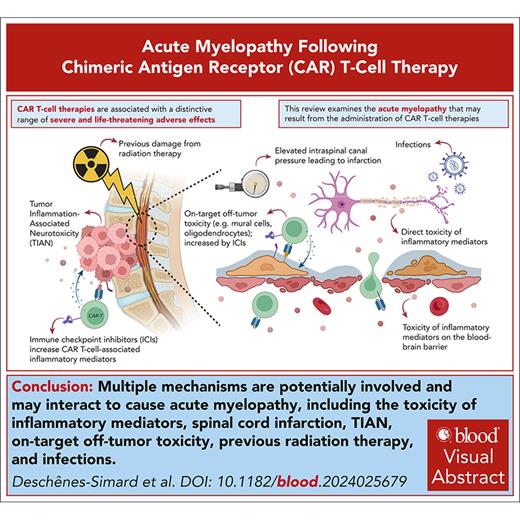
Visual Abstract
Chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment of patients with relapsed or refractory hematologic malignancies, but it comes with unique toxicities, notably cytokine release syndrome and ICANS (immune effector cell–associated neurotoxicity syndrome). As experience with CAR T-cell therapy grows, distinct and infrequent neurologic complications are becoming increasingly evident. Recently, reports of acute myelopathy after the administration of CAR T-cell therapies have been accumulating. Despite the establishment of consensus guidelines for managing ICANS, there remains limited guidance on the appropriate investigations and treatments for this rare complication. In this manuscript, we delve into the clinical features, pathophysiology, and strategies for the optimal management of acute myelitis after CAR T-cell therapy and draw insights from reported cases in the literature.
Introduction
Chimeric antigen receptor (CAR) T-cell therapies mark a groundbreaking advancement in immuno-oncology in which the intrinsic antitumor capabilities of T lymphocytes are merged with cutting-edge genetic innovations. However, this new technology comes with many challenges, including a unique spectrum of severe and life-threatening toxicities.
Cytokine release syndrome (CRS) and ICANS (immune effector cell–associated neurotoxicity syndrome) are well documented, and criteria for their diagnosis and severity assessment have been established.1 However, emerging toxicities, notably rare neurologic complications, are increasingly recognized.2 In recent years, several cases of acute myelopathy following CAR T-cell therapy have been reported. Although guidelines exist for the management of CRS and ICANS, there is limited guidance available for these emerging toxicities.3-5 Therefore, there is an urgent need to raise awareness about these uncommon complications and to establish a framework for the effective management thereof.
Acute myelopathy following CAR T-cell infusion is a rare yet serious complication that leads to significant morbidity and requires urgent intervention. Available data are primarily limited to case reports, which poses challenges for its management. To date, at least 24 cases have been reported, including 20 adult patients with lymphoma and 4 pediatric patients with B-cell acute lymphoblastic leukemia (B-ALL).
In this review, we explore the clinical presentation, pathophysiology, and management of acute myelopathy after CAR T-cell therapy. Our objective was to provide guidance to clinicians who care for these patients and to improve patient outcomes.
Methods
This narrative review aims to provide a broad overview of the current knowledge. A comprehensive and systematic literature search was conducted to identify cases through PubMed and Embase using the following search strategy: (“car t-cell” OR “chimeric antigen receptor”) AND (“myelopathy” OR “myelitis” OR “paraparesis” OR “quadriparesis” OR “spinal cord”). Titles and abstracts were screened to select the relevant publications that reported clinical data on CAR T-cell therapy, which were then reviewed to identify cases of myelopathy. The reference lists of these articles were also examined to maximize case identification.
To be included, a case had to have a clinical presentation clearly attributed to myelitis or myelopathy or provide radiological evidence of myelopathy. Cases with neurologic deficits not clearly associated with a spinal cord defect were excluded. We carefully assessed cases to avoid redundancies, and when multiple reports discussed the same patient, all reports were referenced.
All discussed cases have been reported previously, and we were informed of 2 additional active cases for which details could not be provided.
Clinical presentation
All patients experienced CRS, generally low grade, before neurotoxicity, which occurred between day +1 and day +15 after CAR T-cell infusion (Tables 1 and 2). Clinical features of ICANS developed in almost all patients between day +2 and day +19. Manifestations were typically severe, including encephalopathy and seizures. Acute myelopathy manifested concurrently or shortly after ICANS onset, between day +5 and day +27. Only patient 11 and patient 22 experienced delayed onset myelitis that occurred 23 and 17 days after the onset of ICANS, respectively, and after initial improvement of other manifestations. It is noteworthy that in some cases, the diagnosis of myelopathy might have been delayed because of limited physical examination in patients with severe encephalopathy or convulsions. Myelopathy symptoms were generally acute and severe, including flaccid paraplegia, quadriparesis, loss of bladder control, and hypoesthesia with a sensory level. Only patient 11 presented subacutely with ascending paresthesia that progressed to a T4 sensory level and paraplegia over 22 days.
Adult cases of acute myelopathy following CAR T-cell therapy for lymphoma
| Characteristics . | Patient 16,7 . | Patient 28 . | Patient 39 . | Patient 410 . | Patient 511 . | Patient 612 . | Patient 713 . | Patient 813 . | Patient 914 . | Patient 1015 . | Patient 1116 . | Patient 122,17 . | Patient 1318 . | Patient 1419 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at infusion (y) | 34 | 50 | 52 | 61 | 44 | 30 | 41 | 30 | 48 | 44 | 28 | 33 | 29 | 41 |
| Sex | Female | Female | Male | Male | Male | Male | Female | Female | Female | Female | Female | Female | Female | Female |
| Histology | DLBCL | DLCBL | MCL | DLBCL | DLBCL | PMBL | DLBCL | PMBL | DLBCL | PMBL | PMBL | EBV+ DLBCL | DLBCL | DLBCL |
| Previous treatment | 3 lines of chemotherapy unspecified | 2 lines of chemotherapy unspecified | 3 lines of therapy unspecified, ASCT | R-CHOP, HD-MTX, CYVE | R-CHOP, R-ICE | Chemotherapy unspecified, radiotherapy, ASCT | 4 lines of chemotherapy unspecified, ASCT | 2 lines of chemotherapy unspecified | R-CHOP, another line of chemotherapy unspecified | DA-EPOCH-R, R-ESHAP | R-CHOP, GDP, radiotherapy | DA-EPOCH-R, R-ICE, prophylactic IT and HD-MTX, radiotherapy, nivolumab | Not specified | Not specified |
| Bridging treatment | Not reported | BEAM, ASCT | Obinutuzumab, bendamustine | Intrathecal methotrexate | Hyper-CVAD | Not reported | Not reported | Radiotherapy, rituximab, bendamustine, dexamethasone | Ibrutinib, lenalidomide | Radiotherapy | Radiotherapy, pembrolizumab, dexamethasone | Radiotherapy | Not reported | Not reported |
| CAR T cells | Axi-cel | Allogeneic CD19 + CD22 directed | Brexu-cel | Tisa-cel | CD19-directed unspecified | CD19-directed unspecified | Axi-cel | Axi-cel | Axi-cel | Axi-cel | Axi-cel | Axi-cel | CD19-directed unspecified | Tisa-cel |
| CRS onset | D + 5 | D + 6 | D + 1 | D + 3 | D + 1 | D + 6 | D + 1 | D + 2 | D + 3 | D + 1 | D + 1 | D + 2 | Not specified | D + 1 |
| Max CRS grade | Grade 2 | Grade 2 | Grade 1 | Grade 1 | Grade 1 | Grade 2 | Grade 1 | Grade 2 | Grade 2 | Grade 2 | Grade 1 | Grade 2 | Not specified | Grade 2 |
| ICANS onset | D + 5 | D + 15 | D + 3 | N/A | D + 5 | D + 6 | D + 2 | D + 5 | D + 5 | D + 5 | D + 4 | D + 5 | D + 5 | N/A |
| Max ICANS grade∗ | Grade 4 | Grade 4 | Grade 4 | No ICANS | Grade 4 | Grade 4 | Grade 4 | Grade 4 | Grade 4 | Grade 4 | Grade 4 | Grade 4 | Grade 4 | No ICANS |
| Myelopathy onset | D + 10 | D + 21 | D + 3 | D + 6 | D + 5 | D + 6 | D + 8 | D + 5 | D + 9 | D + 6 | D + 27 | D + 7 | Not specified | D + 14 |
| Presentation | Paraplegia, progression to respiratory failure and death | Back pain, LE weakness | Paraplegia, sensory level, urine retention | Back pain, left leg radiculopathy | Back pain, paraplegia, T10 sensory level | Paraplegia, T10 sensory level | Quadriparesis, urine retention | Quadriparesis, urine retention | Quadriparesis | Paraplegia, T4 sensory level | Ascending paresthesia, progression to T4 sensory level and paraplegia, urine retention | Paraplegia, L1 sensory level, urine retention | LE weakness, progression to quadriparesis, lower cervical sensory level | Urodynamic urges, limbs sensorimotor deficit |
| Suspected etiology | HHV-6 | HHV-6 | Eosinophilic | Possible TIAN | CAR T-cell– mediated | CAR T-cell–mediated | CAR T-cell–mediated | CAR T-cell–mediated | CAR T-cell–mediated | CAR T-cell–mediated | CAR T-cell–mediated | CAR T-cell–mediated | CAR T-cell–mediated | CAR T-cell–mediated |
| Treatment | Tocilizumab, dexamethasone, MP, anakinra, IVIG, plasmapheresis, foscarnet | IVIG, ganciclovir, foscarnet | Dexamethasone, MP, siltuximab | None | Tocilizumab, dexamethasone, MP, anakinra | Tocilizumab, dexamethasone, siltuximab | Tocilizumab, dexamethasone, MP | Tocilizumab, MP | Dexamethasone, MP, anakinra, IVIG | Dexamethasone, MP, anakinra, siltuximab, IVIG, plasmapheresis | Tocilizumab, dexamethasone, MP, IVIG, plasmapheresis | Dexamethasone, IVIG | High-dose steroids, IVIG, plasmapheresis | Tocilizumab |
| Clinical improvement | No | Complete | No | Complete | No | No | Partial | Partial | Partial | No | Partial | Almost complete | Partial | Complete |
| Best oncologic response | N/A | CR | N/A | CR | CR | CR | CR | CR | CR | CR | CR | CR | Not reported | CR |
| LA | D + 24 | 7 mo | D + 11 | 6 mo | 4 mo | 1 mo | 3 y | 18 mo | 18 mo | 3 mo | 5 mo | 6 y | 4 m | 6 m |
| Oncologic response at LA | Death at D + 24 | CR | Death at D + 11 | CR | CR | CR | CR | CR | CR | CR | CR | CR | Not reported | CR |
| Characteristics . | Patient 16,7 . | Patient 28 . | Patient 39 . | Patient 410 . | Patient 511 . | Patient 612 . | Patient 713 . | Patient 813 . | Patient 914 . | Patient 1015 . | Patient 1116 . | Patient 122,17 . | Patient 1318 . | Patient 1419 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at infusion (y) | 34 | 50 | 52 | 61 | 44 | 30 | 41 | 30 | 48 | 44 | 28 | 33 | 29 | 41 |
| Sex | Female | Female | Male | Male | Male | Male | Female | Female | Female | Female | Female | Female | Female | Female |
| Histology | DLBCL | DLCBL | MCL | DLBCL | DLBCL | PMBL | DLBCL | PMBL | DLBCL | PMBL | PMBL | EBV+ DLBCL | DLBCL | DLBCL |
| Previous treatment | 3 lines of chemotherapy unspecified | 2 lines of chemotherapy unspecified | 3 lines of therapy unspecified, ASCT | R-CHOP, HD-MTX, CYVE | R-CHOP, R-ICE | Chemotherapy unspecified, radiotherapy, ASCT | 4 lines of chemotherapy unspecified, ASCT | 2 lines of chemotherapy unspecified | R-CHOP, another line of chemotherapy unspecified | DA-EPOCH-R, R-ESHAP | R-CHOP, GDP, radiotherapy | DA-EPOCH-R, R-ICE, prophylactic IT and HD-MTX, radiotherapy, nivolumab | Not specified | Not specified |
| Bridging treatment | Not reported | BEAM, ASCT | Obinutuzumab, bendamustine | Intrathecal methotrexate | Hyper-CVAD | Not reported | Not reported | Radiotherapy, rituximab, bendamustine, dexamethasone | Ibrutinib, lenalidomide | Radiotherapy | Radiotherapy, pembrolizumab, dexamethasone | Radiotherapy | Not reported | Not reported |
| CAR T cells | Axi-cel | Allogeneic CD19 + CD22 directed | Brexu-cel | Tisa-cel | CD19-directed unspecified | CD19-directed unspecified | Axi-cel | Axi-cel | Axi-cel | Axi-cel | Axi-cel | Axi-cel | CD19-directed unspecified | Tisa-cel |
| CRS onset | D + 5 | D + 6 | D + 1 | D + 3 | D + 1 | D + 6 | D + 1 | D + 2 | D + 3 | D + 1 | D + 1 | D + 2 | Not specified | D + 1 |
| Max CRS grade | Grade 2 | Grade 2 | Grade 1 | Grade 1 | Grade 1 | Grade 2 | Grade 1 | Grade 2 | Grade 2 | Grade 2 | Grade 1 | Grade 2 | Not specified | Grade 2 |
| ICANS onset | D + 5 | D + 15 | D + 3 | N/A | D + 5 | D + 6 | D + 2 | D + 5 | D + 5 | D + 5 | D + 4 | D + 5 | D + 5 | N/A |
| Max ICANS grade∗ | Grade 4 | Grade 4 | Grade 4 | No ICANS | Grade 4 | Grade 4 | Grade 4 | Grade 4 | Grade 4 | Grade 4 | Grade 4 | Grade 4 | Grade 4 | No ICANS |
| Myelopathy onset | D + 10 | D + 21 | D + 3 | D + 6 | D + 5 | D + 6 | D + 8 | D + 5 | D + 9 | D + 6 | D + 27 | D + 7 | Not specified | D + 14 |
| Presentation | Paraplegia, progression to respiratory failure and death | Back pain, LE weakness | Paraplegia, sensory level, urine retention | Back pain, left leg radiculopathy | Back pain, paraplegia, T10 sensory level | Paraplegia, T10 sensory level | Quadriparesis, urine retention | Quadriparesis, urine retention | Quadriparesis | Paraplegia, T4 sensory level | Ascending paresthesia, progression to T4 sensory level and paraplegia, urine retention | Paraplegia, L1 sensory level, urine retention | LE weakness, progression to quadriparesis, lower cervical sensory level | Urodynamic urges, limbs sensorimotor deficit |
| Suspected etiology | HHV-6 | HHV-6 | Eosinophilic | Possible TIAN | CAR T-cell– mediated | CAR T-cell–mediated | CAR T-cell–mediated | CAR T-cell–mediated | CAR T-cell–mediated | CAR T-cell–mediated | CAR T-cell–mediated | CAR T-cell–mediated | CAR T-cell–mediated | CAR T-cell–mediated |
| Treatment | Tocilizumab, dexamethasone, MP, anakinra, IVIG, plasmapheresis, foscarnet | IVIG, ganciclovir, foscarnet | Dexamethasone, MP, siltuximab | None | Tocilizumab, dexamethasone, MP, anakinra | Tocilizumab, dexamethasone, siltuximab | Tocilizumab, dexamethasone, MP | Tocilizumab, MP | Dexamethasone, MP, anakinra, IVIG | Dexamethasone, MP, anakinra, siltuximab, IVIG, plasmapheresis | Tocilizumab, dexamethasone, MP, IVIG, plasmapheresis | Dexamethasone, IVIG | High-dose steroids, IVIG, plasmapheresis | Tocilizumab |
| Clinical improvement | No | Complete | No | Complete | No | No | Partial | Partial | Partial | No | Partial | Almost complete | Partial | Complete |
| Best oncologic response | N/A | CR | N/A | CR | CR | CR | CR | CR | CR | CR | CR | CR | Not reported | CR |
| LA | D + 24 | 7 mo | D + 11 | 6 mo | 4 mo | 1 mo | 3 y | 18 mo | 18 mo | 3 mo | 5 mo | 6 y | 4 m | 6 m |
| Oncologic response at LA | Death at D + 24 | CR | Death at D + 11 | CR | CR | CR | CR | CR | CR | CR | CR | CR | Not reported | CR |
The following patients were not described in the table because of insufficient information: patient 15, patient with PMBL who received an ICI as bridging therapy and who developed acute and irreversible paraplegia at day +7 after axi-cel20; patient 16, patient with PMBL who died 2 months after axi-cel infusion from acute encephalitis and myelitis21; patient 17, 40-year-old woman with DLBCL who developed acute myelopathy at day+10 after infusion of unspecified CAR T cells and who was treated with tocilizumab and steroids and regained function7; patient 18, patient with large B-cell lymphoma who received a fully human scFv containing, CD19-directed CAR T-cell therapy (JCAR021) and who subsequently developed paraplegia associated with spinal cord edema22,23; patients 19 and 20, active cases with large B-cell lymphomas who developed acute myelitis after CD19-directed CAR T-cell therapy (not published). All patients, except patient number 2 with relapsed disease, had primary refractory disease before CAR T-cell infusion. Disease status was not reported for patient number 3. Only patient number 4 had CNS involvement at CAR T-cell infusion. No patient received CAR T-cell therapy before the treatment indicated in this table. None of the patients have been reported to develop IEC-HS (immune effector cell–associated hemophagocytic lymphohistiocytosis-like syndrome). However, because of a lack of data, we cannot exclude the possibility that some cases were associated with this complication.
ASCT, autologous stem cell transplant; Axi-cel, axicabtagene ciloleucel; BEAM, carmustine, etoposide, cytarabine (Ara C) , and melphalan; Brexu-cel, brexucabtagene autoleucel; CR, complete remission; CYVE, cytarabine andetoposide; DA-EPOCH-R, dose-adjusted etoposide, prednison,e vincristine (oncovin), cyclophosphamide, doxorubicin (hydroxydaunorubicin), and rituximab; EBV, Epstein-Barr virus; GDP, gemcitabine, dexamethasone, and cisplatin; HD-MTX, high-dose methotrexate; Hyper-CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin (adriamycin), and dexamethasone; LA, last assessment; LE, lower extremity; Max, maximum; MCL, mantle cell lymphoma; MP, methylprednisolone; N/A, not applicable; Not reported, no information provided on the occurrence; not specified, occurred but no details provided; R-CHOP, rituximab, cyclophosphamide, doxorubicin (hydroxydaunomycin), vincristine (oncovin), and prednisone; R-ESHAP, rituximab, etoposide, methylprednisolone (solu-medrol), high-dose cytarabine (Ara C), and cisplatin (platinol); R-ICE, rituximab, ifosfamide, carboplatin,and etoposide; scFv, single-chain variable fragments; tisa-cel, tisagenlecleucel.
The ICANS grade was defined according to the American Society for Transplantation and Cellular Therapy (ASTCT) consensus grading. According to this definition, deep focal motor weakness, such as hemiparesis or paraparesis, is classified as grade 4.
Pediatric cases of acute myelopathy following CAR T-cell therapy for B-ALL
| Characteristics . | Patient 2124 . | Patient 2224 . | Patient 2324 . | Patient 2424 . |
|---|---|---|---|---|
| Age at infusion (y) | 7 | 14 | 13 | 2.5 |
| Sex | Female | Male | Male | Male |
| Previous CNS disease | Yes | Yes | No | Yes |
| Previous treatment | Chemotherapy unspecified, tisa-cel, CART22-65s, allo-HSCT, radiotherapy | Chemotherapy unspecified, blinatumomab, tisa-cel | Chemotherapy unspecified | Chemotherapy unspecified, allo-HSCT, inotuzumab, radiotherapy |
| Bridging treatment | Not reported | Not reported | Not reported | Not reported |
| CAR T cells | CD22-directed (CART22-65s) | CD22-directed (CART22-65s) | CD19-directed (huCART19) | Tisa-cel |
| CRS onset | D + 15 | D + 4 | D + 1 | Not reported |
| Max CRS grade | Grade 2 | Grade 2 | Grade 4 | Grade 1 |
| ICANS onset | D + 19 | D + 8 | D + 4 | D + 14 |
| Max ICANS grade∗ | Grade 4 | Grade 4 | Grade 4 | Grade 4 |
| Myelopathy onset | D + 22 | D + 25 | D + 10 | D + 15 |
| Presentation | Areflexic quadriparesis | Areflexic paraparesis, decreased sensation to light touch | Areflexic paraparesis, midchest sensory level | Areflexic quadriparesis |
| Suspected etiology | CAR T-cell– mediated | CAR T-cell–mediated | CAR T-cell–mediated | CMV |
| Treatment | Tocilizumab, dexamethasone, anakinra, carbidopa/levodopa | Tocilizumab, dexamethasone, anakinra | Tocilizumab, dexamethasone, MP, anakinra, IVIG, plasmapheresis, carbidopa/levodopa | Dexamethasone, IVIG, plasmapheresis, valganciclovir, ganciclovir, foscarnet, carbidopa/levodopa |
| Clinical improvement | No | No | No | Partial |
| Best oncologic response | Refractory | Refractory | CR | CR |
| LA | D + 31 | D + 30 | 15 mo | D + 189 |
| Oncologic response at LA | PD, death | PD, death | CR | Relapse, death |
| Characteristics . | Patient 2124 . | Patient 2224 . | Patient 2324 . | Patient 2424 . |
|---|---|---|---|---|
| Age at infusion (y) | 7 | 14 | 13 | 2.5 |
| Sex | Female | Male | Male | Male |
| Previous CNS disease | Yes | Yes | No | Yes |
| Previous treatment | Chemotherapy unspecified, tisa-cel, CART22-65s, allo-HSCT, radiotherapy | Chemotherapy unspecified, blinatumomab, tisa-cel | Chemotherapy unspecified | Chemotherapy unspecified, allo-HSCT, inotuzumab, radiotherapy |
| Bridging treatment | Not reported | Not reported | Not reported | Not reported |
| CAR T cells | CD22-directed (CART22-65s) | CD22-directed (CART22-65s) | CD19-directed (huCART19) | Tisa-cel |
| CRS onset | D + 15 | D + 4 | D + 1 | Not reported |
| Max CRS grade | Grade 2 | Grade 2 | Grade 4 | Grade 1 |
| ICANS onset | D + 19 | D + 8 | D + 4 | D + 14 |
| Max ICANS grade∗ | Grade 4 | Grade 4 | Grade 4 | Grade 4 |
| Myelopathy onset | D + 22 | D + 25 | D + 10 | D + 15 |
| Presentation | Areflexic quadriparesis | Areflexic paraparesis, decreased sensation to light touch | Areflexic paraparesis, midchest sensory level | Areflexic quadriparesis |
| Suspected etiology | CAR T-cell– mediated | CAR T-cell–mediated | CAR T-cell–mediated | CMV |
| Treatment | Tocilizumab, dexamethasone, anakinra, carbidopa/levodopa | Tocilizumab, dexamethasone, anakinra | Tocilizumab, dexamethasone, MP, anakinra, IVIG, plasmapheresis, carbidopa/levodopa | Dexamethasone, IVIG, plasmapheresis, valganciclovir, ganciclovir, foscarnet, carbidopa/levodopa |
| Clinical improvement | No | No | No | Partial |
| Best oncologic response | Refractory | Refractory | CR | CR |
| LA | D + 31 | D + 30 | 15 mo | D + 189 |
| Oncologic response at LA | PD, death | PD, death | CR | Relapse, death |
All patients, except patient number 24, had evidence of active disease at CAR T-cell infusion. None of the patients had CNS involvement at the time of CAR T-cell infusion. None of the patients have been reported to develop IEC-HS. However, because of a lack of data, we cannot exclude the possibility that some cases were associated with this complication.
Abbreviations are explained in Table 1.
Allo-HSCT, allogeneic hematopoietic stem cell transplantation; PD, progressive disease.
The ICANS grade was defined based on the ASTCT consensus grading. According to this definition, deep focal motor weakness, such as hemiparesis or paraparesis, is classified as grade 4.
Probable CAR T-cell–mediated acute myelopathy
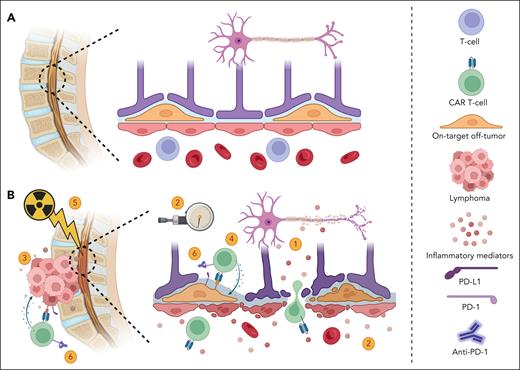
For most documented cases, no secondary causes were reported (Tables 1 and 2). The information available from the investigations was often insufficient to definitively rule out alternative etiologies. Nevertheless, the timing suggests a role for CAR T cells. Because of the limited number of patients, drawing definitive conclusions regarding the pathophysiological role of CAR T cells is challenging. However, several intriguing hypotheses have emerged (Figure 1).
Potential mechanisms of acute myelopathy following CAR T cells. (A) Normal spinal cord and BBB. (B) Acute myelopathy after infusion of CAR T cells. Several potential mechanisms are likely involved and may interact, including: (1) inflammatory mediators disrupt the BBB and exert direct toxicity; (2) a decrease in blood flow to the spinal cord that can potentially lead to infarction can be caused by elevated intraspinal canal pressure secondary to inflammation; (3) local inflammation from an adjacent tumor causes BBB damage and neuronal cell injury (TIAN); (4) on-target off-tumor toxicity of CAR T cells. Potential targets include CD19-expressing mural cells (illustrated), which can lead to BBB disruption, and CD22-expressing oligodendrocytes, which can cause demyelination; and (5) radiation therapy damages the BBB and/or sensitizes neuronal cells to neurotoxicity; and (6) ICIs may enhance on-target off-tumor toxicities or promote increased secretion of inflammatory mediators when CAR T cells are activated at the tumor site. Other contributing factors may include tumor histology, patient sex, and age. Figure created with BioRender.com.
Potential mechanisms of acute myelopathy following CAR T cells. (A) Normal spinal cord and BBB. (B) Acute myelopathy after infusion of CAR T cells. Several potential mechanisms are likely involved and may interact, including: (1) inflammatory mediators disrupt the BBB and exert direct toxicity; (2) a decrease in blood flow to the spinal cord that can potentially lead to infarction can be caused by elevated intraspinal canal pressure secondary to inflammation; (3) local inflammation from an adjacent tumor causes BBB damage and neuronal cell injury (TIAN); (4) on-target off-tumor toxicity of CAR T cells. Potential targets include CD19-expressing mural cells (illustrated), which can lead to BBB disruption, and CD22-expressing oligodendrocytes, which can cause demyelination; and (5) radiation therapy damages the BBB and/or sensitizes neuronal cells to neurotoxicity; and (6) ICIs may enhance on-target off-tumor toxicities or promote increased secretion of inflammatory mediators when CAR T cells are activated at the tumor site. Other contributing factors may include tumor histology, patient sex, and age. Figure created with BioRender.com.
Secondary to severe ICANS
It is worth noting that some patients exhibited rapid resolution of magnetic resonance imaging (MRI) findings, and those with longer follow-up demonstrated significant improvement in their neurologic deficits.2,13,14 It is plausible that acute spinal cord damage is caused by an inflammatory storm, and spinal cord edema may represent an extreme manifestation of ICANS. Supporting this, all patients experienced CRS, and all but 1 had severe ICANS before developing myelopathy. In addition, nearly all patients with lymphoma received CAR T cells that contained a CD28 costimulatory domain, which is known to induce a robust early inflammatory response and a higher incidence of CRS and ICANS than the 4-1BB (CD137 or tumor necrosis factor receptor superfamily member 9 [TNFRSF9]) costimulatory domain.25 This might be a reason why there are no described cases of myelitis with 4-1BB–based CAR T-cell therapies for multiple myeloma. In the case of CAR T cells that used a 4-1BB costimulatory domain for B-ALL, there is a disease-specific response that is associated with high inflammation.26
However, reconciling the rapid resolution of ICANS with the delayed or absent recovery of spinal cord function is challenging. In addition, the late occurrence of myelopathy in patient 11, which followed the complete resolution of ICANS, remains unexplained.16 These observations may indicate a heightened susceptibility of the spinal cord to inflammatory damage or an increased local inflammatory response.
Spinal cord infarction
The spinal cord’s vulnerability to inflammation-induced damage may be attributed to an increased risk for infarction. Although not proven, cord infarction was suspected in patient 5 based on the extensive cord edema and diffusion restriction at T10 on MRI.11 Blood flow to the spinal cord is regulated by perfusion pressure, determined by the difference between the mean arterial pressure and the intraspinal canal pressure. Inflammation could elevate pressure within the cerebrospinal fluid (CSF) space, thereby decreasing blood flow and posing a risk for spinal cord infarction. The sudden onset of symptoms in CAR T-cell–induced myelitis aligns with infarction and possibly explains the incomplete recovery in many cases.
TIAN
Tumor inflammation–associated neurotoxicity (TIAN) is an emerging concept that may explain localized spinal cord inflammation, as evidenced in patient 4, who had vertebral and leptomeningeal disease at the time of tisagenlecleucel infusion.10 In TIAN, neurotoxicity is attributed to focal inflammation at the tumor site as opposed to systemic inflammation in ICANS.2 Local inflammatory–mediated damage, compression from transient immune-mediated pseudoprogression, or disruption of the blood-brain barrier (BBB) may be caused by adjacent intracanal or epidural disease.
On-target off-tumor toxicity
In contrast to the transient nature of ICANS, patients with acute myelitis displayed prolonged neurologic deficits, sometimes accompanied by prolonged abnormal MRI signals, most often linear T2 hyperintensity throughout the central spinal cord.12,16 One possible explanation for this prolonged presentation is on-target off-tumor toxicity. This hypothesis has been proposed to explain a distinct range of rare neurologic complications associated with B-cell maturation antigen (BCMA)-directed and G protein–coupled receptor class C group 5 member D (GPRC5D)-directed CAR T cells with potential toxicity on the basal ganglia and the inferior olivary nucleus, respectively.27,28 Notably, brain mural cells have been shown to express CD19.29 These cells fulfill functions such as regulating central nervous system (CNS) blood flow, immune cell entry into the CNS, and maintaining BBB integrity. It remains unclear whether mural cells in the spinal cord express differing levels of CD19 or if the spinal cord is more vulnerable to mural cell dysfunction induced by CD19-directed CAR T-cell therapy.
Recently, there were 2 reported pediatric cases of acute myelitis following CD22-directed CAR T-cell therapy in B-ALL.24 Both cases exhibited T2 hyperintensity in the posterior cord on MRI. To date, CD22-directed CAR T cells have been associated with a low incidence of ICANS. One hypothesis for this observation is the absence of on-target off-tumor toxicity in mural cells, thus preserving the BBB.30 However, CD22 is expressed in mature oligodendrocytes, which play critical roles in the CNS, such as providing axonal metabolic support and activity-dependent myelination.30-32 Postmortem pathologic examination of 1 patient revealed diffuse leukomyelopathy with extensive vacuolation of the white matter and preserved grey matter, which could suggest specific cytotoxicity in mature oligodendrocytes. However, there was a notable absence of inflammation and a scarcity of T lymphocytes.24
On-target off-tumor toxicities may also explain why acute myelopathy has been described in lymphoma and B-ALL but not in multiple myeloma.
Other etiologies for acute myelopathy
Infections, autoimmune myelopathies, paraneoplastic phenomena, malignancies, metabolic disorders, demyelinating diseases, and drug toxicities may be other potential etiologies. Notably, cases of suspected fludarabine-induced neurotoxicity following lymphodepletion for CAR T-cell therapy have been reported.33,34 However, this toxicity typically presents with progressive, delayed, and widespread neurologic dysfunction, including visual impairments, dementia, ataxia, and motor-sensorimotor symptoms. Therefore, it is unlikely to explain acute myelopathy following CAR T-cell therapy. Only rare reports have identified probable secondary causes in the context of CAR T-cell treatment (Tables 1 and 2).
Viral infections
Encephalitis/myelitis secondary to cytomegalovirus (CMV) or human herpesvirus 6 (HHV-6) are rare but well-documented complications following hematopoietic cell transplantation.35 A few cases of CMV and HHV-6 encephalitis also have been reported after CAR T-cell therapy.8,36-39 However, myelitis attributed to these viruses following CAR T-cell therapy is extremely rare with only 3 documented cases.
Patient 1 developed paraplegia at day +10, which was refractory to steroids, anakinra, IV immunoglobulins (IVIGs), and plasmapheresis.6 Although plasma HHV-6 DNA remained undetected, a commercial polymerase chain reaction (PCR) test on CSF returned positive with 123 copies per mL on day +16, prompting the immediate initiation of foscarnet at a dose of 60 mg/kg every 8 hours. After 7 days of foscarnet therapy, a repeat CSF PCR test showed 1800 copies per mL, and the patient’s clinical status deteriorated to quadriparesis and respiratory failure, which led to her death on day +24.
Patient 2 was enrolled in a clinical trial that investigated the combination of allogeneic CD22 and CD19 CAR T cells after autologous hematopoietic cell transplantation for the management of relapsed diffuse large B-cell lymphoma (DLBCL).8 On day +15, the patient developed grade 1 ICANS, and HHV-6 was detected in the blood by droplet digital PCR at 1172.41 copies per μg cell-free DNA. The patient began treatment with ganciclovir at a dose of 5 mg/kg every 12 hours and foscarnet at a dose of 60 mg/kg every 12 hours on day +16. On day +17, HHV-6 was detected in the CSF by droplet digital PCR at 4694.16 copies per μg cell-free DNA, and the patient was started on IVIG infusions for 7 days. The patient developed weakness in her lower extremities on day +21 after starting treatment for HHV-6 infection. At the 7-month follow-up, the patient had complete clearance of HHV-6 in blood and showed complete resolution of neurologic deficits.
Drawing definitive conclusions to explain the differing outcomes of these 2 patients is challenging. The authors used different techniques and units to measure and report HHV-6 viral loads, making it unclear if viral load alone had a significant impact. However, the patient with the poorer outcome had a low viral load, suggesting that this may not be an important prognostic factor. Key distinctions between the cases seem to be the timing and the intensity of antiviral treatment. Both patients received IVIG, but the patient who achieved complete symptom resolution was treated early, before clinical myelopathy developed, and with a more aggressive combination of ganciclovir and foscarnet.
In these instances, it remains uncertain whether HHV-6 infection stems solely from latent viral reactivation in the setting of immunosuppression or if CAR T cells themselves elevate the risk. Intriguingly, a recent study has demonstrated HHV-6 reactivation in cultures of human CD4+ T cells during CAR T-cell manufacturing. A subset of CAR T cells, referred to as HHV-6 superexpressors, exhibit heightened viral transcriptional activity. These cells have been identified in certain commercial cellular therapy products approved by the US Food and Drug Administration.40 We could postulate that these HHV-6 superexpressor CAR T cells may heighten the risk for HHV-6 encephalitis/myelitis. Additional studies are necessary to ascertain whether preemptive treatment of CAR T cells with antivirals before infusion could be beneficial.
Recently, a case of CMV-mediated myelitis was suspected in patient 24.24 The patient developed CMV viremia before CAR T-cell therapy with CMV detected in the serum by PCR on day −1 at 1855 IU/mL. On day +14, the patient developed encephalopathy and decreased movements, followed by flaccid quadriparesis on day +15. CSF analysis at this time was positive for CMV. Despite treatment with IVIG and therapeutic doses of ganciclovir and foscarnet, the serum CMV viral load increased to 36 331 IU/mL on day +24, and the CSF remained positive. His clinical condition showed minimal improvement, and he died from relapsed disease on day +189.
For the cases discussed here, it is not possible to definitively conclude if acute myelopathy was caused solely by CMV or HHV-6 infections, because viremia alone does not establish causality. All these cases developed low-grade CRS and grade 4 ICANS, and we cannot exclude the possibility that other discussed etiologies at least partially explain the neurologic presentations and differences observed between the cases.
Eosinophilic acute myelopathy
Only 1 case of eosinophilic myelitis following CAR T-cell therapy has been reported. Patient 3, with a high tumor burden but no CNS involvement, received bridging therapy with obinutuzumab plus bendamustine for mantle cell lymphoma.9 The patient received brexucabtagene autoleucel, and on day +2, he was administered cefepime, levetiracetam, and tocilizumab for persistent CRS. By day +3, the patient developed paraplegia and complete loss of sensation in their lower extremities. CSF analysis revealed pleocytosis with 92% eosinophils, whereas microbiologic investigations yielded no remarkable findings. Notably, there was no peripheral blood eosinophilia. The patient’s neurologic symptoms stabilized and a repeat MRI of the spine showed radiologic improvements on steroid treatment. However, the patient succumbed to septicemia on day +11.
Eosinophilic myelitis is rare and has various underlying causes. Infections by neurotropic pathogens, particularly helminthic infections, are the most prevalent.41 Other less common causes include malignancies, autoimmune disorders, certain medications, and reactions to prostheses. Although the role of CAR T cells in this myelopathy case is uncertain, studies have linked interleukin-5 (IL-5) levels to the severity of neurotoxicity in acute lymphoblastic leukemia.42 However, a central role of IL-5 produced by CAR T cells seems improbable because of the presence of isolated eosinophilia in the CSF. Similarly, a reaction stemming from occult CNS involvement of lymphoma seems improbable because of the extensive systemic disease burden and absence of peripheral eosinophilia.
Although the patient received medications that may induce neurotoxicity, such as cefepime and fludarabine, neither have been associated with eosinophilic encephalitis/myelitis. Moreover, a systemic drug reaction typically does not manifest solely with CSF eosinophilia. Despite the absence of evidence of an autoimmune disorder and the absence of a clear explanation for the patient’s presentation, an undetected infectious agent remains plausible.
Potential risk factors for acute myelopathy after CAR T-cell therapy
Given the limited number of patients who have developed acute myelopathy following CAR T-cell therapy, definitive conclusions cannot be drawn. However, as previously mentioned, the characteristics of a CAR construct, such as the costimulatory domain and the specific target, may influence the risk for neurologic complications. In addition, certain observations warrant further investigation.
It should be noted that most patients who developed acute myelopathy after CAR T-cell therapy were diagnosed with lymphomas associated with an inflamed microenvironment, such as primary mediastinal B-cell lymphoma (PMBL) and Epstein-Barr virus–positive DLBCL (Table 1). This suggests that these patients potentially have a heightened susceptibility to neurotoxicity, possibly stemming from a more vigorous response of CAR T cells within an immune-primed tumor microenvironment. Supporting this hypothesis, a recent study revealed that the response was superior in PMBL when compared with DLBCL after axicabtagene ciloleucel; however, there was a higher incidence of grade ≥3 ICANS in PMBL.21 In this study, 4 patients succumbed to grade 5 ICANS, 1 of whom developed refractory encephalomyelitis, and all 4 patients had PMBL. Despite our greater experience with CAR T-cell therapy in lymphoma, specific disease characteristics that influence the CAR T-cell response may explain why acute myelopathy has not been described in multiple myeloma and has only been reported in 4 cases of B-ALL.
Interestingly, 4 patients received bridging with radiation therapy (Table 1), including 2 with spinal canal exposure (patients 10 and 12).2,15 Radiation to the spinal cord can cause the Lhermitte phenomenon that is characterized by transient and nonsevere electrical shock-like paresthesia upon neck flexion that typically occur 2 to 4 months after radiation. Radiation-induced myelitis is a rare complication and is generally not seen earlier than 6 months after radiation with a median latency period of 18 months. The onset is usually insidious with slow progression over several months.43 The timing, radiographic pattern, and acute presentation of myelopathy after CAR T-cell infusion argue against radiation-induced myelitis. Furthermore, the reported radiation doses were well below those known to induce myelitis.43,44 Nonetheless, it remains unknown whether interactions between radiotherapy and elevated levels of immune mediators in the blood and CSF could increase local alterations to the BBB or sensitize to neurotoxicity. In addition, low-dose radiation has been shown to sensitize tumor cells to CAR T-cell–mediated death.45 It is unclear if radiation also enhances the risk for on-target off-tumor toxicity in the CNS. Despite these cases, recent clinical data on the use of radiation as bridging therapy have shown promising outcomes with no concerning neurotoxicity signals.46-50
Three patients received immune checkpoint inhibitors (ICIs) that targeted programmed cell death protein 1 (PD-1) before CAR T-cell therapy.2,16,20 Checkpoint inhibitors can trigger various immune-related adverse events, including rare neurologic complications.51,52 Myelitis associated with ICIs is exceedingly rare, primarily documented in isolated case reports.53-56 Proposed mechanisms include activation of self-reactive T lymphocytes and/or the induction of paraneoplastic processes that involve autoreactive antibodies. Although combining ICIs with CAR T-cell therapy has shown manageable safety profiles, reports of early-onset immune toxicity linked to this combination have emerged.57-59 The question of whether ICIs and CAR T cells exhibit a synergistic effect on the risk for myelitis, perhaps by increasing on-target off-tumor toxicity, remains unanswered. In addition, determining whether acute myelitis in patients who received both ICIs and CAR T-cell therapy stems directly from ICI exposure, the CAR T-cell therapy itself, or a combination of both poses a significant challenge. In general, patients who developed myelopathy after ICIs presented with a progressive or subacute course and showed a propensity to relapse after initial treament.55,56,60-62 This pattern was solely observed in patient 11.16 Although CSF studies were conducted in 2 patients who received CAR T cells after ICIs, there was no mention of autoantibody testing.2,16 In these patients, treatment with steroids and IVIG, with or without plasmapheresis, demonstrated at least partial effectiveness in 1 case and was associated with nearly complete resolution of symptoms in the other.
Another surprising finding is that most adult patients who developed acute myelopathy after CAR T-cell therapy were women (Table 1). Female hormones have been shown to protect the BBB and to decrease lymphocyte trafficking.63,64 Estrogen signaling is also known for its role in regulating inflammation.65,66 It remains unclear whether alterations of these functions during CAR T-cell therapy predisposes to severe neurotoxicity. Nevertheless, inflammatory response has been shown to be consistently higher in females than in males after puberty, and females are more at risk for autoimmune diseases.67 An increased incidence of myelitis in females may thus reflect their susceptibility to autoimmunity and severe inflammation following CAR T-cell infusion.
Finally, although nearly half of the patients with lymphoma with probable CAR T-cell–mediated acute myelopathy were diagnosed with PMBL, they were significantly younger than the median age for a large B-cell lymphoma diagnosis (Table 1). Only 1 patient was older than 50 years, whereas the median age at diagnosis of DLBCL is typically around 65 years.68 One hypothesis is that younger patients may yield more potent lymphocytes for CAR T-cell production or could exhibit a more robust response after infusion because of the absence of immune senescence associated with aging. Immunosenescence is characterized by a chronic, low-grade inflammatory state but decreased responsiveness to appropriate stimuli.69 This aligns with a recent study that demonstrated a shorter event-free survival after CAR T-cell therapy in older patients.70
Recommendations
Given the rarity of this complication, there are no established guidelines for diagnosing and treating acute myelopathy following CAR T-cell therapy. In this study, we offer expert opinions based on our review of the literature and personal experience (Table 3). These recommendations are primarily related to adult cases and may not all be applicable to pediatric populations.
Recommendations for diagnostic workup and treatment of CAR T-cell–related myelopathy
| . | We recommend . | To be considered . | Investigational . |
|---|---|---|---|
| Diagnosis | Brain and spine MRI with and without contrast Evaluation for constipation and urinary retention with bladder scan Early consultations with neurology and infectious disease specialists If no contraindication, lumbar puncture with opening pressure measurement and CSF analysis (biochemistry, cell count, cytology, flow cytometry, and oligoclonal bands) Infectious workup on CSF, including cultures and NAT for HHV-6, CMV, VZV, HSV-1/2, JC-virus, EBV, adenovirus, and enteroviruses Infectious workup on peripheral blood, including cultures, QuantiFERON, HIV and hepatitis testing, Treponema pallidum testing, NAT for HHV-6, EBV, adenovirus, and CMV Serologic investigation, including vitamin B12, folate, methylmalonic acid, homocysteine, vitamin E, copper, ceruloplasmin, ferritin, C-reactive protein, CBC, LDH, liver enzymes, bilirubin, INR, PTT, creatinine, and extended electrolytes | Autoantibody testing (onconeural, AQP4, MOG antibodies) in CSF if previous exposure to ICIs and as per neurology for other patients Extended infectious workup on CSF and peripheral blood guided by an infectious disease specialist Serologic investigations if suspicion of IEC-HS, including triglycerides, fibrinogen, lactate, and soluble IL-2 receptor-α. Other serologic test: thiamine level Serology analysis for rheumatoid disease and NMO, including ANA, anti-Ro/La, anti-AQP4 testing, and other autoantibodies as guided by the clinical picture Electrodiagnostic studies | Extended cytokine panel on peripheral blood and/or CSF Lymphocytes subsets in peripheral blood and/or CSF Measurement of CAR T-cell expansion in peripheral blood and/or CSF |
| Treatment | High-dose steroids: methylprednisolone 1 g IV daily for 3-5 d with a slow taper depending on response and etiologic workup Avoidance of tocilizumab if no concurrent CRS If an infectious cause is identified: targeted antimicrobial therapy Medication review and avoidance of neurotoxic agents Correction of deficiencies in essential micronutrients Correction of hyponatremia Early rehabilitation | Empirical thiamine repletion: for example, 500 mg every 8 h for 72 h, followed by daily maintenance supplementation Empirical treatment for HHV-6 and CMV while awaiting results Other empirical infectious treatments if there are risk factors and if patient is not improving on initial steroid therapy IVIG 2 g/kg administered in divided doses per package insert, or plasmapheresis if previous exposure to ICIs. IVIG could be considered for other patients if refractory to initial treatment with pulse dose steroids Anakinra if refractory to initial management | Siltuximab Emapalumab Canakinumab Basiliximab Ruxolitinib BTK inhibitors Other TKI Salvage therapy (etoposide, cyclophosphamide, alemtuzumab, and ATG) |
| . | We recommend . | To be considered . | Investigational . |
|---|---|---|---|
| Diagnosis | Brain and spine MRI with and without contrast Evaluation for constipation and urinary retention with bladder scan Early consultations with neurology and infectious disease specialists If no contraindication, lumbar puncture with opening pressure measurement and CSF analysis (biochemistry, cell count, cytology, flow cytometry, and oligoclonal bands) Infectious workup on CSF, including cultures and NAT for HHV-6, CMV, VZV, HSV-1/2, JC-virus, EBV, adenovirus, and enteroviruses Infectious workup on peripheral blood, including cultures, QuantiFERON, HIV and hepatitis testing, Treponema pallidum testing, NAT for HHV-6, EBV, adenovirus, and CMV Serologic investigation, including vitamin B12, folate, methylmalonic acid, homocysteine, vitamin E, copper, ceruloplasmin, ferritin, C-reactive protein, CBC, LDH, liver enzymes, bilirubin, INR, PTT, creatinine, and extended electrolytes | Autoantibody testing (onconeural, AQP4, MOG antibodies) in CSF if previous exposure to ICIs and as per neurology for other patients Extended infectious workup on CSF and peripheral blood guided by an infectious disease specialist Serologic investigations if suspicion of IEC-HS, including triglycerides, fibrinogen, lactate, and soluble IL-2 receptor-α. Other serologic test: thiamine level Serology analysis for rheumatoid disease and NMO, including ANA, anti-Ro/La, anti-AQP4 testing, and other autoantibodies as guided by the clinical picture Electrodiagnostic studies | Extended cytokine panel on peripheral blood and/or CSF Lymphocytes subsets in peripheral blood and/or CSF Measurement of CAR T-cell expansion in peripheral blood and/or CSF |
| Treatment | High-dose steroids: methylprednisolone 1 g IV daily for 3-5 d with a slow taper depending on response and etiologic workup Avoidance of tocilizumab if no concurrent CRS If an infectious cause is identified: targeted antimicrobial therapy Medication review and avoidance of neurotoxic agents Correction of deficiencies in essential micronutrients Correction of hyponatremia Early rehabilitation | Empirical thiamine repletion: for example, 500 mg every 8 h for 72 h, followed by daily maintenance supplementation Empirical treatment for HHV-6 and CMV while awaiting results Other empirical infectious treatments if there are risk factors and if patient is not improving on initial steroid therapy IVIG 2 g/kg administered in divided doses per package insert, or plasmapheresis if previous exposure to ICIs. IVIG could be considered for other patients if refractory to initial treatment with pulse dose steroids Anakinra if refractory to initial management | Siltuximab Emapalumab Canakinumab Basiliximab Ruxolitinib BTK inhibitors Other TKI Salvage therapy (etoposide, cyclophosphamide, alemtuzumab, and ATG) |
ANA, antinuclear antibody; AQP4, aquaporin-4; ATG, antithymocyte globulin; BTK, Bruton’s tyrosine kinase; CBC, complete blood count; HSV-1/2, herpes simplex virus 1/2; INR, international normalized ratio; LDH, lactate dehydrogenase; MOG, myelin oligodendrocyte glycoprotein; NAT, nucleic acid testing; NMO, neuromyelitis optica; PTT, partial thromboplastin time; TKI, tyrosine kinase inhibitor; VZV, varicella-zoster virus.
Diagnostic workup
The initial workup necessitates a multidisciplinary approach to rule out secondary causes. It is particularly important to involve neurologists and infectious disease specialists with expertise in CAR T-cell therapies at an early stage.
When neurologic manifestations compatible with myelopathy arise, we recommend conducting a radiologic evaluation with both brain and spine MRIs, with and without contrast. Despite a recent report that suggested that MRI has limited therapeutic impact for ICANS, we emphasize that an MRI is essential for demonstrating spinal cord inflammation.71 Obtaining a baseline MRI also enables objective response assessment following treatment initiation.
In the absence of contraindications, we recommend prompt CSF analysis and opening pressure measurement for all patients, because these tests can guide further etiologic investigations and/or targeted interventions. We underscore the critical role of lumbar puncture in identifying infectious causes. As mentioned earlier, HHV-6 DNA and other infectious markers are sometimes only detectable in the CSF. If CSF eosinophilia is present or if the patient has risk factors for certain pathogens, an extensive infectious workup should be conducted by an experienced infectious disease specialist. Along with basic biochemistry and cell count, cytology and flow cytometry should be performed on the CSF to exclude CNS lymphoma or other neoplasms. In addition, CSF investigation for oligoclonal bands and onconeural, aquaporin-4, and myelin oligodendrocyte glycoprotein antibodies, should be performed for patients exposed to ICIs and may also be considered for other relevant cases. Beyond CSF testing, a lumbar puncture may also be beneficial for CSF diversion, thereby increasing blood flow to the spinal cord and reducing the risk for infarction.
Serologic investigations should be conducted to exclude metabolic causes, such as deficiencies in vitamin B12, folate, vitamin E, copper, and/or thiamine. Infectious workup should be completed under the guidance of an infectious disease specialist, which may include, but is not limited to, testing for HHV-6, CMV, HIV, and syphilis and obtaining blood cultures. Antinuclear antibodies and/or anti-Ro/La Sjögren's syndrome antigen A and B antibodies [SSA and SSB]) testing can be considered if there is clinical suspicion of a rheumatoid disease, such as lupus or Sjögren’s syndrome. Aquaporin-4 should also be considered to exclude neuromyelitis optica. Finally, evaluating toxicity in other organs may help to distinguish whether myelopathy is a manifestation of a systemic process. For instance, findings such as hyperferritinemia, combined with increased liver function enzymes, hyperbilirubinemia, cytopenia, coagulopathy, hypofibrinogenemia, renal insufficiency, electrolyte disorders, and/or lactate dehydrogenase elevation might suggest IEC-HS (immune effector cell–associated hemophagocytic lymphohistiocytosis-like syndrome).72
Management
Acute myelopathy is a medical emergency because of its potential for debilitating long-term neurologic sequelae, and prompt initiation of treatment is essential. A significant observation is that most reported cases achieved excellent oncologic outcomes despite the early and prolonged use of high-dose steroids. Indeed, complete remission was attained by all described patients with lymphoma, and this remission was maintained at their last follow-up, which was several years after CAR T-cell infusion in some cases. Therefore, the key message is that we should not hesitate to employ aggressive steroid treatment, because it is unlikely to compromise the efficacy of CAR T-cell therapy.
In all cases, a comprehensive medication review should be performed, and the avoidance of neurotoxic agents should be prioritized. In addition, other potential contributing factors to neurotoxicity or spinal cord edema, such as deficiencies in essential micronutrients and hyponatremia, should be addressed. In severe cases of ICANS, empirical thiamine repletion has been encouraged. This recommendation is supported by preclinical evidence that indicates that thiamine deficiency compromises BBB integrity and that inflammation is a driver of thiamine depletion.2,73,74 Although there is currently no clinical evidence that supports the use of empirical thiamine supplementation in acute myelopathy, its favorable safety profile warrants consideration. Lastly, early rehabilitation services should be provided to all patients.
Further interventions should be guided by etiologic investigations. Prompt initiation of targeted antimicrobial therapy is imperative in cases of infectious myelitis. While awaiting results, empirical treatments may be justified if there is clinical suspicion of an infectious cause, if the patient has risk factors for a particular pathogen, or if the patient’s condition fails to respond to initial therapy. For patients who have been exposed to ICIs before CAR T-cell infusion and for whom ICI-induced myelopathy cannot be ruled out, we recommend following the published guidelines for managing immune-related adverse events after ICIs, in addition to considering our recommendations for CAR T-cell–associated myelopathy.75-77 This includes a slow taper of steroids because of the protracted course and risk for relapse associated with ICI-induced myelopathy.
The use of the IL-6 receptor inhibitor tocilizumab should be limited to instances in which concurrent CRS is present. Its ability to penetrate the BBB is limited, and it has demonstrated ineffectiveness in treating neurotoxicity.78 Furthermore, there are indications that tocilizumab may exacerbate the risk for ICANS by elevating systemic and CSF IL-6 levels.79 Notably, administration of the IL-6 inhibitor siltuximab to 2 patients with myelitis failed to yield neurologic improvement, suggesting that direct IL-6 inhibition may also be ineffective.12,15 Conversely, emerging evidence from preclinical data and small clinical trials supports the use of the IL-1 receptor antagonist anakinra in managing refractory and/or severe ICANS.80-83 Despite this, the efficacy of anakinra in myelitis cases remains uncertain, because only 1 of 3 documented patients showed improvement following treatment with this agent.11,14,15 However, given its favorable safety profile, we recommend anakinra administration in cases of concomitant ICANS, and it can be considered in severe or refractory myelitis cases. Presently, there is insufficient data to support or refute the use of alternative agents.
The role of IVIG or plasmapheresis in severe ICANS and CAR T-cell–mediated myelitis remains unclear.84 Among the patients discussed above, 3 showed improvement following IVIG administration, whereas 2 others exhibited no response.2,14-16,18 In addition, 1 patient exhibited partial clinical improvement following plasmapheresis.18 However, the potential benefit of IVIG or plasmapheresis seems more evident and is recommended in cases of ICI-induced myelitis.5,54,55,75,76 In our series, 1 patient with previous ICI treatment experienced rapid and significant improvement after IVIG administration.2 Therefore, we believe that IVIG or plasmapheresis should be strongly considered for patients with previous ICI treatment who are refractory to initial high-dose steroid therapy. These modalities can also be considered for other patients who are refractory to steroids on a case-by-case basis. However, caution is warranted if spinal cord infarction is possible because of the risk for increased viscosity and thrombosis associated with IVIG administration and the risk for hypotension associated with plasmapheresis.
Conclusion
CAR T-cell therapies are still relatively new, and as their indications expand, the understanding of their neurotoxicity profile continues to evolve. Although CAR T-cell–related myelopathy is rare, it is likely underreported, and clinicians should maintain a high index of suspicion.
Multiple secondary causes should be considered. However, the pathophysiology of CAR T-cell–mediated acute myelopathy remains largely unexplained. We have explored multiple hypotheses to explain a specific treatment-related toxicity. Nonetheless, acute myelopathy is likely caused by a complex interaction between multiple factors, including the patient’s predisposition to adverse immune reactions, characteristics of the treated disease, previous treatments, and properties of the particular CAR T-cell product. In addition, the concept of TIAN underscores the importance of thorough disease staging before CAR T-cell therapy and bridging therapy to reduce disease burden.
This review aimed to raise awareness of this rare yet significant complication and to offer guidance for its management. Reporting future cases and systematic monitoring in clinical trials is imperative to elucidate the causative mechanisms and to develop preventive and therapeutic strategies.
Acknowledgments
X.D.-S. was supported by a fellowship from the University of Montreal Hospital Centre; a Perras, Cholette & Cholette Clinical Subspecialty Scholarship; and a Detweiler Travelling Fellowship from the Royal College of Physicians and Surgeons of Canada.
Authorship
Contribution: X.D.-S. wrote the initial draft of the manuscript; B.D.S. and P.B.D. edited the manuscript; and all authors reviewed and provided comments on the manuscript and approved the final version for publication.
Conflict-of-interest disclosure: B.D.S. reports receiving consulting honoraria from Celgene, Bristol Myers Squibb, and Kite/Gilead; serving on the data and safety monitoring board for Janssen; and being on the scientific advisory board of IN8bio. The remaining authors declare no competing financial interests.
Correspondence: Xavier Deschênes-Simard, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; email: xavier.deschenes-simard@umontreal.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal