The introduction of BTK inhibitors and BCL2 antagonists to the treatment of chronic lymphocytic leukemia (CLL) has revolutionized therapy and improved patient outcomes. These agents have replaced chemoimmunotherapy as standard of care. Despite this progress, a new group of patients is currently emerging, which has become refractory or intolerant to both classes of agents, creating an unmet medical need. Here, we propose that the targeted modulation of the tumor microenvironment provides new therapeutic options for this group of double-refractory patients. Furthermore, we outline a sequential strategy for tumor microenvironment-directed combination therapies in CLL that can be tested in clinical protocols.
Introduction
The treatment of chronic lymphocytic leukemia (CLL) has evolved rapidly over the last 2 decades. Bruton's tyrosine kinase and BCL2 inhibitors (BTKi, BCL2i) have mostly replaced chemo(immuno)therapy (CIT) as first-line therapy,1-3 with CIT remaining an effective option in fit patients with mutated immunoglobulin heavy chain variable region (IGHV) status. Despite achieving undetectable minimal residual disease (uMRD) by fixed-duration regimens,4,5 disease recurs in a relevant fraction of patients with CLL, and switching from BTKi to BCL2i or vice versa is the current standard of care for refractory patients. Patients refractory to both classes of agents (BTKi and BCL2i) are termed double-refractory (2R). As of today, there is no standard therapy for these patients who are 2R.
The emergence of resistance mutations and clonal selection of resistant cells are leukemia-intrinsic factors driving relapses. In addition, interactions between leukemic cells and their supportive tumor microenvironment (TME) contribute to therapeutic resistance, leukemic survival, and immune evasion. Thus, modulating the bidirectional CLL-TME cross talk should enable new treatment strategies. Therein, combining inhibitors of tumor-intrinsic signaling with immunotherapies and TME modulators might synergistically intercept the enormous plasticity and dynamic adaptation of microenvironmental cells to therapeutic stress. Here, we propose a strategy to target microenvironmental dialogues for treating 2R CLL.
2R CLL: an emerging clinical problem
Today, most patients with CLL are treated in first- and second-line treatments with continuous BTKi monotherapy (ibrutinib/acalabrutinib/zanubrutinib) or time-limited BCL2i (venetoclax) combinations. The choice of first line was recently reviewed and is based on individual risk stratification and patient preference.6 Although continuous BTKi monotherapy effectively controls disease, MRD remains detectable7 and most patients relapse or need subsequent therapy because of adverse events. Fixed-duration BCL2i combinations can induce long-lasting, deep remissions with sustained uMRD. However, a significant fraction of patients who are high-risk ultimately develop detectable MRD and subsequent relapses after first-line therapy.4,8,9 Although patients who achieved long-lasting remission upon fixed-duration therapy may be retreated with the same inhibitor class,10 patients with early relapse or under continuous therapy are switched to the other class of agents as second-line treatment. Although these second-line therapies are usually highly effective, a relevant fraction relapses again and becomes 2R.11 The combination of time-limited BCL2i with BTKi (without mCD20Ab) yields long-lasting disease control as first-line treatment.1,5,12,13 The Front-Line therapy in CLL: Assessment of Ibrutinib-containing Regimes (FLAIR) protocol showed that MRD-guided, time-limited venetoclax plus ibrutinib achieved long-lasting remissions and a survival benefit compared with fludarabine, cyclophoshamide and rituximab (FCR) chemoimmunotherapy as first-line therapy.5 However, at relapse, these patients will be double-exposed to BTKi and BCL2i.14 Hence, future studies need to determine which patients can be successfully retreated with BTKi and/or BCL2i. Certainly, a long, treatment-free remission (≥4 years) will be requested for such retreatment.
To date, no generally accepted treatment standard exists for patients who are 2R.11 Given the increasing use of BTKi and BCL2i and the recent approval of combined ibrutinib and venetoclax as first-line treatment in Europe,12,13 the number of patients who are 2R is expected to increase further. This group is characterized by high-risk genetics and an elevated risk of Richter’s transformation. The median overall survival of 2R CLL was reported to be short, with only 8 to 27 months,15-17 and retreatment with venetoclax plus ibrutinib demonstrated only transient benefit with a median treatment duration of 7.5 to 9.3 months, followed by disease progression.16,18 Although future patients who are 2R will have received fewer prior therapies and demonstrate better outcomes, they will remain a major challenge.
In addition, some patients with CLL will need treatment alternatives as they discontinue BTKi/BCL2i because of side effects (up to 1 out of 3-5 patients on ibrutinib).19 For some patients treated with BTKi, switching to newer-generation BTKi may offer a valid option, with noninferior disease control and fewer cardiovascular side effects,19-22 whereas newer BCL2i are currently under clinical investigation.
Taken together, patients with 2R-CLL represent a major medical need requiring the development of novel treatment strategies.
Treatment of 2R-CLL with agents targeting cell-autonomous mechanisms
Chemoimmunotherapy
There are a few approved therapeutic options for patients with 2R-CLL, including CIT, PI3K inhibitors (PI3Ki), or allogeneic stem cell transplantation (Figure 1). However, data on CIT in 2R patients are sparse, and as they commonly carry genetic TP53 dysfunction, the effectiveness of CIT is limited.23,24 Moreover, a large proportion of 2R patients are old/frail, excluding who undergo intense regimens such as CIT or allogeneic allogeneic stem cell transplantation, which additionally require some remission-inducing therapy.
Overview of 2R-CLL. The different treatment sequences leading to 2R-CLL are shown. In this situation, there are some approved therapeutic options, as well as experimental protocols or the repurposing of drugs approved for other purposes that are outlined in this review. Abs, antibodies; SCT, stem cell transplantation; CDK9i, CDK9 inhibitors.
Overview of 2R-CLL. The different treatment sequences leading to 2R-CLL are shown. In this situation, there are some approved therapeutic options, as well as experimental protocols or the repurposing of drugs approved for other purposes that are outlined in this review. Abs, antibodies; SCT, stem cell transplantation; CDK9i, CDK9 inhibitors.
PI3Ki
There is limited knowledge regarding the use of PI3Ki in 2R-CLL. One study reported an overall response rate of 47% and a median PFS of only 5 months in 17 double-exposed patients.25 Other trials in relapsed/refractory (R/R) CLL demonstrated high response rates to PI3Ki, including in patients with high-risk genetics, suggesting efficacy in patients who are 2R.26,27 However, immune-mediated side effects, including grade 3 diarrhea, colitis, and pneumonitis, remain a concern.26-28
Noncovalent BTKi and BTK degraders
Upon treatment with covalent BTKi (cBTKi; ibrutinib/acalabrutinib/zanubrutinib), resistance mutations drive relapses (eg, detected in 87% of patients who progressed on ibrutinib29). Most commonly, these occur within the inhibitor binding site at cysteine residue 481 (C481) and reduce the binding and efficacy of cBTKi, whereas in other patients, additional or single mutations of the downstream signaling protein PLCγ2 were identified as the cause of BTKi resistance.29,30
Noncovalent BTKi such as pirtobrutinib demonstrated good efficacy in patients who are 2R and cBTKi-pretreated (overall response rate was 70%; median PFS 16.8 months) with a favorable toxic-effect profile and retained its effectiveness in mutated BTK-C481.31 Notwithstanding, patients who are PLCγ2-mutated exhibited reduced response rates,31 and new resistance mutations against pirtobrutinib have been described.32 Hence, noncovalent BTKi alone will not overcome therapeutic resistance in all patients who are 2R.
BTK degraders induce degradation of the target via ubiquitylation, offering a novel mechanism to overcome BTK-resistance mutations. Several compounds effectively degrade BTK in vitro, including those with C481 mutations,33 and demonstrated efficacy in patients with highly pretreated CLL with typical toxicities.34
BCL2 and MCL1i
Besides mutations in the BCL2 gene (eg, G101V), which reduce the binding and effectiveness of venetoclax, a plethora of further mechanisms counteract BCL2i.35 Foremost, deregulation of apoptosis-regulating proteins resulting from leukemia-intrinsic (eg, genetic mutations, amplification, or epigenetic regulation35,36) and/or TME processes promote resistance to BCL2i.37-39 Therefore, clinical trials are evaluating new BCL2i and inhibitors targeting alternative antiapoptotic proteins, such as MCL1 (MCL1 inhibitors [MCL1i]) and Bcl-XL (BclXLi). Although early results suggest a potential to overcome venetoclax resistance,40 not all new-generation BCL2i show activity against BCL2 resistance mutations. Moreover, targeting other antiapoptotic proteins has shown severe side-effects (MCL1i: hematotoxicity, cardiotoxicity, intestinal and liver toxicity, BclXLi: thrombocytopenia), which strongly limit their use in patients with 2R-CLL.41,42
Targeting of ROR1
In contrast to healthy B cells, CLL cells mostly express the surface receptor ROR1.43 ROR1 signaling, induced by WNT5a, activates leukemic cells, and high expression levels are associated with lymphomagenesis, dismal outcome, and venetoclax resistance.43-45 Zilovertamab, a mROR1Ab, disrupts ROR1 signaling and inhibits the growth of BCL2i-resistant cells.44 Therefore, ROR1 offers a promising target for antibodies, BITEs, and chimeric antigen receptor (CAR) T cells and has demonstrated promising efficacy in combination with ibrutinib.46 Similarly, early clinical data evaluating a ROR1-BITE showed promising preliminary results.47
Treatment of 2R-CLL with agents targeting the TME
Cell-specific agents
The survival of CLL cells heavily depends on a supportive TME. The interactions between malignant cells and surrounding cells represent a bidirectional dialogue via direct contact or soluble mediators and may result in malignant growth and resistance to therapy, as recently reviewed in detail.48 In the following, we will briefly summarize key cellular components of the CLL microenvironment with a focus on therapeutic options (Figures 2 and 3).
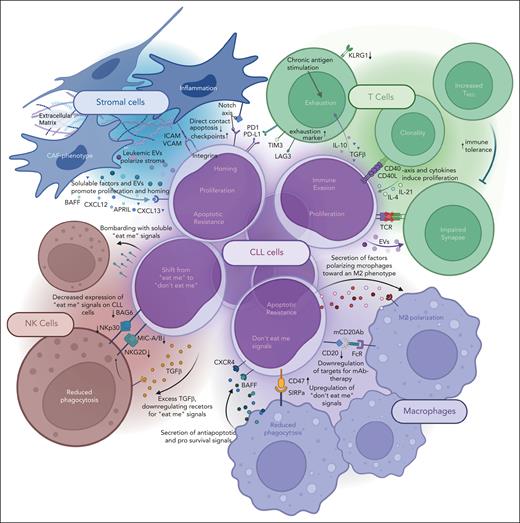
Mechanisms of immune evasion by CLL cells with the different compartments of the TME. Graphical depiction of all mentioned bidirectional mechanisms by which CLL cells and the surrounding cells of the TME interact.
Mechanisms of immune evasion by CLL cells with the different compartments of the TME. Graphical depiction of all mentioned bidirectional mechanisms by which CLL cells and the surrounding cells of the TME interact.
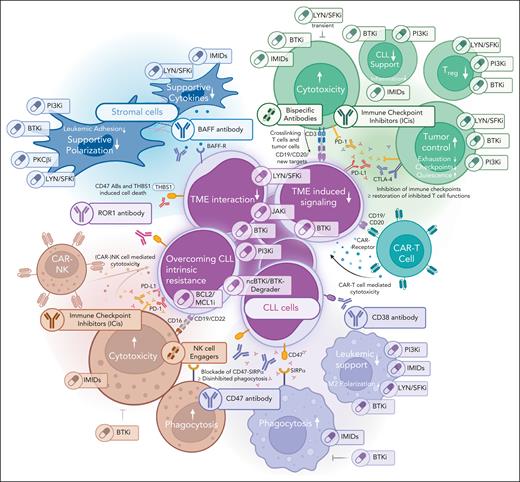
Therapeutic options targeting the different compartments of the TME. Graphical depiction of the different TME compartments, including possibilities of targeting these.
Therapeutic options targeting the different compartments of the TME. Graphical depiction of the different TME compartments, including possibilities of targeting these.
In CLL, T cells show a dysfunctional composition and polarization at several levels, resulting in reduced tumor surveillance or even active tumor support.49 Patients with CLL exhibit increased numbers of peripheral T cells with oligoclonal restriction, differentiation toward effector phenotypes, and loss of naïve subsets due to chronic antigen exposure and contact with tolerogenic, malignant B cells, which lack expression of costimulatory molecules.49 In addition, elevated numbers of immunosuppressive regulatory T cells (Treg) impair an effective T-cell response. The CD4+/CD8+ T-cell ratio is often shifted toward CD8+ cells,50 which express increased levels of exhaustion markers (eg, CD160, CD244, and PD-1) and have reduced proliferative and cytolytic activity.51 In addition, CD4+ T cells may promote proliferation and survival of CLL cells through cytokine secretion (eg, IFNγ) and direct cell-cell interaction.49 Critically, CLL cells use high levels of immune checkpoint proteins like PD-L1 and other B7-family ligands (CD200, CD276) to avoid T-cell killing and induce T-cell dysfunction.49,52 Hence, the restoration of physiological T-cell function and redirecting toward malignant cells seems promising.
Immune checkpoint inhibitors (ICis) block T-cell suppressive molecules (eg, PD-1/PD-L1 or CTLA-4) and have revolutionized cancer therapy.53,54 In contrast to other lymphomas and promising preclinical data,55 ICis showed disappointing results in CLL, with no response to pembrolizumab (mPD-1Ab) observed in an exploratory trial with 16 patients who are R/R.56 The causes of T-cell dysfunction in CLL are not entirely clear, but recent data demonstrated enhanced T-cell senescence characterized by altered metabolism, mitochondrial fitness, disturbed inflammatory cytokine production, and epigenetic reprogramming as potential mechanisms.57-59 Along these lines, the underperformance of CAR T cells in CLL seems to correlate with an immunosenescent phenotype, including increased aerobic glycolysis and pseudohypoxia.59-61
Given the multifaceted dysfunction of T cells in CLL, the addition of other drugs may be needed to sensitize CLL to ICis. The combination of ICis with BTKi may improve CD8+ T-cell function and overcome BTKi resistance.62 A therapeutic activity of this combination was demonstrated in R/R CLL and RT.63-65 Preclinical data also indicate synergistic effects of ICis with BCL2i66,67 and preliminary data demonstrated high early uMRD rates combining atezolizumab (mPD-L1Ab) with venetoclax-obinutuzumab in first-line treatment.68 In addition, novel ICi (targeting LAG3 or TIM3) are tested alone or in combination for the treatment of R/R CLL.
Bispecific antibodies (BsAbs) and T-cell engagers (BITEs) bind 2 different antigens, mostly linking T cells to a target and are currently approved for relapsed hematological malignancies.69,70 Preliminary data indicate good induction of T-cell proliferation, activation, and leukemia clearance in vitro,71 and early clinical results of epcoritamab (CD3 × CD20) show deep responses with 53% ORR in double-exposed CLL.72,73 Because some BsAbs showed improved effectiveness in combination with BTKi/BCL2i in vitro,71,74 combining BsAbs with BTKi/BCL2i and/or ICis appears promising to boost T-cell function.
CAR T cells are autologous T cells engineered to express CAR targeting tumor cells. In CLL, CR rates and long-term responses to CD19 CAR T cells were lower than in other lymphomas.75,76 Explanations include impaired formation of the immune synapse, production of extracellular vesicles attenuating CAR T-cell function,76 and metabolic perturbation of CAR T cells.59,60,77 Importantly, the efficacy of CAR T-cell products is also reduced by production from a largely dysfunctional T-cell pool. Therefore, strategies are being developed to improve T-cell fitness before harvesting, during manufacturing, and after transfusion. In this regard, ibrutinib was shown to restore T-cell function, facilitate production, reduce CRS rates, and increase in vivo efficacy of CAR T cells in CLL.78-81
Macrophages play an essential role in the TME of CLL.82,83 CLL cells promote the generation of anti-inflammatory M2-polarized tumor-associated macrophages (TAMs) from peripheral blood mononuclear cells,84 which inhibit leukemic apoptosis by secreting cytokines (eg, SDF-1α, BAFF, and APRIL) and in direct contact with CLL cells.84-86 The presence of M2-polarized macrophages is associated with an unfavorable outcome, and the depletion of macrophages may restrict leukemic growth in vivo.87-90
Macrophages detect eat-me signals and clear cells by phagocytosis, including mCD20Ab-loaded cells in CIT in CLL.91-93 CLL cells may circumvent phagocytosis by expressing CD47, which binds SIRPα on macrophages and is associated with dismal clinical outcome.94,95 Targeting the CD47/SIRPα axis by antibodies binding to CD47 (eg, magrolimab) or SIRPα (eg, SIRP-1/SIRP-2) can restore efficient phagocytosis and has shown therapeutic effects in different malignancies.94-97 There are limited data on CD47i in CLL. Because magrolimab commonly induces hematotoxicity, especially profound anemia, more specific fusion proteins (eg, SIRPα-Fc) and BsAbs, such as CD47 × CD19 BsAb, are being tested.98 Moreover, inhibition of CD47 shows increased efficacy in combination with mCD20Abs or BCL2i, including the induction of caspase-independent CLL cell death.99,100
PD-L1 is also expressed on macrophages and may promote an M2 phenotype, contributing to immune evasion.52,101 Interestingly, a PD-1 × CD47 BsAb demonstrated promising results in patients with cancer who are heavily pretreated cancer.102,103
A dysfunctional interaction of NK cells with CLL cells also enhances immune evasion.104 NK cells express activating and inhibitory receptors, whose balance is shifted toward an inhibitory phenotype in CLL. The expression of activating receptors (eg, NKG2D) on NK cells is inhibited by TGFβ.104-106 Activating NK-cell receptors may also be downregulated by the shedding of respective ligands, and elevated serum levels of these ligands were associated with a dismal outcome.107 In addition, CLL cells can evade NK-cell clearance by expressing low levels of activating and high levels of inactivating ligands.108,109 In contrast to T cells, microenvironmental NK cells do not show intrinsic defects and efficiently abrogate tumor cells.110 Therefore, combinational therapies to activate microenvironmental NK cells have become a promising element of TME-directed strategies. CD47/SIRPα and PD-1/PD-L1 axes are also relevant for NK-cell function,104,111 and the aforementioned therapeutics may contribute to restore normal NK-cell activation.
NK cells engineered to target tumor cells by CAR expression (CAR-NK cells) can be used off-the-shelf from healthy donors, overcoming some critical issues of allogeneic CAR T cells (reduced risk of allogeneic reaction, lower incidence of severe adverse effects, and graft-versus-host disease112). In addition, CAR-NK cells can eliminate tumor cells, which do not express a specific target antigen.112 The first clinical data on CAR-NK cells in lymphoid malignancies are encouraging. CD19–directed CAR-NK cells demonstrated an ORR of 73% in 11 patients with lymphoid malignancies, including 3 CRs out of 5 patients with heavily pretreated CLL.113 Another study on R/R lymphoma demonstrated objective responses in 8 of 11 patients with CD19-directed CAR-NK cells combined with mCD20Abs.114
Bispecific or multispecific NK-cell engagers, which retarget NK-cell activity, are tested in phase 1/2 trials after promising preclinical results.115 Bispecific (CD16 × CD19) and trispecific (CD16 × CD19 × CD22) antibodies were shown to trigger NK-cell activation and increased cytotoxicity against a human B-cell leukemia cell line.116
Follicular dendritic cell–like stromal cells and nonendothelial mesenchymal stroma cells (MSCs) are present in CLL homing sites and support leukemic survival and proliferation.117 CLL cells induce the formation of follicular dendritic cell networks118 and coculture models with different MSCs revealed a leukemia-supportive reprogramming of stromal cells via extracellular vesicles and the activation of inflammatory stromal pathways.119,120 Consequently, stromal cells promote therapeutic resistance to BCL2i and ICis by upregulating antiapoptotic BCL-family members39 and PD-L1 in CLL cells.121
Similarly, stromal cell kinase inhibition might suppress antiapoptotic and resistance effects, as in vivo models demonstrated that inhibition of stromal PKCβ, subsequent NFκB activation, or LYN activity could reduce leukemic progression. Pharmacological inhibition via midostaurin, enzastaurin (for PKCβ), or dasatinib (for LYN) induced similar effects119,122 and overcame stroma-induced resistance to chemotherapy or BCL2i.39 Given their immunomodulatory capacity, depletion of activated TME stromal cells by CAR T cells in R/R multiple myeloma overcame immunosuppression and enhanced CAR T efficiency in vivo.123 Interruption of stromal activating signaling demonstrated synergistic efficacy with ICis in preclinical models;124,125 thus the combination of kinase inhibition with ICis, CAR T cells, and BsAbs is interesting.
MSCs support leukemic cells via direct contact and soluble factors like CCL2, CXCL12, BAFF, and APRIL, some of which are also secreted by NLCs and T cells.126 Blockade of prosurvival cytokines showed promising preclinical results mostly in combination with BCL2i or BTKi, increasing leukemic treatment susceptibility, although their relevance in resistant CLL is unknown.127-130
Therapeutic agents targeting multiple TME compartments
Within the TME, therapeutic modulation might be achieved by blocking specific receptors governing cell-cell interactions or by drugs that act on multiple cells. In this context, the repurposing of approved kinase inhibitors is getting increasing attention to create novel, TME-directed treatment strategies.131,132
Besides their known antileukemic effects, BTKi can directly act on various cellular TME components. In macrophages, BTK inhibition reduces chemokine secretion, and off-target inhibition of inducible T-cell kinase interferes with phagocytosis as well as T-cell activation and proliferation.133 In patients treated with BTKi, T cells show improved proliferation, cytolytic activity, and reduced expression of exhaustion markers. Specifically, treatment with BTKi reduced PD-1 and CTLA-4 expression on T cells and decreased the number of immunosuppressive Treg.131,134-136 Importantly, such effects may be subpopulation-specific; for example, inducible T-cell kinase inhibition via BTKi especially promoted a Th1 phenotype with increased antitumor activity.131,137 Accordingly, BTKi treatment supported the activity of BsAbs against CLL cells in vitro.71,138 Moreover, ibrutinib was reported to facilitate CAR T-cell production, reduce CRS rates, and increase in vivo efficacy of CAR T cells78-81 and ICis62 in CLL.
Similarly, PI3Ki modulates cellular interactions in the leukemic microenvironment.26,27 PI3Ki can reverse the protumor phenotype in macrophages and augment the efficacy of ICis.139-141 PI3Ki can also reduce inflammatory signaling in macrophages and modulate T-cell phenotype and function by reducing the secretion of proleukemic inflammatory cytokines and the frequency of Treg cells.139-141
Dasatinib is an inhibitor of BCR-ABL and SRC family kinases like LYN, which modulates the activity of downstream kinases BTK and PI3K and is commonly overexpressed in CLL.142 Dasatinib was evaluated in patients who are fludarabine-refractory and showed some therapeutic activity (partial remission [PR], 15%-20%; lymph node [LN] reduction, 44%-60%) at a time when kinase inhibitor–induced lymphocytosis was unknown and seen as progression.143,144 Similarly, the LYN inhibitor bafetinib induced partial LN responses in pretreated CLL.145 Our group demonstrated that LYN plays an essential role in the TME as it modulates the polarization, cellular phenotype, and function of macrophages and stromal cells.122,146 Further, dasatinib treatment helps to overcome CD40L-induced venetoclax resistance in CLL cells.147,148 Dasatinib also showed strong immunomodulatory effects by reversibly inhibiting T-cell activity via LCK and SRC,149 thus reducing the rate of CRS and ICANS in CAR T and BsAb therapy.150-153 In parallel, dasatinib reverted the exhausted T-cell phenotype, reduced Treg abundance, and augmented the efficacy of immunotherapies by intermittently pausing T-cell activity154-157 and increasing NK-cell cytotoxicity.158-160 Given its pleiotropic effects on the TME, dasatinib is an appealing TME modulator. However, known toxicities like pleural/pericardial effusions or infectious complications might limit its use in patients with 2R-CLL.
Similarly, SYK inhibitors (SYKi; eg, entospletinib) demonstrated single-agent efficacy in R/R CLL (ORR, 90%) with sustained PFS in combination with BTKi and mCD20Ab.161 SYKi were also active in patients who were R/R after prior BTKi or PI3Ki therapy162 and might specifically neutralize some PLCγ2 resistance mutations upon BTKi treatment.163 SYKi inhibited BAFF-induced prosurvival signaling164 and reduced activation of CD4+ and CD8+ T cells in patients with CLL,165 which is essential to overcome T-cell–induced venetoclax resistance166 but might increase the risk of infectious complications.
The immunomodulatory drug (IMID) lenalidomide achieves lasting responses in naïve and R/R CLL.167-169 IMIDs show limited cytotoxicity170 and act through modulation of the TME by altering the substrate specificity of CRL4CRBN E3 ubiquitin ligase, resulting in degradation of IKZF1, IKZF3, and CK1α.171 Lenalidomide increases the numbers of NK cells and CD4+ T cells in CLL,172,173 inhibits the nurturing effect of TAMs on CLL cells, and induces proliferation and activation of TAMs.172-174 In other hematological entities, IMIDs stimulate NK-cell proliferation, activation, and cytolytic function and promote anti-tumor T-cell function.175 Importantly, lenalidomide augments the efficacy of T cells in combination with BsAbs in vitro.176 However, known side effects of lenalidomide like serious tumor flare and potential secondary malignancies have strongly reduced its use in CLL.177,178
Inhibitors of Janus kinases, such as ruxolitinib, modulate the hematological microenvironment and immune system and are used to treat myeloproliferative neoplasias and graft-versus-host disease.179,180 Similarly, ruxolitinib was shown to relieve symptoms of patients with CLL.181 The dual SYK/Janus kinases inhibitor cerdulatinib was shown to antagonize B-cell receptors and microenvironmental signaling.182 However, clinical results using ruxolitinib in combination with ibrutinib failed to demonstrate convincing benefit.183
CD38 expression correlates with unfavorable outcomes in CLL,184 and activation of CD38 modulates the TME toward immunosuppression.185 However, in contrast to encouraging preclinical data,186 the combination of daratumumab (mCD38Ab) with ibrutinib did not improve the outcome of patients with very high-risk CLL.187 Nevertheless, daratumumab may still become valuable for CLL therapies when given in an appropriate combination or sequence, as it interferes with the homing of malignant cells by inhibiting CD49d-mediated CLL adhesion.188 Moreover, in multiple myeloma, daratumumab reverts the immunosuppressive T-cell compartment, including elevation of T-cell clonality and abrogation of CD38+ Treg.189
A new concept of clinical trials targeting the TME
Patients with 2R-CLL represent a major medical need, as no standard therapy is available. New noncovalent BTKi are effective for a limited time and might represent an important bridging concept. However, additional strategies are needed, and we propose thst TME-modulating drugs will aid in treating them.
As the microenvironment is highly complex, effective TME–directed therapies will likely need to target multiple instead of single interactions within this network. Accordingly, monotherapies have demonstrated only limited responses so far, especially in R/R patients. Combining potent antineoplastic agents with (multiple) TME-rewiring therapeutics could maximize treatment efficacy, as this approach aims to restore antitumorigenic TME and prevent resistance. Both synergistic and opposing effects of drugs on components of the TME must be anticipated when designing study concepts. This comprises a variety of potential outcomes to consider when combining TME-directed agents: (1) creating synergistic effects by targeting shared effector mechanisms (eg, enhancing T-cell cytotoxicity via ICis and BsAbs or phagocytosis via mAbs and CD47i); (2) preparing the TME for further therapeutic interventions (eg, reducing T-cell exhaustion/dysfunction by BTKi before CAR T-cell therapy); (3) overcoming treatment resistance (eg, combining BCL2i with dasatinib); and (4) adding antileukemic efficacy. Agents that have both direct activity on leukemic cells and synergistic effects on the TME (eg, BTKi, PI3Ki, dasatinib, lenalidomide) might be particularly attractive combination partners.
The TME dynamically adapts to therapies, whereas the initial composition also critically governs treatment outcome. In this regard, the efficacy of immunotherapies depends on the immune cell landscape at treatment initiation and treatment-associated shifts in composition. The presence of active T cells may augment CAR T and ICi efficacy,190 whereas exhausted T cells and Tregs may prevent the success of these, including BsAb.191 Similarly, TAMs inhibit CAR T-cell efficiency,190 and cancer-associated fibroblasts may reduce (CAR)T-cell migration and cytotoxicity.192 For example, a successful modulation of TME composition in CLL has been demonstrated in vitro for epcoritamab by pretreatment with BTKi.74 Repolarizing TAMs by CD47i/kinase inhibitors and/or activating NK cells by NK-cell engagers/CD47i can also augment the efficacy of immunotherapies.99,100 Moreover, reduction of the leukemic burden may shift effector:target ratio and improve the efficacy of CAR T cells, ICis, and BsAbs.193 In conclusion, the sequential use of cytotoxic and/or TME-modulating agents to prepare the TME for more effective immunotherapy may optimize treatment outcome.
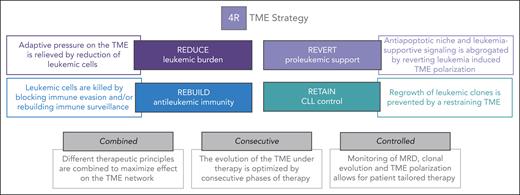
With these translational considerations in mind, we define 4 functionally different steps in our proposed TME–directed therapy for patients with 2R, which we call the 4R strategy (Figure 4).
The 4R TME strategy. Overview of our 4R TME strategy, including the 4 R's (reduce, rebuild, revert, and retain), as well as the main further considerations for studies needed for successful TME–directed therapies.
The 4R TME strategy. Overview of our 4R TME strategy, including the 4 R's (reduce, rebuild, revert, and retain), as well as the main further considerations for studies needed for successful TME–directed therapies.
Reduce
The first step shall achieve a rapid reduction in tumor burden to facilitate the effectiveness of the subsequent steps. This debulking efficiently lessens the constant adaptive pressure CLL cells exert on surrounding cells. Moreover, it reduces the (genetic) heterogeneity of the leukemia population and may already eliminate some resistance-defining clones. In addition, a quantitative shift in the effector:target ratio may increase the susceptibility to immunotherapies, and it was demonstrated that a reduction in leukemic burden restored T-cell function by overcoming metabolic adaptations in T cells.61,194 A rapid debulking can be achieved by classical cytotoxic agents, (next-generation) BCL2i/MCLi, or potent monoclonal antibodies. Although the efficacy of chemotherapy in 2R-CLL is unknown and might also deplete immune cells, preclinical data suggest increased efficacy of BsAbs with cytotoxic agents,195 and current studies evaluate BsAbs plus chemotherapy in various B cell non-Hodgkin lymphoma (B-NHL). Thus, protocols should propose a pretreatment debulking step, especially for patients with a high tumor load, which can be monitored by standard response criteria.
Reverse
As indicated, TME cell types acquire a leukemia-supportive polarization. Various agents may help rewire this leukemia-supportive microenvironmental polarization of fibroblasts, macrophages, and T cells, as shown for kinase inhibitors and IMIDs, and should be combined or sequentially used with other TME-directed or antineoplastic therapies. To monitor this rewiring, panels assessing T-cell exhaustion/senescence markers, including T-cell metabolism, should be added at regular time points to trials with TME-directed approaches. Furthermore, rebiopsies should be performed to study the composition, distribution, and/or polarization of cells in the TME (NK cells, macrophages, etc). This will create important insights into the potential benefits for a specific patient and optimize TME targeting in future clinical trials.
Rebuild
Some of the current immunotherapeutic concepts depend on intact components of the immune system and can generate effective, long-lasting antileukemic immunity. Recent examples of such agents are CAR T or CAR-NK cells, BsAbs, ICis, or mCD47Abs. Chances for success can be increased by the above-mentioned prior treatment steps. Additional agents can be combined to further increase efficacy or prevent immune evasion. At this step, we suggest monitoring of MRD and TME composition by rebiopsies may help guide further treatment decisions and gain mechanistic insights into the dialogue of different (immune) effector cells in the microenvironment.
Retain
We anticipate that novel therapeutic sequences and combinations may induce long-lasting complete remissions with no need for further intervention in some patients. In other patients, treatment intensification or maintenance may be appropriate.196 Treatment decisions in this phase can be facilitated by sequential measurement of MRD, as recently demonstrated,5 a well-established predictive marker of treatment outcome in CLL.7 Understanding and controlling MRD dynamics by repeated sampling to assess growth kinetics and (clonal) mutation status could forecast leukemic growth and relapse for each patient.197 The potency of MRD is supported by the results of the FLAIR trial, which suggest that an MRD-adjusted treatment duration may allow for the design of efficient therapies.5 However, although MRD measurements mostly represent leukemic burden in peripheral blood or bone marrow, they may not fully reflect persistent lymphadenopathy.198 Therefore, the additional measurement of circulating tumor DNA might better reflect leukemic burden across compartments and improve monitoring of clonal evolution.199,200 Further techniques to monitor the nonmalignant TME composition, such as multiplex imaging, are becoming available, and some cellular components (T and NK cells) can be assessed from peripheral blood.51,201-203 Together with the assessment of the leukemic burden,204-207 this might facilitate the development of risk-adapted, patient-tailored maintenance strategies of 2R-CLL.
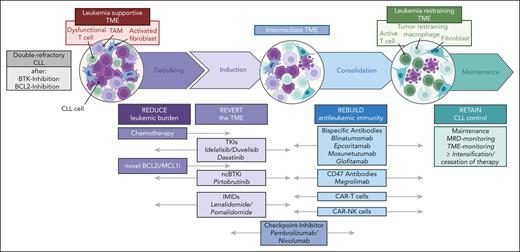
Taken together, study designs like the 4R concept will guide the development of a series of future trials in which we will target the microenvironment of patients with 2R-CLL (Figure 5). This conceptual framework may establish optimized drug combinations that create beneficial modulation of the TME. Although designed for patients with CLL, the 4R concept may be transferred to other B-NHL, where similar mechanisms of resistance and niche formation exist.
Schematic overview of possible novel TME–directed trial designs. The 4R objectives of TME trials are ordered according to the classical phases of CLL therapy design, and novel drugs are allocated to these principles below. Future trials can be designed by rationally choosing the respective drug combinations.
Schematic overview of possible novel TME–directed trial designs. The 4R objectives of TME trials are ordered according to the classical phases of CLL therapy design, and novel drugs are allocated to these principles below. Future trials can be designed by rationally choosing the respective drug combinations.
Acknowledgments
The authors thank Phuong-Hien Nguyen and Paula Cramer for critically reading and discussing the manuscript. All figures were created with BioRender.com.
M.H. is supported by grants from the Deutsche Forschungsgemeinschaft, SFB 1530, Z01, and B01.
Authorship
Contribution: All authors designed and wrote the manuscript; and M.H. designed some of the major ideas of the manuscript.
Conflict-of-interest disclosure: M.H. reports receiving institutional research support by Roche, Janssen, BeiGene, and AbbVie. The remaining authors declare no competing financial interests.
Correspondence: Michael Hallek, Department I of Internal Medicine, University Hospital of Cologne, Kerpener Str. 62, D-50937 Cologne, Germany; email: michael.hallek@uni-koeln.de.
References
Author notes
R.I.L. and A.F.v.S. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal