Key Points
MTV affected outcomes of second-line treatment in both arms, and axi-cel improved EFS and PFS over standard care, irrespective of MTV.
Baseline MTV associated with grade ≥3 CRS and neurologic events after axi-cel treatment.
Visual Abstract
Metabolic tumor volume (MTV) assessed using 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography, a measure of tumor burden, is a promising prognostic indicator in large B-cell lymphoma (LBCL). This exploratory analysis evaluated relationships between baseline MTV (categorized as low [median or less] vs high [greater than median]) and clinical outcomes in the phase 3 ZUMA-7 study (NCT03391466). Patients with LBCL relapsed within 12 months of or refractory to first-line chemoimmunotherapy were randomized 1:1 to axicabtagene ciloleucel (axi-cel; autologous anti-CD19 chimeric antigen receptor T-cell therapy) or standard care (2-3 cycles of chemoimmunotherapy followed by high-dose chemotherapy with autologous stem cell transplantation in patients who had a response). All P values are descriptive. Within high- and low-MTV subgroups, event-free survival (EFS) and progression-free survival (PFS) were superior with axi-cel vs standard care. EFS in patients with high MTV (vs low MTV) was numerically shorter with axi-cel and was significantly shorter with standard care. PFS was shorter in patients with high MTV vs low MTV in both the axi-cel and standard-care arms, and median MTV was lower in patients in ongoing response at data cutoff vs others. Median MTV was higher in patients treated with axi-cel who experienced grade ≥3 neurologic events or cytokine release syndrome (CRS) than in patients with grade 1/2 or no neurologic events or CRS, respectively. Baseline MTV less than or equal to median was associated with better clinical outcomes in patients receiving axi-cel or standard care for second-line LBCL. The trial was registered at www.clinicaltrials.gov as #NCT03391466.
Introduction
First-line chemoimmunotherapy for large B-cell lymphoma (LBCL) is curative in many patients, but ∼40% experience disease progression or relapse, require new antilymphoma therapy, or die within 2 years in the postrituximab era.1 Until recently, second-line standard care was high-dose chemotherapy (HDT) with autologous stem cell transplantation (ASCT) for those who respond to salvage chemoimmunotherapy.2 However, based on results of the phase 3 ZUMA-7 and TRANSFORM studies, autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy may replace salvage chemoimmunotherapy and HDT/ASCT as standard of care for patients with LBCL who are refractory to first-line therapy or relapsed within 12 months.3,4 In ZUMA-7, second-line therapy with axicabtagene ciloleucel (axi-cel) significantly prolonged event-free survival (EFS) vs standard care in patients with relapsed/refractory (R/R) LBCL (hazard ratio [HR], 0.398; stratified log-rank P < .0001).5 The primary overall survival (OS) analysis of ZUMA-7 (median follow-up, 47.2 months) demonstrated a statistically significant improvement in OS with axi-cel over standard care. Median OS was not reached in the axi-cel arm and was 31.1 months in the standard-care arm, with 4-year OS rates of 54.6% and 46.0%, respectively (HR, 0.73; stratified log-rank P < .03).6 Axi-cel was superior to standard care across negative prognostic subgroups, including patients with elevated lactate dehydrogenase (LDH) and those with high tumor burden (TB) determined by sum of the product of perpendicular diameters (SPD).7
High TB has long been recognized as an independent risk factor for poor outcome in LBCL8 and other lymphomas.9,10 TB measurement has evolved from assessing maximal tumor dimension using clinical examination, chest radiography, and lymphography to determining total tumor volume in nodal and extranodal sites by combining positron emission tomography (PET) and computed tomography (CT; PET-CT).11 Still, there is no standard procedure for quantifying TB in lymphoma. Cheson et al12 described the estimation of TB using SPD for up to 6 target measurable lesions on CT. Although SPD is widely used to evaluate TB in LBCL,13 this CT-based method does not account for nonmeasured lesions or metabolic activity. In [18F]fluoro-d-glucose (FDG)–avid lymphomas, 2-deoxy-2-FDG PET-CT, which achieves precise anatomic localization of metabolically active tissues, is considered the gold standard for staging and response assessment13-15 and has emerged as a basis for measuring TB.11
TB based on metabolic tumor volume (MTV) assessed using FDG PET-CT is a promising prognostic indicator in LBCL. Although there clearly is a need for standard methods for quantifying MTV across tumor and treatment types, higher pretreatment MTV has consistently predicted shorter progression-free survival (PFS) and OS in patients with LBCL receiving standard first-line chemoimmunotherapy16-20 or platinum-based salvage chemotherapy.21 Furthermore, higher pretreatment MTV was associated with poorer clinical outcomes in patients receiving anti-CD19 CAR T-cell therapy for R/R LBCL after ≥2 lines of therapy.22-26 Here, we present exploratory analyses of relationships between whole-body MTV at baseline and clinical outcomes for patients treated with axi-cel or standard care in the ZUMA-7 study.
Methods
Patients
Full details on the ZUMA-7 study (ClinicalTrials.gov identifier: NCT03391466) were previously reported.5 Briefly, eligible patients were aged ≥18 years (with no upper age limit) and had LBCL confirmed by histology according to World Health Organization 2016 classification criteria.27 Patients were refractory to adequate first-line treatment consisting of an anti-CD20 monoclonal antibody and anthracycline-containing regimen or had relapsed ≤12 months after completing first-line chemoimmunotherapy.
Study design
Patients were randomized 1:1 to axi-cel or investigator-selected standard chemoimmunotherapy, stratified by response to first-line therapy and second-line age-adjusted International Prognostic Index (IPI).5 Patients in the axi-cel arm received a single infusion of axi-cel (target dose, 2 × 106 CAR T cells per kg) after undergoing leukapheresis followed by lymphodepleting chemotherapy with cyclophosphamide (500 mg/m2 per day) and fludarabine (30 mg/m2 per day) 5, 4, and 3 days before axi-cel infusion. Bridging therapy was optional and limited to corticosteroids.5 Patients randomized to standard care received 2 to 3 cycles of protocol-defined, investigator-selected platinum–based chemoimmunotherapy, and those who had a complete response (CR) or partial response (PR) proceeded to HDT-ASCT. Disease assessments per Lugano classification13 occurred at time points specified from randomization. Although crossover between treatment groups was not planned, patients in either arm could receive off-protocol treatment, including cellular immunotherapy. The trial was conducted after institutional review board approval of the protocol and in compliance with the Declaration of Helsinki, and all patients provided written, informed consent.
End points and assessments
The primary end point of the study was EFS, defined as the time from randomization to the earliest date of disease progression according to the Lugano classification,13 new lymphoma therapy, death from any cause, or a best response of stable disease (SD) up to and including the response on day 150 assessment after randomization, per blinded central review. Key secondary end points were objective response rate and OS. Additional secondary end points were PFS and incidence and severity of adverse events.
TB based on SPD (per International Working Group 2007 criteria12) was assessed by the central imaging laboratory, as previously described.5 MTV was based on attenuation-corrected, whole-body FDG PET-CT scans at screening. Although a standardized approach for quantification of MTV is lacking in the field, our procedures for MTV determination were carried out with a predefined and consistent methodology across patients, similar to that used by others.19,20,23,28 Briefly, whole-body FDG PET-CT scans were performed in ZUMA-7 patients who underwent at least a 4-hour fast before FDG administration. Patients with acceptable blood glucose values (<200 mg/dL), received an IV dose of FDG (recommended range: 370-740 MBq [10-20 mCi] with weight-based adjustments allowed) per institutional standard procedures. After a 60 (±10) minute incubation period, low-dose CT for attenuation correction was obtained (70-80 mA; 120-140 kvP) followed by PET emission scanning in 2D or 3D mode at 2 to 5 minutes per bed position. Reconstruction algorithms (iterative reconstruction, with time of flight, if available) and postacquisition filtering were performed per manufacturer’s recommendation based on local institutional practices. Images were uploaded to a centralized site to determine MTV. Whole-tumor volumes of interest were placed on individual tumors using a predefined, semiautomated approach that included semiautomated placement of outlines around regions of abnormal FDG uptake at least moderately greater than that of normal liver (visual Lugano score, >3) followed by manual adjustments of the lesion contours by a single PET radiologist per patient to ensure entire tumor lesions were included and/or nontumorous/normal tissue regions were excluded. Subsequent radiologist-defined adjustments of volumes of interest placement included adding regions of tumor not initially captured and excluding normal tissue. MTV was calculated as the number of voxels or volume picture elements, with standardized uptake value (SUV) measurements between 41% and 100% of tumor maximum SUV (SUVmax; per European Association of Nuclear Medicine guidelines29) and reported as total MTV (mL) per patient. Low and high MTV were defined as MTV less than or equal to median and greater than median, respectively. Associations between MTV and baseline characteristics and clinical outcomes were assessed. Safety analyses were limited to cytokine release syndrome (CRS) and neurologic events.
Total lesion glycolysis (TLG) was defined as the product of MTV and SUVmean for a given lesion. Total TLG is reported as the sum of all lesion TLG for a given patient. The International Metabolic Prognostic Index (IMPI) was also applied in ZUMA-7 patients with baseline data for MTV, age, and Ann Arbor stage.30 For IMPI, patients were divided into 2 groups (low and high IMPI) of the same size as the age-adjusted IPI categories (0-1 and 2-3). For this purpose, patients were ranked by their absolute IMPI, and patient numbers were matched accordingly with the number of the corresponding age-adjusted IPI categories. Associations between IMPI and EFS and PFS were evaluated to determine whether age and Ann Arbor stage would improve the predictive value of MTV.
Statistical analyses
ZUMA-7 primary efficacy and safety analyses were previously reported.5 The analyses presented here were exploratory. Analyses evaluating relationships between MTV and efficacy outcomes were based on the full analysis set, whereas analyses of MTV and safety outcomes were based on the safety set. All P values are descriptive.
Baseline MTV data were summarized by treatment arm and were pooled for the analysis of MTV by baseline characteristics: age (<65 vs ≥65 years), germinal center B-cell like vs nongerminal center B-cell like, prognostic subgroups (high-grade B-cell lymphoma vs non–high-grade B-cell lymphoma), elevated LDH vs normal LDH, primary refractory vs relapsed, CD19 positivity by immunohistochemistry (yes vs no), and CD19 H-score by immunohistochemistry (less than or equal to median vs greater than median). Two-sided P values for each categorical comparison were calculated using the Wilcoxon rank sum test. Spearman correlation estimates and P values from a Fisher z transformation were used to summarize relationships between baseline MTV and continuous baseline covariates (ie, SPD and LDH).
Kaplan-Meier estimates were provided for EFS and PFS. Estimated HRs, 95% confidence intervals (CIs), and descriptive 2-sided P values were calculated from a Cox proportional-hazards model. For these analyses, patients were categorized into either high or low MTV. The same methods were used to evaluate associations between IMPI (high vs low based on median) and time-to-event outcomes.
Exploration of alternative MTV thresholds included evaluation of EFS and PFS by baseline MTV quartiles and by best MTV threshold. The best MTV threshold was based on log-rank statistics that resulted in the greatest separation (ie, lowest P value) between the high-MTV and low-MTV curves for EFS and PFS within each arm (ie, 4 different thresholds). Additionally, an unstratified Cox regression model with the log2 of baseline MTV as a continuous variable was used to provide estimated HRs, 95% CIs, and P values for EFS and PFS in the axi-cel and standard-care arms.
Several analyses were also conducted to compare MTV with other measures of TB using the ZUMA-7 data set. Spearman correlation estimates and P values from a Fisher z transformation were used to evaluate relationships between baseline MTV and SUVmax or SPD or TLG. Similar to the Kaplan-Meier methods described for MTV, EFS and PFS were evaluated for both arms based on high vs low SUVmax, SPD, and TLG, with median as the threshold for each measure.
We used multivariate analyses to determine the potential predictive value of MTV for time-to-event outcomes after adjustment for other prognostic factors (ie, age, LDH, and second-line age-adjusted IPI). An unstratified Cox regression model with baseline MTV (high vs low according to the median or best threshold), derived second-line age-adjusted IPI (2-3 vs 0-1), baseline age group (≥65 vs <65 years), and baseline LDH status (elevated vs normal) as covariates was used to provide estimated HRs, 2-sided 95% CIs, and P values.
The Wilcoxon rank sum test was used to compare baseline MTV within treatment arms for the following patient groups: responders (CR + PR) vs nonresponders (SD + progressive disease); CR vs others (PR + SD + progressive disease); and ongoing response vs others (progression after response + nonresponder). Logistic regression was performed to assess the association between MTV and clinical outcome (eg, CR vs others) within the treatment arms. The Wilcoxon rank sum test was also used to compare MTV data for patients with neurologic events (grade ≥3 vs grades 2, 1, and none) and CRS (grade ≥3 vs grades 2, 1, and none).
The trial was conducted after institutional review board approval of the protocol at each site.
Results
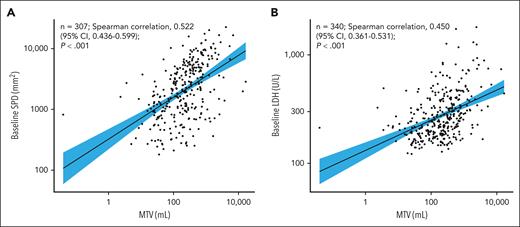
Of 359 patients randomized in the ZUMA-7 study between 25 January 2018 and 4 October 2019 (full analysis set), 340 (axi-cel, n = 175; standard care, n = 165) were evaluable for MTV (ie, had baseline and 1 postbaseline scan available). The median follow-up from randomization to the data cutoff date (18 March 2021) for all randomized patients was 24.9 months. Median MTV at baseline was 231.07 mL (range, 0.04-16 669.3) overall, and was comparable in the axi-cel and standard-care arms (Table 1). Representative images of patients with high and low MTV are shown in Figure 1. Median MTV was significantly higher in patients aged <65 years vs ≥65 years and in patients with elevated vs normal LDH. MTV was moderately correlated with both SPD (Spearman correlation, 0.522; 95% CI, 0.436-0.599; Figure 2A) and LDH (Spearman correlation, 0.450; 95% CI, 0.361-0.531; Figure 2B).
Baseline MTV by treatment arm and characteristics
| Treatment arm or characteristic . | n/N . | Median MTV (range), mL . | Descriptive P value∗ . |
|---|---|---|---|
| Treatment arm | |||
| Axi-cel | 175†/180 | 228.66 (2.3-16 669.3) | .66 |
| Standard care | 165/179 | 231.90 (0.04-2 811.2) | |
| Age, y | |||
| <65 | 235/250 | 256.57 (0.04-16 669.3) | <.01 |
| ≥65 | 105/109 | 176.71 (6.8-4 101.8) | |
| Molecular subgroup, per central laboratory | |||
| GCB | 202/208 | 229.45 (3.5-16 669.3) | .55 |
| Non-GCB | 53/56 | 242.60 (6.9-5 488.5) | |
| Disease type, per central laboratory | |||
| HGBL | 54/57 | 307.71 (8.5-6 823.5) | .31 |
| Non-HGBL | 251/256 | 228.48 (0.04-16 669.3) | |
| LDH | |||
| Elevated | 185/195 | 371.17 (2.3-16 669.3) | <.01 |
| Normal | 155/164 | 126.96 (0.04-3 712.8) | |
| Response to first-line therapy at randomization | |||
| Primary refractory | 252/265 | 236.88 (0.04-16 669.3) | .64 |
| Relapse ≤12 mo after completion of 1L therapy | 87/92 | 215.33 (3.6-5 317.7) | |
| CD19 positive on IHC staining | |||
| Yes | 270/278 | 237.92 (0.04-13 527.0) | .86 |
| No | 25/25 | 248.89 (3.6-16 669.3) | |
| CD19 H-score | |||
| Less than or equal to median‡ | 149/152 | 241.75 (0.04-16 669.3) | .79 |
| Greater than median | 146/151 | 229.72 (2.3-13 527.0) |
| Treatment arm or characteristic . | n/N . | Median MTV (range), mL . | Descriptive P value∗ . |
|---|---|---|---|
| Treatment arm | |||
| Axi-cel | 175†/180 | 228.66 (2.3-16 669.3) | .66 |
| Standard care | 165/179 | 231.90 (0.04-2 811.2) | |
| Age, y | |||
| <65 | 235/250 | 256.57 (0.04-16 669.3) | <.01 |
| ≥65 | 105/109 | 176.71 (6.8-4 101.8) | |
| Molecular subgroup, per central laboratory | |||
| GCB | 202/208 | 229.45 (3.5-16 669.3) | .55 |
| Non-GCB | 53/56 | 242.60 (6.9-5 488.5) | |
| Disease type, per central laboratory | |||
| HGBL | 54/57 | 307.71 (8.5-6 823.5) | .31 |
| Non-HGBL | 251/256 | 228.48 (0.04-16 669.3) | |
| LDH | |||
| Elevated | 185/195 | 371.17 (2.3-16 669.3) | <.01 |
| Normal | 155/164 | 126.96 (0.04-3 712.8) | |
| Response to first-line therapy at randomization | |||
| Primary refractory | 252/265 | 236.88 (0.04-16 669.3) | .64 |
| Relapse ≤12 mo after completion of 1L therapy | 87/92 | 215.33 (3.6-5 317.7) | |
| CD19 positive on IHC staining | |||
| Yes | 270/278 | 237.92 (0.04-13 527.0) | .86 |
| No | 25/25 | 248.89 (3.6-16 669.3) | |
| CD19 H-score | |||
| Less than or equal to median‡ | 149/152 | 241.75 (0.04-16 669.3) | .79 |
| Greater than median | 146/151 | 229.72 (2.3-13 527.0) |
GCB, germinal center B-cell like; HGBL, high-grade B-cell lymphoma; IHC, immunohistochemistry.
Two-sided P values for 2-group comparisons were calculated using Wilcoxon rank sum test.
The initial presentation of these analyses31 inadvertently included data from a patient who was retreated with axi-cel. Those data were removed, and only data from randomized treatment were included.
Median CD19 H-score was 150.
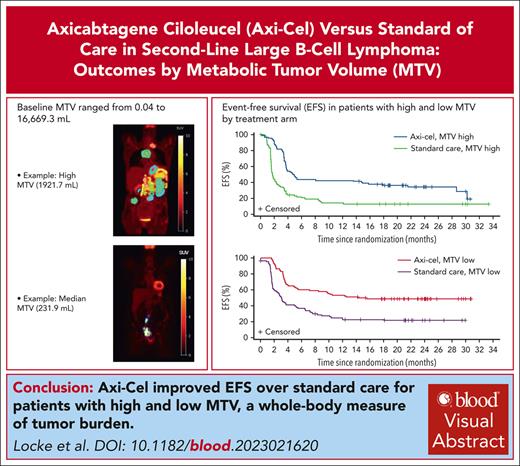
Baseline MTV estimates from whole-body FDG PET images. Single-slice coronal FDG PET images (A,E) demonstrating hypermetabolic uptake in the original image data sets. (A-D) Hypermetabolic FDG uptake in a limited number of lesions in the abdomen and pelvis with an estimated total whole-body MTV of 231.9 mL. (E-H) Hypermetabolic FDG uptake in soft-tissue and nodal lesions in the chest and abdomen with an estimated total whole-body MTV of 1921.7 mL. The segmented lesions containing the individual lesion masks (colored regions in panels B,F) are shown. Within each of these segmented lesions, masks of the FDG PET voxels with SUV values 41% to 100% of SUVmax are delineated on the parametric maps (C,G). The total whole-body MTV is then calculated from the sum of delineated voxels. The arrows represent normal physiologic activity in the bladder (A,E) and gastrointestinal tract (A). Panels D,H are maximum intensity projection images that represent the extent of disease on the whole-body FDG PET.
Baseline MTV estimates from whole-body FDG PET images. Single-slice coronal FDG PET images (A,E) demonstrating hypermetabolic uptake in the original image data sets. (A-D) Hypermetabolic FDG uptake in a limited number of lesions in the abdomen and pelvis with an estimated total whole-body MTV of 231.9 mL. (E-H) Hypermetabolic FDG uptake in soft-tissue and nodal lesions in the chest and abdomen with an estimated total whole-body MTV of 1921.7 mL. The segmented lesions containing the individual lesion masks (colored regions in panels B,F) are shown. Within each of these segmented lesions, masks of the FDG PET voxels with SUV values 41% to 100% of SUVmax are delineated on the parametric maps (C,G). The total whole-body MTV is then calculated from the sum of delineated voxels. The arrows represent normal physiologic activity in the bladder (A,E) and gastrointestinal tract (A). Panels D,H are maximum intensity projection images that represent the extent of disease on the whole-body FDG PET.
Associations between baseline MTV and known prognostic factors. Spearman correlation estimates and P values from a Fisher z transformation were used to summarize relationships between baseline MTV and continuous baseline covariates. (A) Baseline SPD vs MTV. (B) Baseline LDH vs MTV. The fit line is based on linear regression with 95% CI limit.
Associations between baseline MTV and known prognostic factors. Spearman correlation estimates and P values from a Fisher z transformation were used to summarize relationships between baseline MTV and continuous baseline covariates. (A) Baseline SPD vs MTV. (B) Baseline LDH vs MTV. The fit line is based on linear regression with 95% CI limit.
EFS in the axi-cel arm was superior to that of standard care both in patients with high MTV (HR, 0.417; 95% CI, 0.293-0.592) and patients with low MTV (HR, 0.421; 95% CI, 0.286-0.619; Figure 3A). Among axi-cel–treated patients, EFS was numerically shorter in those with high (vs low) MTV (HR, 1.448; 95% CI, 0.980-2.139). Similarly, EFS was shorter in standard care–treated patients with high (vs low) MTV (HR, 1.486; 95% CI, 1.055-2.093). PFS in the axi-cel arm was superior to that of standard care in patients with high MTV (HR, 0.523; 95% CI, 0.357-0.765) and low MTV (HR, 0.501; 95% CI, 0.324-0.773; Figure 3B). PFS was shorter in those with high (vs low) MTV in both the axi-cel (HR, 1.660; 95% CI, 1.097-2.513) and standard-care arms (HR, 1.635; 95% CI, 1.098-2.433).
Kaplan-Meier plots of survival outcomes by MTV and treatment arm. (A) EFS per central assessment. (B) PFS per investigator assessment. Estimated HRs, 95% CIs, and descriptive 2-sided P values were calculated from a Cox proportional-hazards model.
Kaplan-Meier plots of survival outcomes by MTV and treatment arm. (A) EFS per central assessment. (B) PFS per investigator assessment. Estimated HRs, 95% CIs, and descriptive 2-sided P values were calculated from a Cox proportional-hazards model.
A significant EFS benefit of axi-cel over standard care was maintained across quartiles of MTV (supplemental Figure 1, available on the Blood website). The standard-care group demonstrated more pronounced EFS worsening from MTV quartile 2 to MTV quartile 4 (supplemental Figure 1) than the axi-cel group. Best-threshold analysis confirmed that MTV was associated with EFS and PFS in both the axi-cel (supplemental Figure 2A,C) and standard-care (supplemental Figure 2B,D) arms (all P ≤ .01). Consistent with these findings, the continuous variable analysis demonstrated that 1 unit increase in MTV on a log2 scale (equivalent to doubling of MTV on a raw scale) resulted in a 10% increase in the risk for EFS events in the axi-cel arm (P = .02) and a 14% increase in the standard-care arm (P = .01). Similar trends were observed for PFS (supplemental Table 1).
Associations observed between IMPI, which combines MTV, age, and Ann Arbor stage, and both EFS and PFS (supplemental Figure 3) were similar to those observed with MTV alone and time-to-event outcomes. Baseline MTV moderately correlated with baseline SUVmax and SPD, whereas a very strong correlation was observed between MTV and TLG (supplemental Table 2). Comparison of descriptive P values suggested that MTV was a better predictor of EFS than SUVmax, SPD, or TLG in the axi-cel arm and outperformed SUVmax and TLG in the standard care arm (supplemental Table 3). MTV was a better predictor of PFS than SUVmax or SPD in both the axi-cel and standard-care arms (supplemental Table 3), whereas MTV was comparable with TLG for the prediction of PFS. Furthermore, within high and low groups defined by median SUVmax (supplemental Figure 4), SPD (supplemental Figure 5), or TLG (supplemental Figure 6), axi-cel consistently prolonged EFS and PFS over standard care.
In the multivariate analysis, when high and low MTV were based on the median, P values for EFS and PFS did not reach significance by descriptive statistics. However, comparison of P values suggested that, in both arms, MTV was more predictive of EFS than second-line age-adjusted IPI, age, and LDH when adjusted for the other 3 factors. MTV was also more predictive for PFS than the other factors in the axi-cel arm (supplemental Table 4). When multivariate analysis was repeated with high and low MTV based on the best threshold, MTV was significantly predictive of EFS and PFS after adjustment for second-line age-adjusted IPI, age, and LDH. After adjustment of each individual covariate for the remaining 3, only MTV had significant impact on EFS and PFS in the axi-cel and standard-care arms (supplemental Table 5).
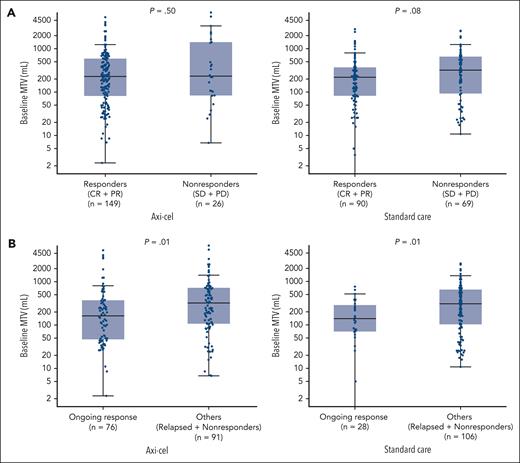
Median MTV was similar between axi-cel responders (228.66 mL; range, 2.3-16 669.3) and nonresponders (233.11 mL; range, 6.8-6823.5; Figure 4A). The difference in median MTV between standard-care responders and nonresponders also did not meet statistical significance (219.32 mL; [range, 0.04-2811.2] vs 320.34 mL [range, 10.8-2593.9]). In both the axi-cel and standard-care arms, median MTV was lower in patients who were in ongoing response at data cutoff than others (axi-cel: 163.05 mL [range, 2.3-5317.7] vs 322.82 mL [6.8-16 669.3]; standard care: 139.48 mL [range, 0.04-760.2] vs 305.98 mL [range, 10.8-2593.9]; Figure 4B). Median MTV was also lower among patients with CR than patients not in CR receiving axi-cel (199.95 mL [range, 2.3-13 527.0] vs 322.82 mL [range, 6.8-16 669.3]; P = .02) or standard care (192.86 mL [range, 0.04-1354.7] vs 296.24 mL [range, 3.6-2811.2]; P = .01; supplemental Figure 7). Consistently, logistic regression analyses demonstrated significant negative associations between CR rate and baseline MTV in the axi-cel (P = .01; supplemental Figure 8A) and standard-care (P = .02; supplemental Figure 8B) arms.
Baseline MTV by response group and treatment arm. (A) Responders vs nonresponders per central assessment. (B) Patients with ongoing response vs others per central assessment. Descriptive 2-sided P values for 2-group comparisons were calculated using Wilcoxon rank sum test. Extreme values are not shown.
Baseline MTV by response group and treatment arm. (A) Responders vs nonresponders per central assessment. (B) Patients with ongoing response vs others per central assessment. Descriptive 2-sided P values for 2-group comparisons were calculated using Wilcoxon rank sum test. Extreme values are not shown.
The safety set included 338 patients. Among axi-cel–treated patients, median MTV was higher for patients who experienced grade ≥3 neurologic events vs patients who experienced grade 1/2 or no neurologic events (320.93 mL [range, 24.3-13 527.0] vs 195.46 mL [range, 2.3-16 669.3]; Figure 5A). Median MTV was also higher for axi-cel–treated patients who experienced grade ≥3 CRS than patients who experienced grade 1/2 or no CRS (582.93 mL [range, 114.6-2508.6] vs 205.73 mL [range, 2.3-16 669.3]; Figure 5B). In the standard-care arm, no association between MTV and neurologic events was observed, and no CRS was reported.
Baseline MTV by grade of neurologic events and CRS in patients treated with axi-cel. (A) Neurologic events. (B) CRS. Descriptive 2-sided P values for 2-group comparisons were calculated using the Wilcoxon rank sum test. Extreme values are not shown.
Baseline MTV by grade of neurologic events and CRS in patients treated with axi-cel. (A) Neurologic events. (B) CRS. Descriptive 2-sided P values for 2-group comparisons were calculated using the Wilcoxon rank sum test. Extreme values are not shown.
Except for modest correlations with baseline and peak interleukin-7 levels, baseline MTV did not associate with pharmacokinetic, pharmacodynamic, or product parameters. In the axi-cel arm, neither CAR T-cell peak nor area under the curve within the first 28 days after treatment (AUC0-28) was significantly associated with ongoing response, even when adjusted for MTV (ratio between CAR T-cell peak or AUC0-28 and MTV; supplemental Figure 9), indicating that the extent of in vivo CAR T-cell expansion was not a strong limiting factor for durable responses (vs others) across the range of MTV in the ZUMA-7 study.
Discussion
This analysis is, to our knowledge, the first to evaluate MTV in a large, randomized study of patients with R/R LBCL. Similar to the ZUMA-7 primary analysis and results in subgroups defined by SPD, LDH, and other established prognostic factors,5,7 the EFS benefit of axi-cel vs standard care was maintained in both high- and low-MTV subgroups. Higher MTV was associated with shorter EFS, shorter PFS, and a reduced likelihood of ongoing response in both arms and more severe neurologic events and CRS in axi-cel–treated patients. These findings confirm previous observations of worse outcomes in patients with high vs low baseline TB for both CAR T-cell therapy and standard care. However, the strength of relationships between TB and the efficacy of CAR T-cell therapy differs across patient populations and the methods used to quantify TB.32 In the multivariate analyses of data from the ZUMA-1 study, baseline TB measured by SPD was negatively correlated with the probability of durable response in patients with R/R LBCL treated with axi-cel in third or later lines.33 Univariate analyses revealed a significant association between SPD and probability of grade ≥3 neurologic events but not grade ≥3 CRS.33 In ZUMA-7, high TB measured via SPD (greater than median) was predictive of poorer EFS in the standard-care arm (HR, 1.5; P < .02) but not in the axi-cel arm (HR, 0.95; P = .68).7 Thus, MTV is a better predictor of outcomes with second-line therapy in patients with R/R LBCL than SPD. MTV also outperformed both SUVmax and TLG with respect to the prediction of EFS in both arms of ZUMA-7.
TB defined by SPD takes into account the dimensions of only 6 target, measurable lesions on CT12 and thus is not a comprehensive reflection of total tumor load. Because CT scans lack functional information, osseous and bone marrow lesions and those located within normal-sized organs may not be detected. FDG PET is a whole-body imaging method with the sensitivity to detect metabolic changes in the involved areas before structural changes are visible.34 Thus, MTV based on FDG PET-CT is a more sensitive and accurate measure of TB than SPD and, for that reason, may be a better prognostic marker.
Standardization of MTV quantification (eg, software package, SUV threshold, and manual intervention to delimit tumors from adjacent sites of physiological uptake), application, and interpretation are needed to improve clinical utility and facilitate cross-study comparisons.11 It should be noted that the range of MTV differs between data sets, different methodologies may yield different MTV values for the same patient, and MTV may differentially associate with the outcomes of one treatment vs another. Thus, our results only apply to the ZUMA-7 data set. Establishment of an absolute threshold for delineating high vs low MTV in routine practice was not our objective and may not be feasible.
Several groups have demonstrated that the addition of other baseline factors improves the prognostic potential of MTV for survival outcomes in LBCL.35,36 Mikhaeel et al30 introduced the IMPI based on the finding that adding age and Ann Arbor stage (a measure of disease dissemination) to MTV improved the prediction of PFS and OS in patients with LBCL receiving first-line chemoimmunotherapy. In this analysis, the IMPI outperformed the conventional IPI in predicting PFS and OS.30 In patients receiving CAR T-cell therapy for later-line R/R LBCL, IMPI was a better predictive factor than IPI for PFS, but neither score significantly associated with objective response rate, duration of response, or OS.37 Although the predictive value of the IMPI (high vs low) was similar to MTV alone in ZUMA-7 patients treated with axi-cel or standard care, findings to date support MTV as a better reflection of disease burden and biology than the surrogate measures included in the IPI (ie, LDH and extranodal involvement).30,37 The results of our multivariate analyses also support the predictive value of MTV beyond that provided by other prognostic indices, including second-line age-adjusted IPI.
Unlike CT, FDG PET-CT–based methods allow for the discrimination of viable tumors from necrotic/fibrotic lesions, which is particularly beneficial during interim and posttreatment assessments.34 Although our analyses focused on the predictive value of baseline MTV for clinical outcomes in R/R LBCL, several groups have shown the value of MTV assessment at early postinfusion time points.26,38,39 For example, Hong et al39 identified high MTV 1 month after CAR T-cell infusion as a significant risk factor for poor PFS among 41 patients with R/R LBCL receiving CAR T-cell therapy in the third or later line. Future analyses of amenable data from axi-cel studies may assess the predictive potential of MTV at postinfusion time points.
This analysis is limited because it was not prespecified and therefore not statistically powered for definitive conclusions. Furthermore, alternative methods of MTV determination and alternative analyses that incorporate morphology, intensity, or special distribution of lesions and/or a different threshold for categorizing high vs low MTV might have yielded different results when applied to the ZUMA-7 data set. For example, a more time-consuming manual approach to MTV determination may provide more accuracy regarding TB.22,40
Despite the lack of a global, standardized approach to MTV quantification (which was beyond the scope of this investigation), our median, quartile, best-threshold, continuous variable, and multivariate analyses all support MTV as biologically and clinically relevant. Finally, baseline MTV was determined at screening in ZUMA-7, before optional bridging therapy with corticosteroids during axi-cel manufacturing. The impact of bridging, reported for 36% of the axi-cel arm, on relationships between MTV and clinical outcomes in ZUMA-7 patients receiving axi-cel was not determined.
In conclusion, in the first analysis of the relationship between MTV and clinical outcome in a large, randomized study of CAR T-cell therapy in R/R LBCL, axi-cel demonstrated superiority over standard care for both high- and low-MTV groups. For both arms, however, baseline MTV differentiated patients with R/R LBCL who experienced more vs less benefit from second-line therapy. MTV was also positively associated with the severity of CAR T-cell–related toxicities. Although standardization of MTV assessment is needed to facilitate broader clinical use, using high MTV to identify patients with poor prognosis has the potential to inform treatment planning and monitoring and may prompt earlier changes in therapy.
Acknowledgments
The authors thank the patients who participated in this study and their families, caregivers, and friends; the study investigators, coordinators, and health care staff at each site; Melinda Ramsey of Nexus Global Group Science, for medical writing assistance, with funding provided by Kite; and all employees of Kite involved over the course of the study for their contributions.
F.L.L. is a clinical scholar of the Leukemia and Lymphoma Society.
Authorship
Contribution: F.L.L., S.F., C. To, P.C., M.S., and R.K. designed the study; F.L.L., O.O.O., J.K., C. Thieblemont, F.M., G.S., S.P.R., S.V., J.W., R.K., and M.J.K. enrolled and treated patients and collected data; and all authors participated in analyzing and interpreting the data, writing the manuscript, and approving the final submitted version.
Conflict-of-interest disclosure: F.L.L. reports consulting/advisory role for Allogene, Amgen, bluebird bio, Bristol Myers Squibb, Celgene, Calibr, Cellular Biomedicine Group, Cowen, EcoR1 Capital, Emerging Therapy Solutions Gerson Lehman Group, GammaDelta Therapeutics, Iovance, Janssen, Kite, Legend Biotech, Novartis, Umoja Biopharma, and Wugen; research funding from Allogene, Kite, and Novartis; and patents, royalties, and other intellectual property from several patents held by the institution in their name (unlicensed) in the field of cellular immunotherapy. O.O.O. reports research funding from Kite; and consulting/advisory role for Janssen, Pfizer, Novartis, Curio Science, ADC Therapeutics, and TG Therapeutics. J.K. reports honoraria from Amgen, AstraZeneca, Bristol Myers Squibb, Celgene, Gilead, Janssen, Karyopharm, Merck, Novartis, Roche, and Seagen; consulting/advisory role for AbbVie, Bristol Myers Squibb, Gilead, Karyopharm, Merck, Roche, and Seagen; and research funding from Roche and Janssen. C. Thieblemont reports honoraria from and consulting/advisory role for AbbVie, Bristol Myers Squibb, Celgene, Incyte, Kite, Novartis, Roche, and Takeda; and travel support from Bristol Myers Squibb, Celgene, Kite, Novartis, Roche, and Takeda. F.M. reports consulting/advisory role for AbbVie, Bristol Myers Squibb, Epizyme, Genmab, Gilead Sciences, Novartis, and Roche; speakers’ bureau participation for Roche; and expert testimony for Roche and Genentech. G.S. reports honoraria from AbbVie, Amgen, Bayer, Epizyme, Regeneron, Roche, MorphoSys, Kite, and Novartis; consultancy/advisory role for Bristol Myers Squibb, Celgene, Incyte, Ipsen, Janssen, Kite, Loxo, Miltenyi Biotec, MorphoSys, Novartis, and Rapt; participation on a data safety monitoring board or advisory board for AbbVie, BeiGene, Bristol Myers Squibb, Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Kite, Miltenyi Biotec, MorphoSys, Takeda, and VelosBio. S.P.R. reports employment with, stock, or other ownership in and patents, royalties, other intellectual property from Precision Molecular and PlenaryAI; honoraria from, speakers’ bureau participation for, and travel support from Lantheus Pharmaceuticals; and consultancy/advisory role for and research funding from Precision Molecular, Lantheus Pharmaceuticals, and PlenaryAI. S.V. reports employment with and research funding from Kite; and stock or other ownership in Gilead Sciences. J.W. reports employment with and research funding from Kite; and stock or other ownership in Gilead. S.F. reports employment and stock or ownership with Kite; and patents, royalties, and other intellectual property from Tusk Therapeutics. C. To reports employment with Kite; and stock or other ownership in Gilead Sciences. P.C. reports employment with Kite; stock or other ownership in Gilead Sciences; and travel support from Kite. M.S. reports employment with, honoraria from, travel support from, and other relationships with Kite; and stock or other ownership in Gilead Sciences. R.K. reports employment with Imaging Endpoints; stock or other ownership in Teladoc, Teleview, Globavir, Verve, and Renibus; consulting or advisory role for Dynamicure, Fore Biotherapeutics, SonALAsense, Dracen, Day One, and FibroGen; and patents, royalties, and other intellectual property with Imaging Endpoints. M.J.K. reports honoraria from and consulting/advisory role for Bristol Myers Squibb, Celgene, Kite, Miltenyi Biotech, Novartis, and Roche; research funding from Kite, Roche, Takeda, and Celgene; and travel support from Kite, Miltenyi Biotech, Novartis, and Roche.
Correspondence: Frederick L. Locke, Moffitt Cancer Center, 12902 USF Magnolia Dr, Tampa, FL 33612; email: frederick.locke@moffitt.org.
References
Author notes
Kite is committed to sharing clinical trial data with external medical experts and scientific researchers in the interest of advancing public health, and access can be requested by contacting medinfo@kitepharma.com.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal