In this issue of Blood, Kawano et al1 identify interleukin-1 receptor type 1 (IL-1R1) and IL-18 as key regulatory factors for mesenchymal stromal cell (MSC) dysfunction in a myelodysplastic syndrome (MDS) model in aged mice, thereby uncovering potential therapeutic targets.
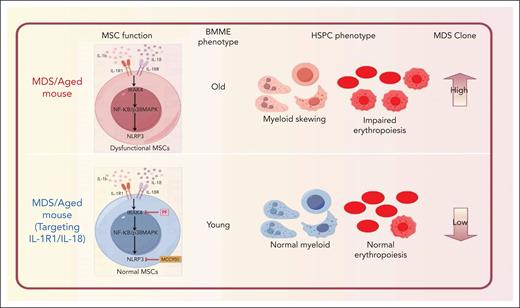
MDSs are hematopoietic stem cell (HSC) disorders associated with bone marrow (BM) dysfunction and a high risk of progressing to acute leukemia.2 Despite significant attention paid to the genetic drivers of MDS,3 increasing evidence highlights the essential role of the bone marrow microenvironment (BMME) in disease initiation and progression of MDS.4,5 Kawano et al demonstrate that inflammatory cytokines IL-1 and IL-18 play a pivotal role in MDS progression and provide compelling evidence for targeting IL-1R1 signaling to reverse MDS-associated changes in BM-derived MSCs and mitigate disease phenotypes in an aged murine model (see figure).
IL-1R1/NLRP3 signaling in the MDS/aged-associated mouse influences MSC dysfunction, BMME aging, skewing hematopoietic lineage differentiation, and promoting MDS clonal expansion. Targeting IL-1R1/IL-18 with IRAK4 inhibitor (PF06650833) and NLRP3 inhibitor (MCC950) reverses these phenotypes, restoring BM niche functionality.
IL-1R1/NLRP3 signaling in the MDS/aged-associated mouse influences MSC dysfunction, BMME aging, skewing hematopoietic lineage differentiation, and promoting MDS clonal expansion. Targeting IL-1R1/IL-18 with IRAK4 inhibitor (PF06650833) and NLRP3 inhibitor (MCC950) reverses these phenotypes, restoring BM niche functionality.
Although previous studies have demonstrated that some pro-inflammatory cytokines, such as tumor necrosis factor-α, IL-6, and IL-8, are markedly elevated in peripheral blood of MDS patients,6,7 there is still a lack of necessary evidence to prove which factors are the most critical for the eventual predominance of mutant hematopoietic stem and progenitor cells (HSPCs) in MDS. This study reveals that the levels of IL-1 family cytokines, including IL-18, were elevated in the BM plasma of MDS patients. Both IL-1β and IL-18 were shown to exert significant effects on MSCs, disrupting their capacity to support HSPCs, and promoting skewed lineage differentiation. These findings emphasize that the pro-inflammatory milieu is a driver of MSC dysfunction in MDS. In addition, the authors demonstrate that IL-1β robustly activates the NF-κB signaling pathway in MSCs, enhancing their capacity to support HSCs and erythroid progenitors. In contrast, IL-18 activated the mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt pathways without affecting NF-κB signaling. These 2 cytokines indirectly promote the proliferation of MDS clone cells by affecting MSCs. These findings highlight the importance of the niche in shaping the progression of MDS.
Finally, using a murine model of MDS,8 the authors demonstrated that targeting IL-1R1 and NLRP3 signaling with inhibitors such as PF06650833 (IRAK4 inhibitor) and MCC950 (NLRP3 inhibitor) not only mitigated the expansion of dysfunctional MSCs but also selectively suppressed MDS clonal cells while sparing non-MDS cells, emphasizing its potential as a dual-action therapy for MDS. NLRP3 inhibition, on the other hand, mitigated aging-associated megakaryocytic skewing of HSCs, further supporting the therapeutic potential of inflammation-targeted therapies.
A key strength of the study lies in its comprehensive approach to examining the effects of IL-1R1 and IL-18 signaling on MSCs. The authors demonstrate that these pathways influence MSC functionality, particularly their ability to support HSPCs and erythroid progenitors. By employing highly purified primary MSCs, the researchers helped to control the confounding effects of other BM cell populations, thereby enhancing the specificity of their findings.
Although other studies have addressed the role of aging and inflammation on MSCs and MDS,9,10 Kawano et al explored the regulation of MSCs in BMME via targeting IL-1R and IL-18 signals, which can mitigate MDS in age-appropriate MDS model. Their work represents a significant advancement in our understanding of the inflammatory BM niche in MDS. By elucidating the roles of IL-1R1 and IL-18 signaling in MSC dysfunction and identifying viable therapeutic targets, the authors have paved the way for innovative treatments that address both the clonal and microenvironmental aspects of the disease. Although challenges remain in translating these findings into clinical practice, the study provides a robust foundation for future research and underscores the importance of targeting inflammation in age-related hematological disorders. The integration of molecular, cellular, and therapeutic insights sets a high bar for research in the field and offers hope for improving outcomes in patients with MDS. With further validation and clinical investigation, the findings could contribute to a paradigm shift in the management of this challenging disease.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal