH), with data processing performed as previously published.26 Low/intermediate GC was defined as the presence of 3 to 4 CNAs and high GC as ≥5 CNAs.19,20 High-density aCGH assessed the biomarker-evaluable subset of patients. IGHV mutational status was assessed by PCR and TP53 status by next-generation sequencing (NGS; whole-exome sequencing with a variant allele frequency cutoff of 5%). Assessment of NOTCH1 mutations was performed as previously published.26
Deep NGS was performed on MRD samples (without prior cell selection) from a subset of VenR-treated patients who received up to 2 years of VenR and had, at any time, high MRD+ in PB. Further details on the NGS panel and the pretreatment sample used for comparison have been described previously.35 Digital droplet PCR was performed as previously published.36
Statistical analysis
There was no alpha spending allocated to this analysis; all P values are therefore descriptive. Kaplan-Meier estimates were used to analyze time-to-event data, including landmark analyses from EOCT and EOT according to MRD status. Survival outcomes are presented as median values with 95% confidence intervals (CIs). Log-rank test and Cox proportional hazards regression model were used to compare overall PFS and OS across treatment arms. Fisher’s exact test was performed to compare MRD status at EOCT and EOT, as well as clinical and cytogenetic risk factors in VenR-treated patients with and without PD after EOT. Statistical analysis was performed using SAS version 9.04.
Results
Patient characteristics and follow-up
Overall, 389 patients enrolled into the main study; 194 received VenR, and 195 received BR. Baseline demographics and disease characteristics are reported in Table 1. Overall, 130 patients completed 2 years of venetoclax without PD; reasons for stopping earlier are detailed in the supplemental Results.31 The median duration of follow-up from enrollment for this analysis was 85.7 months (range, 0.0-99.2); the median duration with VenR was 86.8 months (range, 0.3-99.2) vs 84.4 months (range, 0.0-95.0) with BR.
Baseline demographics and disease characteristics of patients in the main study and substudy
| . | Main study (N = 389) . | Substudy (n = 34) . | ||
|---|---|---|---|---|
| VenR (n = 194) . | BR (n = 195) . | Retreatment with VenR (n = 25) . | Crossed over to VenR (n = 9) . | |
| Mean age (SD), y | 63.9 (10.5) | 64.4 (9.6) | 65.8 (8.3) | 62.3 (16.8) |
| Sex, n (%) | ||||
| Male | 136 (70.1) | 151 (77.4) | 19 (76.0) | 9 (100.0) |
| Female | 58 (29.9) | 44 (22.6) | 6 (24.0) | 0 |
| No. of prior cancer therapies, n (%) | ||||
| 1 | 111 (57.2) | 117 (60.0) | 20 (80.0) | 7 (77.8) |
| 2 | 58 (29.9) | 43 (22.1) | 4 (16.0) | 0 |
| ≥3 | 25 (12.9) | 35 (17.9) | 1 (4.0) | 2 (22.2) |
| del(17p) and/or TP53 mutation, n (%) | ||||
| Yes | 72 (37.1) | 75 (38.5) | 9 (36.0) | 0 |
| No | 106 (54.6) | 95 (48.7) | 12 (48.0) | 8 (88.9) |
| Unknown | 16 (8.2) | 25 (12.8) | 4 (16.0) | 1 (11.1) |
| del(17p), n (%) | ||||
| Yes | 46 (23.7) | 46 (23.6) | 4 (16.0) | 0 |
| No | 127 (65.5) | 123 (63.1) | 16 (64.0) | 8 (88.9) |
| Unknown | 21 (10.8) | 26 (13.3) | 5 (20.0) | 1 (11.1) |
| TP53 mutation, n (%) | ||||
| Yes | 48 (24.7) | 51 (26.2) | 7 (28.0) | 0 |
| No | 144 (74.2) | 132 (67.7) | 18 (72.0) | 8 (88.9) |
| Unknown | 2 (1.0) | 12 (6.2) | 0 | 1 (11.1) |
| IGHV, n (%) | ||||
| Mutated | 53 (29.4) | 51 (28.3) | 1 (4.0) | 3 (33.3) |
| Unmutated | 123 (68.3) | 123 (68.3) | 22 (88.0) | 5 (55.6) |
| Unknown | 4 (2.2) | 6 (3.3) | 2 (8.0) | 1 (11.1) |
| GC, n (%) | ||||
| 0-2 | 106 (54.4) | 114 (58.5) | 11 (44.0) | 7 (77.8) |
| 3-4 | 34 (17.4) | 29 (14.9) | 9 (36.0) | 1 (11.1) |
| ≥5 | 14 (7.2) | 17 (8.7) | 2 (8.0) | 0 |
| Unknown | 40 (20.5) | 35 (17.9) | 3 (12.0) | 1 (11.1) |
| . | Main study (N = 389) . | Substudy (n = 34) . | ||
|---|---|---|---|---|
| VenR (n = 194) . | BR (n = 195) . | Retreatment with VenR (n = 25) . | Crossed over to VenR (n = 9) . | |
| Mean age (SD), y | 63.9 (10.5) | 64.4 (9.6) | 65.8 (8.3) | 62.3 (16.8) |
| Sex, n (%) | ||||
| Male | 136 (70.1) | 151 (77.4) | 19 (76.0) | 9 (100.0) |
| Female | 58 (29.9) | 44 (22.6) | 6 (24.0) | 0 |
| No. of prior cancer therapies, n (%) | ||||
| 1 | 111 (57.2) | 117 (60.0) | 20 (80.0) | 7 (77.8) |
| 2 | 58 (29.9) | 43 (22.1) | 4 (16.0) | 0 |
| ≥3 | 25 (12.9) | 35 (17.9) | 1 (4.0) | 2 (22.2) |
| del(17p) and/or TP53 mutation, n (%) | ||||
| Yes | 72 (37.1) | 75 (38.5) | 9 (36.0) | 0 |
| No | 106 (54.6) | 95 (48.7) | 12 (48.0) | 8 (88.9) |
| Unknown | 16 (8.2) | 25 (12.8) | 4 (16.0) | 1 (11.1) |
| del(17p), n (%) | ||||
| Yes | 46 (23.7) | 46 (23.6) | 4 (16.0) | 0 |
| No | 127 (65.5) | 123 (63.1) | 16 (64.0) | 8 (88.9) |
| Unknown | 21 (10.8) | 26 (13.3) | 5 (20.0) | 1 (11.1) |
| TP53 mutation, n (%) | ||||
| Yes | 48 (24.7) | 51 (26.2) | 7 (28.0) | 0 |
| No | 144 (74.2) | 132 (67.7) | 18 (72.0) | 8 (88.9) |
| Unknown | 2 (1.0) | 12 (6.2) | 0 | 1 (11.1) |
| IGHV, n (%) | ||||
| Mutated | 53 (29.4) | 51 (28.3) | 1 (4.0) | 3 (33.3) |
| Unmutated | 123 (68.3) | 123 (68.3) | 22 (88.0) | 5 (55.6) |
| Unknown | 4 (2.2) | 6 (3.3) | 2 (8.0) | 1 (11.1) |
| GC, n (%) | ||||
| 0-2 | 106 (54.4) | 114 (58.5) | 11 (44.0) | 7 (77.8) |
| 3-4 | 34 (17.4) | 29 (14.9) | 9 (36.0) | 1 (11.1) |
| ≥5 | 14 (7.2) | 17 (8.7) | 2 (8.0) | 0 |
| Unknown | 40 (20.5) | 35 (17.9) | 3 (12.0) | 1 (11.1) |
SD, standard deviation.
Clinical outcomes
The median PFS with VenR was 54.7 (95% CI, 52.3-59.9) vs 17.0 months (95% CI, 15.5-21.7) with BR (hazard ratio [HR], 0.23; 95% CI, 0.18-0.29; P < .0001; Figure 1A). The 7-year PFS rate with VenR was 23.0% (95% CI, 16.1-29.9); no BR-treated patients remained progression free at this time point. In VenR-treated patients with high-risk features (del(17p) and/or TP53 mutation, unmutated IGHV, high GC, or mutated NOTCH1), patients with mutated TP53 and/or del(17p) had the poorest 7-year PFS rate at 5.0% (95% CI, 0.0-13.2; n = 53), followed by mutated NOTCH1 without mutated TP53 and/or del(17p) at 11.1% (95% CI, 0.0-25.4; n = 21). The 7-year PFS rate in those with unmutated IGHV was 16.3% (95% CI, 8.8-23.8; n = 123). No patients with high GC were progression free at 7 years.
Kaplan-Meier estimates of efficacy end points in the overall intent-to-treat population. Investigator-assessed PFS (A), OS (B), EFS (C), and DOR (D). Log-rank test and Cox proportional hazards regression model were used to compare overall PFS and OS across treatment arms. EFS, event-free survival.
Kaplan-Meier estimates of efficacy end points in the overall intent-to-treat population. Investigator-assessed PFS (A), OS (B), EFS (C), and DOR (D). Log-rank test and Cox proportional hazards regression model were used to compare overall PFS and OS across treatment arms. EFS, event-free survival.
The median OS with VenR was not reached (NR) vs 87.8 months (95% CI, 70.1 to NR) with BR (HR, 0.53; 95% CI, 0.37-0.74; P = .0002; Figure 1B). The 7-year OS rates with VenR were 69.6% (95% CI, 62.8-76.5) vs 51.0% (95% CI, 43.3-58.7) with BR. Among VenR- vs BR-treated patients with high-risk features, the 7-year OS rates were 50.6% (95% CI, 35.9-65.4; n = 53) vs 47.0% (95% CI, 31.4-62.6; n = 55) with mutated TP53 and/or del(17p) (by aCGH); 67.7% (95% CI, 58.9-76.6; n = 123) vs 50.1% (95% CI, 40.1-60.0; n = 123) with unmutated IGHV; 63.5% (95% CI, 37.9-89.1; n = 14) vs 33.3% (95% CI, 9.5-57.2; n = 17) with high GC; and 64.4% (95% CI, 42.9-85.8; n = 21) vs 69.2% (95% CI, 51.2-87.1; n = 32) with mutated NOTCH1. The 7-year PFS and OS rates for patients with low-risk genetic features are described in the supplemental Results. Multivariate Cox analyses in VenR-treated patients showed that independent factors associated with PFS were IGHV mutation and TP53 mutation or del(17p) (supplemental Table 1). For OS, independent prognostic factors were number of prior therapies (1 vs >1) and TP53 mutation or del(17p) (supplemental Table 2).
The median event-free survival with VenR was 53.7 months (95% CI, 48.5-59.3) vs 16.4 months (95% CI, 14.2-21.0) with BR (HR, 0.22; 95% CI, 0.17-0.29; P < .0001; Figure 1C). The median DOR was 53.6 months (95% CI, 49.1-57.0) for the 181 of 194 responders (93.3%) to VenR and 19.1 months (95% CI, 16.1-23.6) for the 132 of 195 responders (67.7%) to BR (HR, 0.23; Figure 1D).
MRD status and MRD conversion among VenR-treated patients
As previously reported, 83 VenR-treated patients (70.3%) had uMRD at EOT without PD, and 35 (29.7%) were MRD+.32 The median PFS from EOT for patients with uMRD at EOT was 52.5 months (95% CI, 44.5-61.5) vs 18.0 months (95% CI, 8.5-29.3) in those who were MRD+ at EOT (P < .0001; Figure 2A); the 5-year from EOT PFS rates were 40.7% (95% CI, 28.5-52.9) in patients who were uMRD at EOT vs 12.5% (95% CI, 0.2-24.9) in patients who were MRD+ at EOT. The median OS from EOT was NR for patients who were uMRD at EOT and MRD+ at EOT (Figure 2B); the 5-year from EOT OS rates were 85.7% (95% CI, 77.8-93.6) in patients who were uMRD at EOT vs 73.6% (95% CI, 58.7-88.5) in patients who were MRD+ at EOT.
Kaplan-Meier estimates of efficacy end points by MRD∗ response status at EOT in the overall intent-to-treat population. (A) PFS; (B) OS. Fisher’s exact test was performed to compare MRD status at EOCT and EOT. ∗MRD was categorized as uMRD (<1 CLL cell per 10 000 leukocytes; MRD value <0.0001 [10–4]) and MRD+ (≥10–4).
Kaplan-Meier estimates of efficacy end points by MRD∗ response status at EOT in the overall intent-to-treat population. (A) PFS; (B) OS. Fisher’s exact test was performed to compare MRD status at EOCT and EOT. ∗MRD was categorized as uMRD (<1 CLL cell per 10 000 leukocytes; MRD value <0.0001 [10–4]) and MRD+ (≥10–4).
At this 7-year update, 63 (75.9%) had MRD conversion; 14 VenR-treated patients (16.9%) had no PD or confirmed MRD conversion, and 6 (7.2%) had PD or died. Favorable baseline characteristics were overrepresented among 14 patients with enduring uMRD, 13 of 144 with wild-type TP53 (9.0%) had sustained uMRD vs 1 of 48 with TP53 mutation (2.1%); 7 of 53 with mutated IGHV (13.5%) had sustained uMRD vs 6 of 123 with unmutated IGHV (4.9%). Among 63 patients with MRD conversion, the median time from EOT to conversion was 19.4 months (95% CI, 8.7-28.0; Figure 3A); 39 subsequently had PD or died, and the median time from conversion to PD was 28.3 months (95% CI, 23.2-35.0; Figure 3B). MRD conversion with subsequent PD occurred ∼4 years after EOT. For the 36 patients with PD after MRD conversion, the median time from PD to next treatment was 4.5 months (95% CI, 3.3-6.4; Figure 3C).
Kaplan-Meier estimates by MRD status and MRD conversion among VenR-treated patients in the overall intent-to-treat population. (A) Time to MRD∗ conversion; (B) time from conversion to PD; (C) time from PD to next treatment. ∗MRD was categorized as uMRD (<1 CLL cell per 10 000 leukocytes; MRD value <0.0001 [10–4]) and MRD+ (≥10–4).
Kaplan-Meier estimates by MRD status and MRD conversion among VenR-treated patients in the overall intent-to-treat population. (A) Time to MRD∗ conversion; (B) time from conversion to PD; (C) time from PD to next treatment. ∗MRD was categorized as uMRD (<1 CLL cell per 10 000 leukocytes; MRD value <0.0001 [10–4]) and MRD+ (≥10–4).
At 21 to 42 months after treatment initiation (before progression), 107 PB MRD+ samples from 42 VenR-treated patients were collected to assess the nature/frequency of acquired mutations in BCL2 family genes and TP53. The impact of mutations on TTNT and subsequent response were also analyzed. Responses to next treatment per acquired mutation and the impact of TP53 mutations on TTNT are summarized in the supplemental Results.
Results previously published using a targeted sequencing panel (limit of detection, variant allele frequency 1%) showed acquisition of BAX and PMAIP1 mutations (encoding the BCL2 homology 3-only proteins, BAX and Nova, respectively), but not BCL2, in 4 and 2 of 28 patients at the 5-year data cut, respectively.35 Follow-up analyses using digital droplet PCR (limit of detection of 0.1%) identified 3 distinct point mutations in BCL2 in 4 patients (G101V, D103Y, A113G, and G101V and A113G [n = 1 each]). These were observed across 2 time points for 2 patients; for the remaining 2 patients, mutations were observed at a single time point only (supplemental Table 3). Patient characteristics and responses to treatment are outlined in supplemental Table 4 and supplemental Results, respectively.
TTNT, time to second PFS event, and response to next-line therapy
After cessation of the main study treatment, 95 of 194 VenR-treated (49.0%) and 131 of 195 BR-treated patients (67.2%) received subsequent treatment after PD. In total, 73 (37.6%) in the VenR arm had not received next-line therapy at the final cutoff, and 26 (13.4%) died without subsequent therapy. The median TTNT with VenR was 63.0 months (95% CI, 56.1-73.6) vs 24.0 months (95% CI, 20.7-29.5) with BR (HR, 0.30; 95% CI, 0.23-0.39; P < .0001); the median time off therapy was 28.3 months (range, –0.1-68.6) vs 17.9 months (range, 0.7-82.4), respectively.
Two VenR-treated patients received non-CLL therapy for another malignancy and were excluded from subsequent analysis. Of the remaining 93 VenR-treated patients receiving next-line therapy, 30 (32.3%) received a BTKi (ibrutinib [n = 25], acalabrutinib [n = 4], and zanubrutinib [n = 1]), with 18 ongoing at cutoff; 47 (50.5%) received venetoclax-based therapy (supplemental Results), with 12 ongoing at cutoff; 14 (15.1%) received chemoimmunotherapy (supplemental Table 5); and 2 (2.6%) received other novel agents. Of the 131 BR-treated patients receiving next-line therapy, 79 (60.3%) received a BTKi (ibrutinib [n = 69], acalabrutinib [n = 6], and zanubrutinib [n = 4]), with 20 ongoing at cutoff; 17 (13.0%) received venetoclax-based therapy, with 3 ongoing at final cutoff; 24 (18.3%) received chemoimmunotherapy (supplemental Table 5); and 11 (8.4%) received other novel agents. The median landmark PFS and OS were numerically longer in patients previously randomized to VenR vs BR after the initiation of subsequent therapies (supplemental Figure 1). After a median follow-up of 84 months, the median time from randomization to second PFS event was NR (95% CI, 87.1 months to NR) in initially VenR-treated patients and 77.8 months (95% CI, 61.1 to NR) in initially BR-treated patients (P < .0001; Figure 4A).
Kaplan-Meier estimates by time to second PFS event. Investigator-assessed time to second PFS event for patients receiving subsequent therapy previously randomized to VenR and BR (A) and patients previously randomized to VenR and BR receiving subsequent therapy by treatment type (B).
Kaplan-Meier estimates by time to second PFS event. Investigator-assessed time to second PFS event for patients receiving subsequent therapy previously randomized to VenR and BR (A) and patients previously randomized to VenR and BR receiving subsequent therapy by treatment type (B).
The median time to second PFS event from subsequent therapy in the VenR arm was 42.9 months (95% CI, 27.2 to NR) for patients receiving a BTKi, 59.9 months (95% CI, 34.0 to NR) for patients receiving venetoclax-based therapy, and 12.1 months (95% CI, 7.4-46.7) for patients receiving chemoimmunotherapy (Figure 4B). The median time to second PFS event from subsequent therapy in the BR arm was 44.6 months (95% CI, 32.8-50.3) for patients receiving a BTKi, NR (95% CI, 41.1 to NR) for patients receiving venetoclax-based therapy, and 14.6 months (95% CI, 9.0-26.9) for patients receiving chemoimmunotherapy (Figure 4B).
Among evaluable patients previously treated with VenR and BR, the best overall response rate (ORR) of subsequent venetoclax-based regimens was 76.2% (32/42) and 88.2% (15/17), respectively, whereas the best ORR of subsequent BTKi therapy was 82.6% (19/23) and 78.5% (51/65), respectively (supplemental Figure 2).
Substudy
Overall, 34 patients were enrolled in the substudy; 25 were retreated with VenR, and 9 crossed over to VenR from BR (supplemental Figure 3). Baseline demographics were similar to the main study (Table 1). Of the 25 VenR-retreated patients, 92.0% had ≥1 of the following high-risk features: del(17p) and/or TP53 mutation, IGHV unmutated disease, or GC (Table 1). CNAs and prevalence of high GC increased from the main study baseline to the retreatment baseline (supplemental Figure 4). No crossover patients acquired TP53 mutations, but a meaningful increase in del(17p) clone size was observed in 2 patients. Increase in GC was less prevalent than those observed in the retreatment arm (supplemental Table 6).
The median time between the last venetoclax dose in the main study and the first venetoclax dose in the substudy was 2.3 years (range, 1.2-3.1). Before VenR retreatment, patients with ≥2 years off treatment from the main study had longer PFS vs those with <2 years off treatment from the main study (supplemental Figure 5). Overall, the median follow-up was 33.4 months (range, 2.7-44.0); the median follow-up in the retreatment arm was 32.3 months (range, 2.7-44.0) vs 36.1 months (range, 24.0-39.8) in the crossover arm.
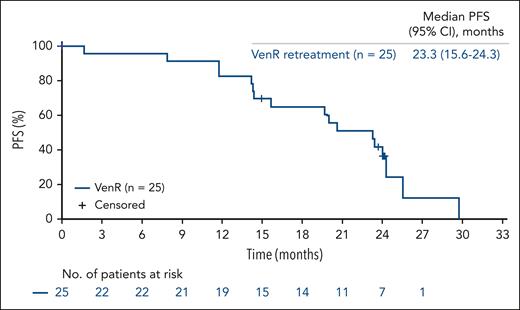
The median PFS in VenR-retreated patients was 23.3 months (95% CI, 15.6-24.3; Figure 5) vs 26.7 months (95% CI, 21.3 to NR) in patients who crossed over to VenR (supplemental Figure 6). The median OS was NR for both arms. Estimated OS rates at years 1, 2, and 3 were 96.0% (95% CI, 88.3-100.0), 79.6% (95% CI, 63.6-95.6), and 53.1% (95% CI, 25.1-81.0) in the retreatment arm, respectively, and 100.0% (95% CI, 100.0-100.0) for each year in the crossover arm. In the retreatment arm, 6 patients died due to PD, and 2 died due to AEs (COVID-19, myelodysplastic syndrome [MDS] progression, and sepsis). In the crossover arm, 1 patient died due to congestive heart failure.
Kaplan-Meier estimate of investigator-assessed PFS in the VenR retreatment population.
Kaplan-Meier estimate of investigator-assessed PFS in the VenR retreatment population.
The best ORR to VenR retreatment was 72.0%; 6 achieved CR, and 12 achieved PR. The median DOR was 15.5 months (95% CI, 11.5 to NR). The best ORR to crossover VenR was 88.9%; 2 patients with CR, 1 with CR with incomplete marrow recovery, 2 with nodular PR, and 3 with PR. The median DOR was 22.5 months (95% CI, 12.7 to not estimable).
Among VenR-retreated patients, 13 (52.0%) completed the full treatment course. Eight (32.0%) achieved uMRD at retreatment EOCT (4 had CR, 3 had PR, and 1 had stable disease); no patients retained uMRD at retreatment EOT. All 8 retreated patients who achieved uMRD at EOCT had unmutated IGHV, and 6 had normal TP53. Among crossover patients, 6 (66.7%) completed the full treatment course. Five (55.6%) had uMRD at crossover EOCT (3 had CR and 2 had PR), and 2 (22.2%) retained uMRD at crossover EOT.
Safety
An overview of safety in the main study and substudy is provided in Table 2; common AEs (≥2%) in the substudy are shown in supplemental Table 7. With longer follow-up, the safety profiles for VenR and BR remained consistent with the primary manuscript, and no new safety findings were observed beyond the 5-year data cut.
Safety summary for safety-evaluable patients in the main study and substudy
| . | Main study (N = 382) . | Substudy (n = 34) . | ||
|---|---|---|---|---|
| VenR (n = 194) . | BR (n = 188) . | Retreatment with VenR (n = 25) . | Crossed over to VenR (n = 9) . | |
| Total no. of patients with ≥1 AE, n (%) | 194 (100.0) | 185 (98.4) | 19 (76.0) | 9 (100.0) |
| Total no. of AEs | 2368 | 1877 | 60 | 26 |
| Total no. of deaths, n (%) | 60 (30.9) | 84 (44.7) | 8 (32.0) | 1 (11.1) |
| Total no. of patients withdrawn from study due to an AE, n (%) | 1 (0.5) | 0 | 0 | 0 |
| Total no. of patients with ≥1 AE with fatal outcome, n (%) | 18 (9.3) | 17 (9.0) | 1 (4.0) | 0 |
| Total no. of patients with ≥1 serious AE, n (%) | 101 (52.1) | 84 (44.7) | 13 (52.0) | 5 (55.6) |
| Total no. of patients with ≥1 related serious AE, n (%) | 44 (22.7) | 51 (27.1) | 4 (16.0) | 4 (44.4) |
| Total no. of patients with ≥1 AE leading to withdrawal from any treatment, n (%) | 37 (19.1) | 18 (9.6) | 2 (8.0) | 1 (11.1) |
| Total no. of patients with ≥1 AE leading to dose interruption, n (%) | 136 (70.1) | 76 (40.4) | 10 (40.0) | 8 (88.9) |
| Total no. of patients with ≥1 AE leading to dose reduction, n (%) | 30 (15.5) | 28 (14.9) | 2 (8.0) | 0 |
| Total no. of patients with ≥1 related AE, n (%) | 170 (87.6) | 170 (90.4) | 10 (40.0) | 7 (77.8) |
| Total no. of patients with ≥1 grade 3/4 AE, n (%) | 150 (77.3) | 121 (64.4) | 16 (64.0) | 8 (88.9) |
| . | Main study (N = 382) . | Substudy (n = 34) . | ||
|---|---|---|---|---|
| VenR (n = 194) . | BR (n = 188) . | Retreatment with VenR (n = 25) . | Crossed over to VenR (n = 9) . | |
| Total no. of patients with ≥1 AE, n (%) | 194 (100.0) | 185 (98.4) | 19 (76.0) | 9 (100.0) |
| Total no. of AEs | 2368 | 1877 | 60 | 26 |
| Total no. of deaths, n (%) | 60 (30.9) | 84 (44.7) | 8 (32.0) | 1 (11.1) |
| Total no. of patients withdrawn from study due to an AE, n (%) | 1 (0.5) | 0 | 0 | 0 |
| Total no. of patients with ≥1 AE with fatal outcome, n (%) | 18 (9.3) | 17 (9.0) | 1 (4.0) | 0 |
| Total no. of patients with ≥1 serious AE, n (%) | 101 (52.1) | 84 (44.7) | 13 (52.0) | 5 (55.6) |
| Total no. of patients with ≥1 related serious AE, n (%) | 44 (22.7) | 51 (27.1) | 4 (16.0) | 4 (44.4) |
| Total no. of patients with ≥1 AE leading to withdrawal from any treatment, n (%) | 37 (19.1) | 18 (9.6) | 2 (8.0) | 1 (11.1) |
| Total no. of patients with ≥1 AE leading to dose interruption, n (%) | 136 (70.1) | 76 (40.4) | 10 (40.0) | 8 (88.9) |
| Total no. of patients with ≥1 AE leading to dose reduction, n (%) | 30 (15.5) | 28 (14.9) | 2 (8.0) | 0 |
| Total no. of patients with ≥1 related AE, n (%) | 170 (87.6) | 170 (90.4) | 10 (40.0) | 7 (77.8) |
| Total no. of patients with ≥1 grade 3/4 AE, n (%) | 150 (77.3) | 121 (64.4) | 16 (64.0) | 8 (88.9) |
At 7 years, a numerically higher rate of patients with ≥1 SPM was observed with VenR (18.0%) vs BR (13.8%), with a total of 56 events in each arm (supplemental Table 8). The rate of SPMs per 100 patient-years was higher with BR (6.3%) vs VenR (4.9%). Four patients developed MDS (3 with VenR and 1 with BR). One patient in each arm developed acute myeloid leukemia. Since the 5-year data cut, 1 additional VenR-treated patient developed Richter transformation, resulting in a total of 8 patients (4.1%) in the VenR arm and 6 patients (3.2%) in the BR arm. The safety profile of VenR in the substudy remained consistent with that of the main study, with no new safety signals observed. Overall, the safety profile of VenR was acceptable and generally consistent with the known safety profiles of venetoclax and rituximab as single agents.
Discussion
This final long-term analysis of the MURANO trial, with a median follow-up of 85.7 months, continues to demonstrate clinically meaningful benefits for fixed-duration VenR over BR in relapsed/refractory CLL. VenR was associated with a clinically meaningful prolongation of PFS, an improvement in OS, and a longer TTNT vs BR. Most patients who completed 2 years of VenR had uMRD at EOT, and achievement of uMRD was associated with prolonged PFS; generally, MRD conversion with subsequent PD did not occur until ∼4 years after EOT.
Although the structural/functional consequences of acquired BCL2 mutations reported in literature vary, they are typically associated with reduced binding affinity and/or diminished susceptibility to venetoclax inhibition in vitro.37 However, in some cases, these variants remain sensitive to clinically relevant concentrations of venetoclax, especially in combination with CD20 antibodies.36 In this cohort, we showed that the acquisition of BCL2 mutations was rare and did not preclude attainment of disease response to retreatment. More studies, especially exploring serial changes in disease subpopulations during therapy, are required to evaluate their impact on the durability of these responses.
In the substudy, with a median follow-up of 33.4 months, both retreated patients and those who crossed over to VenR had high ORR, and over one-third achieved uMRD at EOCT. PFS was almost 2 years in retreated patients; because early progressors in the BR arm were not captured, PFS in patients who crossed over to VenR was slightly higher at just over 2 years. Overall, VenR retreatment is a viable option after initial VenR. Larger cohorts will be required to identify the influence of duration of time off venetoclax and biologic features that may predict favorable responses to VenR retreatment, but it is biologically plausible that retreatment may be optimal in patients with longer time (such as ≥2 years) off venetoclax or in patients achieving uMRD at EOT with initial VenR.
Safety data were consistent with those reported previously, with no new safety signals observed. The safety profile of VenR continues to be manageable, predictable, and consistent with the known safety profile of both agents. There were no clinically meaningful differences in rates of SPM, including myeloid malignancies or Richter transformation, between treatment arms.
Although patients in our study had relapsed/refractory CLL, they had not previously received novel agents; only 5 (VenR) and 2 (BR) patients received B-cell receptor inhibitors (BCRi) before enrollment,32 so outcomes in this study may not be applicable to the current relapsed/refractory CLL population. Of note, the VENICE-1 study demonstrated deep and durable responses with venetoclax monotherapy for both BCRi-naïve and BCRi-pretreated patients.11 Due to the frequent use of fixed-duration regimens and the combination of targeted therapies, more relapsed patients will have been exposed to venetoclax. It was hypothesized that among previously exposed patients, BTKis at the time of relapse would result in longer PFS vs retreatment with venetoclax-based therapies. However, this was not observed in MURANO; the median time to second PFS event was 42.9 and 59.9 months, respectively. Of note, only a few BR-treated patients received venetoclax-based next therapy, because these were not approved/available when patients needed next therapy (the US Food and Drug Administration granted approval for use in relapsed/refractory CLL in June 2018). Another feature of the study population that reflects the era of conduct was that some patients received further chemoimmunotherapy at PD (17.2% after VenR and 18.3% after BR), which is no longer recommended38 and may have compromised OS outcomes; however, these rates were similar between treatment arms.
Current literature on venetoclax retreatment is limited. Among 9 patients retreated with VenR in a phase 1b study, all achieved PR or better.39 Similarly, a retrospective study of venetoclax retreatment in heavily pretreated patients (n = 46) demonstrated high ORR (79.5%) and a median PFS of 25 months.40 Our substudy results are consistent with the following: retreated patients had high ORR and a median PFS of almost 2 years. Although PFS was longer in crossover vs retreatment patients, the increased toxicity from numerous chemoimmunotherapies may increase the risk of secondary MDS/acute myeloid leukemia and drive the development of unfavorable prognostic clones compared with targeted combination therapy.
Despite limited data on BTKis after venetoclax-based therapy in first-line or relapsed/refractory CLL, some studies suggest that BTKis could be a good option for salvage therapy.41-43 A large retrospective study demonstrated higher ORR (84%) in BTKi-naïve patients receiving BTKis after venetoclax vs those who had previously received BTKis (54%).41 BTKis provided durable disease control after PD on venetoclax in a retrospective evaluation of 23 patients with relapsed/refractory CLL.42 In the CAPTIVATE study, 21 of 22 patients retreated with ibrutinib after PD following ibrutinib and venetoclax were evaluable: all responded except for 1 with stable disease and 1 with PD.44 Furthermore, pirtobrutinib demonstrated high ORR (73.3%) in patients with heavily pretreated CLL or small lymphocytic lymphoma (247 had previously received a BTKi and 100 had also received a B-cell lymphoma 2 inhibitor such as venetoclax).6 Sustained benefits after venetoclax cessation may provide an opportunity for patients to experience a treatment-free interval before BTKi initiation.
In a retrospective analysis of heavily pretreated patients with CLL, the ORR was 79% in patients who received venetoclax after ibrutinib discontinuation vs 46% in those who received idelalisib.45 Similarly, in a study of 144 ibrutinib-treated patients with CLL, salvage therapy with venetoclax-based treatments resulted in longer OS and treatment-free survival vs phosphatidylinositol 3-kinase inhibitor–based treatment, chemoimmunotherapy, and anti-CD20 treatment.46 In a real-world study, switching from a BTKi to venetoclax-based therapy resulted in higher ORR (84%) vs a different BTKi (63%).6 Altogether, these indicate the positive potential for venetoclax regimens after progression on BTKis.
Conclusions
This final long-term analysis of the MURANO trial continues to demonstrate clinically meaningful results for fixed-duration VenR in patients with relapsed/refractory CLL. Based on the substudy, retreatment with VenR is a viable option after initial VenR treatment; its implementation in routine clinical practice deserves further investigation. Overall, these data continue to support the use of fixed-duration VenR in patients with relapsed/refractory CLL.
Acknowledgments
The authors thank the patients and their families and all individuals involved in the study.
Venetoclax is being developed in a collaboration between Genentech, Inc and AbbVie. Genentech, Inc and AbbVie provided financial support for the study and participated in the design, study conduct, analysis, and interpretation of data, as well as the writing, review, and approval of the manuscript. Third-party medical writing assistance under the direction of the authors was provided by Vanessa Poon and Rachel Dobb of Ashfield MedComms, an Inizio company, and was funded by F. Hoffmann-La Roche Ltd.
Authorship
Contribution: J.F.S., R.H., T.J.K., and A.P.K. designed the study; J.F.S., R.H., T.J.K., J.d.l.S., and A.P.K. conducted the study; J.F.S., R.H., T.J.K., B.E., C.J.O., S.A., N.L., T.R., J.d.l.S., U.J., G.C., M.M., and A.P.K. were responsible for recruitment and follow-up of patients; J.F.S., R.H., T.J.K., B.E., S.A., T.R., J.d.l.S., U.J., M.M., C.M., A.W.L., Y.J., R.M., M.L., M.T.-M., M.B., and A.P.K. collected data; J.F.S., R.H., T.J.K., B.E., S.A., T.R., J.d.l.S., U.J., M.M., C.M., A.W.L., B.C., R.P., Y.J., R.M., M.L., M.T.-M., M.B., and A.P.K. analyzed data; J.F.S., R.H., T.J.K., B.E., C.J.O., S.A., N.L., T.R., J.d.l.S., U.J., G.C., M.M., B.C., R.P., Y.J., R.M., M.L., M.T-M., M.B., and A.P.K. interpreted data; and all authors contributed to manuscript review and approval and agreed to be accountable for the contents.
Conflict-of-interest disclosure: A.P.K. reports current employment with Amsterdam University Medical Centers, University of Amsterdam; leadership roles in Haemato-Oncology Cooperative Group for Adults in the Netherlands (HOVON) (president of executive board, chairman of the Chronic Lymphocytic Leukemia [CLL] Working Group), Amsterdam University Medical Center (Chairman, Good Research Practice Committee), European Hematology Association (Chairman, Scientific Working Group on CLL), and European Research Initiative on CLL (ERIC; member executive board); consulting or advisory role in and research funding from AbbVie, AstraZeneca, Bristol Myers Squibb (BMS), Janssen, Genmab, Lava Therapeutics, and Roche/Genentech; speaker’s bureau fees from AbbVie and Janssen; and travel, accommodation, and expenses from AbbVie, AstraZeneca, Janssen, and Roche/Genentech. R.H. reports consulting or advisory role fees from AstraZeneca (20 July 2021). T.J.K. reports research funding and/or advisory role fees from Ascerta/AstraZeneca, Celgene, Roche/Genentech, Gilead, Janssen, Loxo Oncology, TG Therapeutics, Verastem, Pharmacyclics/AbbVie, Oncternal Therapeutics Inc, Leukemia and Lymphoma Society, California Institute for Regenerative Medicine, National Cancer Institute (NCI)/National Institutes of Health (NIH), and VelosBio, Inc (research agreement); travel/honoraria fees from Pharmacyclics/AbbVie, Roche/Genentech, Janssen, Gilead, NCI/NIH, Celgene, ERIC, Dava Oncology, International Workshop on Non-Hodgkin Lymphoma National Comprehensive Cancer Network CLL/Small Lymphocytic Lymphoma (SLL) Hairy Cell Leukemia Panel, and OncLive; and holds patents, royalties, or other intellectual property. Cirmtuzumab was developed by T.J.K. in the T.J.K. Laboratory and licensed by the University of California to Oncternal Therapeutics, Inc., which provided stock options and research funding to the T.J.K. Laboratory B.E. reports current employment with University Hospital Cologne; honoraria from Roche, AbbVie, BeiGene, AstraZeneca, and Merck Sharp & Dohme (MSD); consulting or advisory role with Janssen, AbbVie, Gilead, AstraZeneca, BeiGene, MSD, and Lilly; speakers bureau fees from Roche, AbbVie, BeiGene, AstraZeneca, and MSD; research funding from Janssen, Roche, AbbVie, BeiGene, and AstraZeneca; and travel, accommodation, and expenses from BeiGene. C.J.O. reports honoraria from AbbVie, AstraZeneca, BeiGene, Janssen, Merck, Incyte, Novartis, Seattle Genetics, and Roche. S.A. reports honoraria and consulting or advisory role fees from AbbVie, Roche, AstraZeneca, BMS, Paladin, Novartis, Pfizer, and Janssen and research funding from Novartis. N.L. reports consulting or advisory role fees from AbbVie, AstraZeneca, BeiGene, Eli Lilly/Loxo, Genentech, Janssen, and Pharmacyclics and research funding to institution from AbbVie, AstraZeneca, BeiGene, Eli Lilly/Loxo, Genentech, MingSight, Octapharma, Oncternal, and TG Therapeutics. T.R. reports current employment with Medical University of Lodz, Copernicus Memorial Hospital, Lodz, Poland; honoraria and research funding from AbbVie, Janssen, AstraZeneca, BeiGene, Regeneron, and Octapharma; consulting or advisory role fees from AstraZeneca and BeiGene; and travel, accommodation, and expenses from Janssen and AstraZeneca. J.d.l.S. reports consulting or advisory role fees from AbbVie, AstraZeneca, BeiGene, and Roche and travel, accommodation, and expenses from AbbVie and AstraZeneca. U.J. reports honoraria from Roche and AbbVie. G.C. reports honoraria from Gilead Sciences, Janssen, Celgene, Roche, AbbVie, and Novartis; consulting or advisory role fees from Roche, Celgene, Mabqi, and MedXCell; and travel, accommodation, and expenses from Roche. M.M. reports current employment as a consultant in hematology and honoraria from AbbVie and Janssen. C.M. reports current employment with Amsterdam University Medical Centers as cytogeneticist in Human Genetics and research funding for financing for array-analysis MURANO sample from Roche. A.W.L. reports current employment as medical immunologist with Erasmus University Medical Center, Rotterdam; research funding for financing for molecular minimal residual disease analysis of MURANO samples from Roche; and honoraria and research funding from Roche/Genentech and Janssen. B.C. and R.P. report current employment and stock ownership in AbbVie. M.T.-M. reports current employment and stock ownership in Roche. M.L. reports current employment with BeiGene (ended employment in the past 24 months) and held stock ownership with Roche. Y.J. reports current employment and stock ownership in Roche/Genentech. R.M. reports current employment with AstraZeneca; ended employment in the past 24 months with Roche; ended employment in the past 24 months with and honoraria from Hubrecht Institute; and research funding from Oncode Institute. M.B. reports current employment, stock ownership, and honoraria from Roche/Genentech. J.F.S. reports honoraria from AbbVie, AstraZeneca, BeiGene, BMS, Gilead, Janssen, and Roche; consulting or advisory role fees from AbbVie, AstraZeneca, BeiGene, BMS, Genor Bio, Gilead, Janssen, Roche, and TG Therapeutics; speakers bureau fees and travel, accommodation, or expenses from AbbVie, AstraZeneca, and Roche; research funding from AbbVie, BMS, Janssen, and Roche; patents, royalties, and other intellectual property from AbbVie; and expert testimony fees from BMS and TG Therapeutics.
Correspondence: John F. Seymour, The Royal Melbourne Hospital, Peter MacCallum Cancer Centre and The University of Melbourne, 305 Grattan St, Melbourne, VIC 3000, Australia; email: john.seymour@petermac.org.
References
Author notes
Presented as an oral presentation at the 28th European Hematology Association annual meeting, Frankfurt, Germany, 8-11 June 2023, and as an oral presentation at the 17th International Conference on Malignant Lymphoma meeting, Lugano, Switzerland, 13-17 June 2023.
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/).
Further details on Roche's criteria for eligible studies are available at https://vivli.org/members/ourmembers/. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.




![Kaplan-Meier estimates of efficacy end points by MRD∗ response status at EOT in the overall intent-to-treat population. (A) PFS; (B) OS. Fisher’s exact test was performed to compare MRD status at EOCT and EOT. ∗MRD was categorized as uMRD (<1 CLL cell per 10 000 leukocytes; MRD value <0.0001 [10–4]) and MRD+ (≥10–4).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/145/23/10.1182_blood.2024025525/2/m_blood_bld-2024-025525-gr2.jpeg?Expires=1764959661&Signature=SG5u3tybPvtD2QSY7BkCD9-sND~7FviM8nGQti~hn9MSRvNnm~NJd6PxBnHVQLr-xNYwjv6NHCXP3jPce6u460g5rXIPGLSn0fRe7tmyOxO2LwZdPg43uw9As5Sn7i9-8hk3OJlPzOuySrRWHhM2zPecCexLLzVU1gwXkJ8J6qpH9NX1xf-pUZpHC~HQPcRg-5YyquA8eRKdmayRN1Aja~x3stXoT1XkXkqjUuAbxvhKdkpCdr~fV8A6bb6p5TuwoKlAUZ-nzinbE~RPCQdk0HfKutBExBOb254numTL16lOWataPsoEUnDRgMo3iQazQ0fka5AEe4Oj7jRVFVBJ1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Kaplan-Meier estimates by MRD status and MRD conversion among VenR-treated patients in the overall intent-to-treat population. (A) Time to MRD∗ conversion; (B) time from conversion to PD; (C) time from PD to next treatment. ∗MRD was categorized as uMRD (<1 CLL cell per 10 000 leukocytes; MRD value <0.0001 [10–4]) and MRD+ (≥10–4).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/145/23/10.1182_blood.2024025525/2/m_blood_bld-2024-025525-gr3.jpeg?Expires=1764959661&Signature=XCzQKohGatUl9Pvdq1004SliuJ9yzfwsoW5S9WjspEf3k7wHTz1gVd0uDQ~SWo3-GwEqczWFtubweWhu5jcxSWdOoOx9dSyD1hrk9NXMqa9JtTKTtK8zFSILN86ahe7o7SGiBvd~Vf7z~M0livbBd7k3qno~h0B4CivZ36SX4agIWUwL1guvOAsZaSOPadJVQf3NIy0d9i3VlhvRvCtDVnhvwcMF6pLdPnT9ICuesGDsLSFGM1XHtNI4COh0WoyJFpRMRSba-onA5wovRMRgzAyuVOuEbT0LlqDwI-QjMN36z11om9OuyuNEHyXq6742ZGnlgZpN09A1mCoSeq3fZg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal