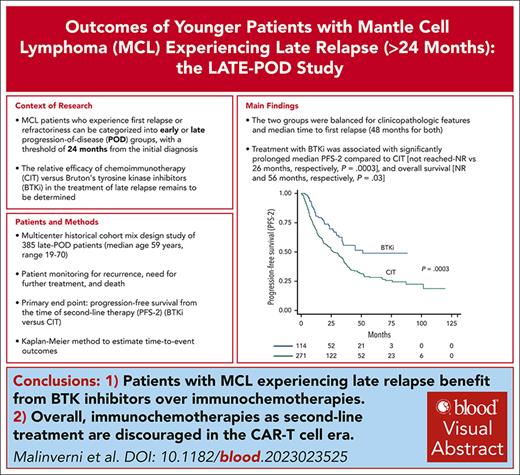
Key Points
Patients with MCL experiencing late relapse benefit from BTK-inhibitors over chemoimmunotherapies.
Overall, chemoimmunotherapies as second-line treatment are discouraged in the era of chimeric antigen receptor T-cell therapies.
Visual Abstract
Patients with mantle cell lymphoma (MCL) who experience first relapse/refractoriness can be categorized into early or late progression-of-disease (POD) groups, with a threshold of 24 months from MCL diagnosis. Bruton tyrosine kinase inhibitors (BTKi) are the established standard treatment at first relapse, but their effectiveness compared with chemoimmunotherapy (CIT) in late-POD patients remains unknown. In this international, observational cohort study, we evaluated outcomes among patients at first, late POD beyond 24 months. The primary objective was progression-free survival from the time of second-line therapy (PFS-2) of BTKi vs CIT. Overall, 385 late-POD patients were included from 10 countries. Their median age was 59 years (range, 19-70), and 77% were male. Median follow-up from the time of second-line therapy was 53 months (range, 12-144). Overall, 114 patients had second-line BTKi, whereas 271 had CIT, consisting of rituximab-bendamustine (R-B; n = 101), R-B and cytarabine (R-BAC; n = 70), or other regimens (mostly cyclophosphamide-hydroxydaunorubicin-vincristine-prednisone]- or platinum-based; n = 100). The 2 groups were balanced in clinicopathological features and median time to first relapse. Overall, BTKi was associated with significantly prolonged median PFS-2 than CIT (not reached [NR] vs 26 months, respectively; P = .0003) and overall survival (NR and 56 months, respectively; P = .03). Multivariate analyses showed that BTKi was associated with lower risk of death than R-B and other regimens (hazard ratio, 0.41 for R-B and 0.46 for others), but similar to R-BAC. These results may establish BTKi as the preferable second-line approach in patients with BTKi-naïve MCL.
Introduction
For younger patients with mantle cell lymphoma (MCL), the standard first-line treatment typically includes rituximab and high-dose cytarabine (HD-AraC), usually followed by autologous stem cell transplantation1 consolidation. This treatment ensures prolonged remissions for most patients, and has been recently successfully improved by the Bruton tyrosine kinase inhibitor (BTKi) ibrutinib in association with chemoimmunotherapy (CIT).2 Despite continuous improvement in survival expectations by the addition of new drugs, a plateau in the survival curves has not been observed to date, and virtually all patients experience relapse.3 For patients who are refractory to induction or relapse within 24 months from initial diagnosis (early progression of disease [POD]), BTKi has been demonstrated to be superior to CIT,4,5 and has become a standard second-line approach, as reported by treatment guidelines.6,7 Indeed, POD within 24 months can identify almost half of the patients with MCL who have significantly shorter survival (among whom there is a high proportion of high-risk features) and may benefit from investigational approaches.8-10 In contrast, in the late-POD group, in which expected survival is much longer, BTKi and CIT have both been considered as valid second-line options depending on age and disease characteristics at relapse. So far, the only study comparing CIT with BTKi in this setting failed to demonstrate any difference in survival outcomes between the 2 approaches, but was limited by the small number of patients treated with ibrutinib (n = 50; 19% of the sample) and relatively short follow-up.4 Late-POD patients have therefore not been systematically investigated in the literature. Through this multinational effort, we aimed to compare BTKi and CIT in a substantial cohort of late-POD patients, who were followed up prospectively to prolong median follow-up. Our findings indicate that late-POD patients benefit from second-line treatment with BTKi, similarly to early-POD patients.
Patients and methods
Study design
This multicenter, historical cohort, mixed-design study incorporates retrospective data collection based on clinical records, and proposes a sequential data collection in line with a prospective approach for a cohort of patients with relapsed/refractory MCL, who experienced late-POD. Data for this study were contributed by a total of 30 centers affiliated with the Fondazione Italiana Linfomi, and 9 additional European centers that are part of the European Mantle Cell Lymphoma Network. Patients with MCL were included in this study if they met the following inclusion criteria: (1) the first recurrence of the disease occurred at least 24 months after the initial diagnosis; (2) they received up-front treatment with intensive regimens that included rituximab and HD-AraC (defined as single cytarabine dose >1 g/m2); (3) they were not observed for >3 months after initial diagnosis, to exclude cases with indolent clinical behavior; (4) the diagnosis of MCL was made between 1 January 2008 and 30 June 2019; and (5) they were treated at the time of relapse based on the initial treatment intent, with at least 1 cycle of CIT, BTKi, or alternative drug combinations. Patients treated upfront with BTKi were excluded, as were patients with central nervous system involvement.11 Overall, 134 patients were previously included in the MANTLE-FIRST study,4 but their follow-up was updated and prolonged for the specific purpose of the current study.
Patients were monitored until the end of the follow-up date (10 December 2022) for recurrence, need for further treatment, and death. All events were validated by reviewing each patient’s medical record. Treatment at diagnosis and recurrence was determined by the attending physician. The diagnosis of MCL and the morphological classification were based on World Health Organization criteria12 at each center. The protocol was approved by the institutional review boards of the participating centers or national ethical committees.
Treatment
All patients were treated first-line with rituximab- and HD-AraC–containing regimens that were divided into 3 groups, as previously described.4 Based on the second-line therapy received, 2 categories were defined. One group was composed of patients treated in second-line with BTKi (ibrutinib monotherapy for the vast majority of patients) and the other of patients treated with CIT, consisting of rituximab-bendamustine (R-B), R-B and cytarabine (R-BAC), or alternative combination regimens. Consolidation with autologous or allogeneic transplantation was permitted after a second or further remission, provided that a specific analysis was conducted to censor the follow-up of these patients at the time of transplantation, thus limiting the confounding effect of these therapies on the final outcome.
Outcome measures and sample size calculation
The primary aim of the study was to assess the outcomes of patients with late-POD MCL based on the type of treatment they received at the first relapse. The primary end point was progression-free survival (PFS) associated with the use of BTKi vs CIT as second-line therapy, calculated from the initiation of second-line therapy (PFS-2) until lymphoma relapse, progression, death from any cause, or end of the studies (administrative censoring). Overall survival from the initiation of second-line therapy (OS-2) was defined as the time until death from any cause, with censoring at the last contact for patients who survived beyond the follow-up period. TP53 mutations were searched in a proportion of patients with lymphoma diagnoses by conventional Sanger sequencing or by high-throughput next-generation sequencing.
The sample size was calculated in a 2:1 CIT-to-BTKi ratio to reject the null hypothesis that the PFS curves of the 2 groups were equal, with a probability (statistical power) equal to 0.80. The type I error associated with testing the null hypothesis was 0.05. On the basis of our hypothesis and previous observations of an expected median PFS-2 for patients treated with CIT and BTKi of 36 and 60 months, respectively, 200 patients treated with CIT and 100 treated with BTKi were needed to compare the PFS-2 associated with the 2 approaches. It was required that, after the accrual period of 1 month, all patients were prospectively followed up to reach a median follow-up from the time of relapse of 48 months for the final analysis.
Statistical analyses
The Kaplan-Meier method was applied to estimate time-to-event outcomes (OS-2 and PFS-2), and comparisons between treatment groups were performed using the stratified log-rank test. All variables listed in Table 1 were computed in univariate analysis, as was calendar time, such as calendar year or period of diagnosis or relapse in the model. Proportional Cox regression models were used to perform comparisons between treatment groups, and the results were expressed as hazard ratios (HRs), representing the relative risk of computed variables based on comparison of event rates. Patients who did not log an event in the trial time frame were censored on the last valid lymphoma assessment date. In the OS-2 analysis, patients still alive at the time of the last follow-up were censored on the last date known to be alive. All confidence intervals (CI) were calculated at the 95% level and all P values reported were 2-sided. Statistical analyses were performed using Stata 13 (StataCorp, College Station, TX).
Clinical and pathological characteristics of 385 late-POD patients, divided by second-line therapies
| . | All patients N = 385 . | BTKi n = 114 . | CIT n = 271 . | P value for comparison . |
|---|---|---|---|---|
| Median age (range) | 59 (19-70) | 57 (19-70) | 59 (35-70) | |
| <60 | 200 (52%) | 63 (55%) | 137 (50%) | .37 |
| Male sex | 292 (77%) | 86 (75%) | 206 (76%) | .94 |
| MIPI score | ||||
| Low | 144 (39%) | 43 (42%) | 101 (39%) | .71 |
| Intermediate | 131 (36%) | 40 (39%) | 91 (25%) | |
| High | 90 (25%) | 20 (19%) | 70 (26%) | |
| Morphology | ||||
| Blastoid or pleomorphic∗ | 40 (12%) | 12 (11%) | 28 (12%) | .68 |
| Ki-67, ≥30%∗ | 99 (44%) | 31 (38%) | 68 (47%) | .16 |
| TP53 mutational status∗ | 9 (15%) | 3 (17%) | 6 (14%) | .75 |
| Median time to POD | ||||
| Months (range) | 48 (24-165) | 48 (24-145) | 48 (24-165) | .49 |
| Up-front treatment | ||||
| RHyperCVAD/MTXHDAC | 53 (14%) | 15 (13%) | 66 (24%) | .06 |
| R-CHOP/DHAP + ASCT | 107 (28%) | 41 (36%) | 38 (14%) | |
| Nordic/R-HDS | 226 (58%) | 58 (51%) | 168 (62%) | |
| Country of enrollment | ||||
| Italy | 201 (52%) | 62 (54%) | 139 (51%) | .58 |
| Other countries | 184 (48%) | 52 (46%) | 132 (49%) |
| . | All patients N = 385 . | BTKi n = 114 . | CIT n = 271 . | P value for comparison . |
|---|---|---|---|---|
| Median age (range) | 59 (19-70) | 57 (19-70) | 59 (35-70) | |
| <60 | 200 (52%) | 63 (55%) | 137 (50%) | .37 |
| Male sex | 292 (77%) | 86 (75%) | 206 (76%) | .94 |
| MIPI score | ||||
| Low | 144 (39%) | 43 (42%) | 101 (39%) | .71 |
| Intermediate | 131 (36%) | 40 (39%) | 91 (25%) | |
| High | 90 (25%) | 20 (19%) | 70 (26%) | |
| Morphology | ||||
| Blastoid or pleomorphic∗ | 40 (12%) | 12 (11%) | 28 (12%) | .68 |
| Ki-67, ≥30%∗ | 99 (44%) | 31 (38%) | 68 (47%) | .16 |
| TP53 mutational status∗ | 9 (15%) | 3 (17%) | 6 (14%) | .75 |
| Median time to POD | ||||
| Months (range) | 48 (24-165) | 48 (24-145) | 48 (24-165) | .49 |
| Up-front treatment | ||||
| RHyperCVAD/MTXHDAC | 53 (14%) | 15 (13%) | 66 (24%) | .06 |
| R-CHOP/DHAP + ASCT | 107 (28%) | 41 (36%) | 38 (14%) | |
| Nordic/R-HDS | 226 (58%) | 58 (51%) | 168 (62%) | |
| Country of enrollment | ||||
| Italy | 201 (52%) | 62 (54%) | 139 (51%) | .58 |
| Other countries | 184 (48%) | 52 (46%) | 132 (49%) |
MIPI, Mantle Cell Lymphoma International Prognostic Index; Nordic/R-HDS, rituximab, cyclophosphamide, vincristine, doxorubicin, prednisone (maxi-CHOP), alternating with rituximab and HD-AraC, followed by autologous stem cell transplantation; RHyperCVAD/MTXHDAC, fractionated cyclophosphamide, doxorubicin, vincristine, and dexamethasone alternated with high-dose methotrexate and cytarabine; R-CHOP/DHAP + ASCT, R-CHOP alternated with cisplatin-cytarabine and dexamethasone, followed by autologous stem cell transplantation. Fifty-two patients received rituximab maintenance as consolidation of ASCT.
Data not available for all patients: MIPI available for 365; morphology for 335; Ki67 for 226; and TP53 mutation for 62. Differences between CIT and BTKi were similar when the analysis was limited to the 259 patients who were included and treated after 2014, when BTKi were available (P = .26 for age; P = .78 for sex; P = .58 for morphology; P = .29 for Ki67; and P = .33 for time to POD).
The protocol was approved by the institutional review boards of the participating centers or national ethical committees.
Results
Patient characteristics and second-line treatment allocation
The patient cohort examined included clinical records of 385 patients with MCL who relapsed after a minimum period of 24 months from diagnosis (late POD). The median age of the cohort was 59 years (range, 19-70), 77% were male, and the median time from MCL diagnosis to first relapse was 48 months (range, 24-165). Clinical and pathological characteristics of the patients are shown in Table 1. Second-line treatment consisted of BTKi (ibrutinib monotherapy in 99% of cases) in 114 patients (30%), whereas 271 had CIT. CIT consisted of R-B in 101 patients (26%), R-BAC in 70 patients (18%), and a variety of different treatments in 100 patients (26%). These treatments, all including rituximab, also included cyclophosphamide-hydroxydaunorubicin-vincristine-prednisone (R-CHOP) or CHOP-like regimens with or without radiotherapy (n = 34), platinum-based chemotherapy (n = 19), bortezomib-based combinations (n = 14), HD-AraC plus dexamethasone (n = 10), lenalidomide-based combinations (n = 7), or a variety of alkylator-based, temsirolimus, or gemcitabine/fludarabine–containing combinations (n = 16). Overall, 25 patients (6%) were consolidated with autologous transplantation, whereas 72 (19%) had allogeneic transplantation in the second (n = 60) or further remission (n = 12). The distribution of patients who underwent transplantation according to previously defined groups of second-line treatment was reported in supplemental Table 1, available on the Blood website. Patients who received an autologous transplant after second-line treatment were similarly distributed in the different therapeutic groups (7% of the CIT and 5% of the BTKi group; supplemental Table 1). Allogeneic transplant was instead administered more frequently after R-BAC (31%; P = .003) than after other second-line treatments, with 27 patients (24%) in the BTKi group consolidated with allogeneic transplantation.
Given that this was not a randomized study, clinical or pathological differences in distribution of patients between the CIT and the BTKi groups were expected, but, as shown in Table 1, none were statistically significant. Significant differences emerged in the subgroups of CIT-treated patients (R-BAC vs R-B). As shown in supplemental Table 2, R-BAC-treated patients were younger than R-B-treated patients (median 54 vs 62 years), and differed when stratified according to their first-line treatment.
Time-to-event outcomes
With a median follow-up of 53 months (range, 1-144) from the time of first relapse, the median OS-2 and PFS-2 for the entire series were 60 months (95% CI, 51-72) and 33 months (95% CI, 26-36), respectively (Figure 1). The median follow-up was significantly longer for patients treated with CIT (64 months [range, 3-144]) than for patients treated with BTKi (34 months [range, 1-85]; P = .001), which reflects the recent shift toward the use of BTKi in patients with MCL (available in Europe since 2014). Median time on active BTKi treatment for all patients was 28 months (range, 1-85), with 51 patients (45%) who were continuing treatment at the point of data collection cutoff, 36 patients (31%) discontinuing because of disease progression, 9 (8%) because of toxicity, and 18 (16%) because of stem cell transplantation or death. The use of BTKi as second-line treatment was associated with significantly longer median PFS-2 compared with CIT (not reached vs 26 months [95% CI, 20-35], respectively; P = .0003; Figure 2A). Importantly, BTKi was also associated with better OS-2 compared with CIT (not reached vs 56 [95% CI, 43-69] months, respectively; P = .04; Figure 2B). When patients treated with CIT were divided into the 3 predefined groups, we observed that BTKi and R-BAC showed comparable outcomes both in terms of PFS-2 and OS-2, whereas treatment with R-B and/or other regimens was associated with significantly worse PFS-2 and OS-2 (P = .0003 and P = .018, respectively; Figure 3). Further subanalysis of outcomes according to treatment subgroups is shown in supplemental Figure 1. The same analysis performed by censoring patients at the time of allogeneic transplantation demonstrated that treatment consolidation did not significantly modify the effect of the treatments on OS-2, confirming the significant benefit of BTKi compared with CIT (P = .004; supplemental Figure 2). PFS-2 and OS-2 of 106 late-POD patients treated with BTKi in second-line treatment and with available response evaluations are shown in supplemental Figure 3.
Survival curves of 385 patients MCL experiencing late POD. (A) PFS from time of second-line therapy (PFS-2; median, 33 months). (B) Overall survival from time of second-line therapy (OS-2; median, 60 months). Median follow-up for survivors from time of second-line therapy was 53 months (range, 1-144).
Survival curves of 385 patients MCL experiencing late POD. (A) PFS from time of second-line therapy (PFS-2; median, 33 months). (B) Overall survival from time of second-line therapy (OS-2; median, 60 months). Median follow-up for survivors from time of second-line therapy was 53 months (range, 1-144).
Survival curves of 385 patients with MCL experiencing late POD divided according to second-line treatment. (A) PFS from time of second-line therapy (PFS-2) of BTKi (median not reached) vs CIT (median, 26 months). (B) Overall survival from time of second-line therapy (OS-2) of BTKi (median, 88 months) vs CIT (median, 56 months).
Survival curves of 385 patients with MCL experiencing late POD divided according to second-line treatment. (A) PFS from time of second-line therapy (PFS-2) of BTKi (median not reached) vs CIT (median, 26 months). (B) Overall survival from time of second-line therapy (OS-2) of BTKi (median, 88 months) vs CIT (median, 56 months).
Survival curves of 385 patients with MCL experiencing late POD divided according to second-line treatment. (A) PFS from time of second-line therapy (PFS-2) of BTKi vs R-BAC, R-B, or other approaches. (B) Overall survival from time of second-line therapy (OS-2) of BTKi vs R-BAC, R-B, or other approaches.
Survival curves of 385 patients with MCL experiencing late POD divided according to second-line treatment. (A) PFS from time of second-line therapy (PFS-2) of BTKi vs R-BAC, R-B, or other approaches. (B) Overall survival from time of second-line therapy (OS-2) of BTKi vs R-BAC, R-B, or other approaches.
Univariate analysis for PFS-2 and OS-2 revealed that advanced age (>60 years), high Mantle Cell Lymphoma International Prognostic Index score at diagnosis, male gender, blastoid morphological variant, and shorter time to POD were associated with impaired survival outcomes. Except for time to POD, which had an impact on outcome of BTKi-treated patients, as detailed below, none of the listed clinical and pathological variables had statistically significant impact when restricting the analysis to BTKi-treated patients. No difference in treatment outcomes was observed between patients treated in Italy vs other countries (supplemental Figure 4), or when dividing patients in time frames for treatment period (2008-2014 and 2014-2018). A TP53 mutation was detected at diagnosis in 9 of 62 evaluable patients (15%). The presence of a TP53 mutation was associated with significantly shorter OS-2 than patients with wild-type TP53 (Figure 4).
Survival curves of 62 patients with MCL experiencing late POD divided according to TP53 mutational status at lymphoma diagnosis. (A) PFS from time of second-line therapy (PFS-2) of TP53 wild-type (WT; median, 25 months) vs mutated (median, 19 months). (B) Overall survival from time of second-line therapy (OS-2) of TP53 WT (median not reached) vs mutated (median, 24 months).
Survival curves of 62 patients with MCL experiencing late POD divided according to TP53 mutational status at lymphoma diagnosis. (A) PFS from time of second-line therapy (PFS-2) of TP53 wild-type (WT; median, 25 months) vs mutated (median, 19 months). (B) Overall survival from time of second-line therapy (OS-2) of TP53 WT (median not reached) vs mutated (median, 24 months).
Multivariate analysis
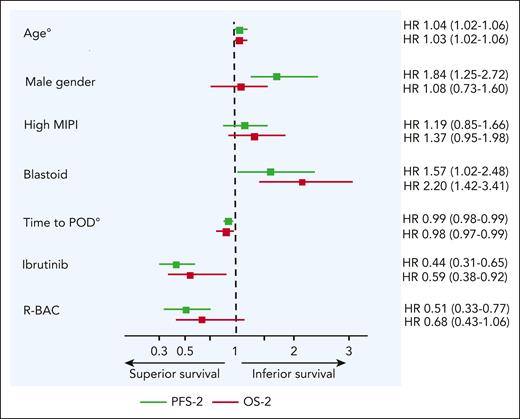
Multivariate Cox regression was performed to analyze PFS-2 and OS-2, including all variables that were significantly associated with the respective outcomes in univariate analysis, as shown in Figure 5. The analysis was conducted on 324 patients for whom all data were available. Independent predictive features associated with impaired PFS-2 and OS-2 in late-POD patients were advanced age (HR, 1.04 and 1.03, respectively) and blastoid morphology (HR, 1.57 and 2.20, respectively). In contrast, longer time to POD (HR, 0.99 and 0.98) and the use of BTKi as second-line therapy (HR, 0.44 and 0.59) were associated with independently superior survival. Male gender was associated with significantly worse PFS-2 (HR, 1.84), whereas the use of R-BAC as a second-line regimen was associated with significantly better PFS-2 (HR, 0.51), but not OS-2. All HRs and 95% CIs are shown in Figure 5. Overall, the use of BTKi was associated with lower risk of death than R-B and other regimens (HR, 0.41 for R-B and 0.46 for others), but similar to R-BAC (HR, 0.85).
HR and corresponding 95% CI in terms of PFS-2 and OS-2. Estimates were obtained from multivariable Cox proportional hazard model, including all variables that were significant at univariate analysis. Analysis was performed on 324 patients with available data. °Age and time to POD were computed as continuous variables. MIPI, Mantle Cell Lymphoma International Prognostic Index.
HR and corresponding 95% CI in terms of PFS-2 and OS-2. Estimates were obtained from multivariable Cox proportional hazard model, including all variables that were significant at univariate analysis. Analysis was performed on 324 patients with available data. °Age and time to POD were computed as continuous variables. MIPI, Mantle Cell Lymphoma International Prognostic Index.
Influence of time to POD and treatments after second line
As shown by multivariable analysis, time to POD computed as a continuous variable was an independent predictor of both PFS-2 and OS-2, indicating that the longer the interval between MCL diagnosis and first relapse, the better the expected survival outcomes. For this reason, we analyzed the impact of longer time to POD on OS-2, and found that both 36 and 48 months (P < .0001 and P = .02, respectively), but not 60 months (P = .51), maintained a prognostic impact for the whole cohort (supplemental Figure 5A-C). When we restricted this analysis to BTKi-treated patients, we observed that a 36-month cutoff had prognostic relevance (P = .04); however, no other differences were found at the 48- or 60-month cutoffs (supplemental Figure 5D-F). Identical results were obtained when censoring patients receiving an allogeneic transplant at any time during follow-up (data not shown). Similar curves with time-to-event cutoffs for CIT vs BTKi are reported in supplemental Figure 6.
OS from the time of second relapse (OS-3) was estimated for 206 patients relapsing or progressing after second-line therapy. Of these patients, for 177 we had data on third-line treatment, which consisted of BTKi in 70 (40%), R-B in 20 (10%), R-BAC in 17 (10%), and other approaches in 70 (40%) patients. A beneficial effect of BTKi compared with CIT (P = .004) in terms of OS-3 was observed, as shown in supplemental Figure 7A-B. This effect was identified when BTKi was administered as third-line treatment, confirming the importance of BTKi in the treatment journey of patients with MCL. Thirty-four patients relapsing or progressing after second-line BTKi were then analyzed, confirming the low overall OS-3 survival prognosis of these patients regardless of administered CIT (supplemental Figure 7C). Indeed, the 12 patients treated with alternative approaches (3 chimeric antigen receptor T cell [CAR-T] therapy, 6 clinical trials with noncovalent BTKi, and 3 lenalidomide-based combinations) achieved a better OS-3, although this difference was not statistically significant.
Discussion
To our knowledge, this study represents the first report comparing CIT with targeted therapy in patients with MCL experiencing late first relapse. The hypothesis formulated by the study was met, with BTKi proving superior to CIT as second-line therapy in patients with late-POD, thus confirming the observations already reported in early-POD patients.4
The clinical course of MCL remains largely heterogeneous throughout its clinical history, from diagnosis to subsequent relapses. For patients relapsing after first-line CIT, use of covalent BTKi is recommended.6 Prospective and retrospective observations,13 including comparison with alternative available options,4 have established BTKi as the preferable choice. This is also the case of the uncommon occurrence of central nervous system relapse, in which ibrutinib has been associated with better survival than the blood-brain barriercrossing CIT.11 Nevertheless, no specific data have been available to date for patients experiencing late relapse. The behavior of patients at first relapse, based on time-to-event is gaining interest with the advent of CAR-T therapy, to prioritize patients who are candidates for such a complex and resource-intensive treatment. Indeed, the predicted durability of the response to covalent BTKi as second-line therapy can be assumed by time to POD, as outlined by the results of the present study. Although the median duration of response of a late-POD patient would be in the average of 5 years, a substantial group of late-POD patients (approximately one-third, as reported in Figure 2) will fail BTKi treatment within 2 years from relapse. There is a need to identify these patients as early as possible by carefully detecting suboptimal response to BTKi, or with biological studies, to refer them for consideration and workup of CAR-T therapy in a timely manner. Clinical models incorporating time to POD and pathological features have been constructed to predict the durability of response to BTKi as second-line therapy, which may become useful in routine clinical practice.14 Although ibrutinib is still the recommended BTKi in most European countries, this drug is no longer available in the United States for use in MCL. Nevertheless, we believe our results should be applied without concern to alternative BTKi.
R-BAC confirmed its clinical activity in the relapsed setting, and was associated with PFS-2 and OS-2 similar to those of BTKi in late-POD patients. The performance of the R-BAC regimen, as one would expect to see more toxicity, and therefore potentially negative effects on OS-2, was surprisingly good in late-POD patients. R-BAC patients were significantly younger than R-B patients in this series (supplemental Table 2), although similar to BTKi-treated patients. Conversely, we observed an established trend among hematologists of reducing R-BAC doses (and delivering 2 days of cytarabine instead of 3) compared with the original protocol, according to hemotoxicity, thus potentially limiting the negative impact that was observed in early-POD patients on OS-2.4 However, in the present era of availability of CAR-T therapy, R-BAC use is hampered by the well-known toxicity conferred by using bendamustine on T-cells before leukapheresis. It may be assumed, for future guidelines, that R-BAC could be reserved after BTKi failure, either as a bridge to CAR-T (after apheresis), or as a standalone reinduction treatment. McCulloch et al demonstrated how R-BAC was able to produce high response rates in patients relapsing after BTKi (overall response rate, 83%; complete response rate, 60%; median PFS, 10 months; median OS, 12.5 months).15
Our data are consistent with the long-term analysis conducted by Rule et al reporting excellent PFS-2 in late relapsing patients treated with ibrutinib, with a significant number in remission after 5 years, and an increase in PFS of 36%16 compared with early-POD patients. Other retrospective large-scale analyses have been reported in the literature,3,17 with survival results comparable with our observations, although more focused on patients’ journey, and not designed for analyzing different treatments specifically as second-line therapies.
We analyzed the subpopulation of patients for whom TP53 status was recorded at diagnosis, showing that mutant TP53 negatively affected OS-2. Although the median OS-2 of patients harboring the wild-type form was 5.3 years, patients with the mutant form had a median OS-2 of 2.8 years. We found a 14.5% TP53 mutation rate in our small cohort of tested patients (n = 62), suggesting that among late-POD patients, intrinsically characterized by a better prognosis than early-POD patients, a proportion of patients carry a TP53 mutation, which seems to maintain its adverse prognostic impact over time. Due to the low numbers, it was not possible to establish whether the adverse effect of TP53 mutation was mitigated by different treatments, as it was not possible to add this factor to multivariable analysis. Our data are hypothesis-generating given the low numbers and the retrospective nature of this selection. We believe it would be worthwhile to investigate the biological characteristics of late-POD patients as opposed to early-POD patients, concerning mutational status and biological features. Following the LATE-POD study, future studies should definitely rely on biology and possibly start from BTKi treatment onward.
This study has several limitations, starting from a lack of central pathological review to address tumor morphology. In addition, the retrospective nature of this analysis may have created selection and treatment bias in patient enrollment from different centers and countries.
With the therapeutic landscape evolving rapidly in MCL, including the early use of BTKi,2 our data may help to guide clinicians for patients who did not receive BTKi as their initial therapy.
In conclusion, the present study seems to indicate a superiority of BTKi compared with CIT in late-POD patients. Among CIT, only R-BAC seems to deserve further investigation in the relapsed/refractory setting. Our data consolidate the view that administration of BTKi in earlier lines of therapy results in more optimal outcomes in patients with MCL. Given the increasing availability of small molecules, bispecific antibodies, and cellular therapies, future studies should focus on biological and clinical aspects of BTKi resistance, hopefully providing novel effective options for BTKi-refractory patients.
Acknowledgments
The authors thank Emilia Elizbieta Florea for administrative assistance, and the Associazione Italiana contro Leucemie, Linfomi e Mieloma section of Verona.
This study was partially supported by research funding from Johnson & Johnson Innovative Medicine to the University of Verona.
Authorship
Contribution: C.V., C.M., and M.M. designed the research study, analyzed the data, and wrote the article; C.V. and M.M. performed the statistical analysis; and all authors contributed with study materials, provided substantial contributions to research analysis, revised the article critically, and approved the submitted final version.
Conflict-of-interest disclosure: I.G. has received educational honoraria and support to the department from Johnson & Johnson Innovative Medicine, Takeda, and Lokon Pharma. M.C.T. has served on speakers’ bureaus for Incyte, Gilead Sciences, Novartis, Janssen, and Sobi; has served on advisory boards for Incyte, Bristol Myers Squibb (BMS), Gilead Sciences, Novartis, and BeiGene. E.G. has received educational honoraria from Johnson & Johnson Innovative Medicine, Genmab, Gilead, and Lilly; advisory board honoraria from Gilead and Miltenyi; nonfinancial support from Johnson & Johnson Innovative Medicine and Gilead; and research support from Johnson & Johnson Innovative Medicine. A.D.R. has served as a consultant for Roche, Kite Gilead, and AbbVie; on speakers’ bureaus for Roche, Kite Gilead, Johnson & Johnson Innovative Medicine, Incyte, Eli Lilly, and Sobi; and on advisory boards for Kite Gilead, BMS, Novartis, Takeda, and AbbVie. F.M. has served on advisory boards and has received speakers’ honoraria from AbbVie, Gilead, Johnson & Johnson Innovative Medicine, Merck & Co, Roche, and Sobi. A.R. has served on advisory boards for Takeda, Incyte, and Italfarmaco; and on speakers’ bureaus for Takeda, Servier, and Sobi. I.F. has received research funding from AbbVie, BeiGene, and Eli Lilly. F.M.Q. has served on speakers’ bureaus for AstraZeneca and Kite Gilead; and has served as a consultant for Sandoz. T.A.E. has received educational honoraria from Roche and Kite; advisory board honoraria from Roche, Kite, Loxo Oncology, BeiGene, Incyte, Secura Bio, and Autolus; has traveled to scientific conferences for Roche, Gilead, AbbVie, and AstraZeneca; has received research support from Gilead, AstraZeneca, and BeiGene; and has been a member of the trial steering committee for Loxo Oncology. P.M.S. has served on speakers’ bureaus for Johnson & Johnson Innovative Medicine, Gentili, Incyte, Sanofi, AstraZeneca, Roche, Kite Gilead, Takeda, and Kyowa Kirin. L.N. has served as a consultant for Takeda; has served on advisory boards for Kiowa Kirin, Takeda, Incyte, and Roche; and on speakers’ bureaus for Kiowa Kirin, Johnson & Johnson Innovative Medicine, Incyte, Eli Lilly, Roche, AbbVie, BeiGene, and EUSA Pharma. V.R.Z. has received research support from Kite Gilead; has served as consultant for Roche; has served on speakers’ bureaus for Johnson & Johnson Innovative Medicine, Lilly, Sobi, and Takeda; has served on advisory boards for Kite Gilead, Merck & Co, Roche, and Takeda; and has received support for travel expenses and accommodations from BeiGene, Johnson & Johnson Innovative Medicine, Roche, and Takeda. A.A. has served on advisory boards for Takeda, Incyte, AbbVie, and Johnson & Johnson Innovative Medicine; and on speakers’ bureaus for Novartis and Gentili. M.J. has received support, personal fees, and nonfinancial support from AbbVie; grants and nonfinancial support from Celgene and Johnson & Johnson Innovative Medicine; and grants, personal fees, and nonfinancial support from Kite Gilead, BeiGene, AstraZeneca, Roche, and Pierre Fabre. C.V. has received research support from AbbVie and Johnson & Johnson Innovative Medicine; has served as a consultant for Johnson & Johnson Innovative Medicine and Pfizer; on speakers’ bureaus for AbbVie, Janssen, Gentili, Pfizer, Incyte, Servier, AstraZeneca, and Kyowa Kirin; and on advisory boards for AbbVie, Kite Gilead, Janssen, Gentili, Novartis, Pfizer, Roche, Incyte, and BMS. The remaining authors declare no competing financial interests.
The current affiliation of C.W.E. is Genmab A/S, Copenhagen, Denmark.
Correspondence: Carlo Visco, Department of Engineering for Innovation Medicine, Section of Hematology, University of Verona, Piazzale L. A. Scuro 10, 37134 Verona, Italy; email: carlo.visco@univr.it.
References
Author notes
Data from this work have not been deposited to publicly accessible databases.
Data are available on request from author Carlo Visco (carlo.visco@univr.it) and Massimo Mirandola (massimo.mirandola@univr.it).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal