Visual Abstract
The DNA damage response (DDR) encompasses the detection and repair of DNA lesions and is fundamental to the maintenance of genome integrity. Germ line DDR alterations underlie hereditary chromosome instability syndromes by promoting the acquisition of pathogenic structural variants in hematopoietic cells, resulting in increased predisposition to hematologic malignancies. Also frequent in hematologic malignancies are somatic mutations of DDR genes, typically arising from replication stress triggered by oncogene activation or deregulated tumor proliferation that provides a selective pressure for DDR loss. These defects impair homology–directed DNA repair or replication stress response, leading to an excessive reliance on error-prone DNA repair mechanisms that results in genomic instability and tumor progression. In hematologic malignancies, loss-of-function DDR alterations confer clonal growth advantage and adverse prognostic impact but may also provide therapeutic opportunities. Selective targeting of functional dependencies arising from these defects could achieve synthetic lethality, a therapeutic concept exemplified by inhibition of poly-(adenosine 5′-diphosphate ribose) polymerase or the ataxia telangiectasia and Rad 3 related-CHK1-WEE1 axis in malignancies harboring the BRCAness phenotype or genetic defects that increase replication stress. Furthermore, the role of DDR defects as a source of tumor immunogenicity, as well as their impact on the cross talk between DDR, inflammation, and tumor immunity are increasingly recognized, thus providing rationale for combining DDR modulation with immune modulation. The nature of the DDR–immune interface and the cellular vulnerabilities conferred by DDR defects may nonetheless be disease-specific and remain incompletely understood in many hematologic malignancies. Their comprehensive elucidation will be critical for optimizing therapeutic strategies to target DDR defects in these diseases.
Introduction
The integrity of the human genome is continuously challenged by DNA damage. To survive, human cells rely on the DNA damage response (DDR) that recognize and repair damaged DNA. DNA damage signals are propagated through 2 signaling pathways, the ataxia telangiectasia mutated (ATM)-Chk2 pathway that responds to DNA double-strand breaks (DSBs), and the ataxia telangiectasia and Rad 3 related (ATR)-Chk1 pathway that responds primarily to replication stress. Two possible outcomes ensue, either repair and resolution of the incipient DNA damage, or induction of p53-mediated apoptosis in the case of irreparable damage. As the most deleterious of DNA lesions, DSBs are repaired through nonhomologous end-joining (NHEJ) and homologous recombination repair (HRR). The removal of replication obstacles necessitates other repair mechanisms such as nucleotide excision repair (NER) and ribonucleotide excision repair in addition to DSB repair.
As critical tumor suppressors, DDR signaling and DNA repair mechanisms are frequently inactivated in hematologic malignancies, promoting tumor progression, and conferring an adverse prognostic impact. Although therapeutic vulnerabilities resulting from DDR defects present opportunities for precision medicine, this has yet to be realized in hematologic malignancies. In this review, we present an account of the DDR defects that frequently occur in hematologic malignancies, focusing on how these defects contribute to tumorigenesis, how they interact with the immune microenvironment, and how they can be exploited as therapeutic targets. We discuss recent advances and identify key unresolved questions as opportunities for future research.
The spectrum of DDR defects in hematologic malignancies
The connection between defective DDR and hematologic malignancies was first established through the characterization of germ line DDR alterations that underlie constitutional syndromes with an inherited predisposition to hematologic malignancies (Figure 1). However, more frequent in hematologic malignancies are acquired DDR defects arising from somatic mutations and copy number alterations.
Major developments in our understanding of DDR in hematologic malignancies. Key milestones are shown encompassing the discovery of chromosome instability syndromes and functions of DDR pathway genes, the identification of DDR defects as cancer drivers and the development of therapies to target these defects.
Major developments in our understanding of DDR in hematologic malignancies. Key milestones are shown encompassing the discovery of chromosome instability syndromes and functions of DDR pathway genes, the identification of DDR defects as cancer drivers and the development of therapies to target these defects.
Inherited DDR defects predisposing to hematologic malignancies
Chromosomal instability syndromes are predominantly autosomal recessive disorders resulting in defects within proteins important for HRR (Table 1; Figure 2).1 In Fanconi anemia (FA), FANC mutations undermine the HRR of DNA interstrand crosslinks arising from endogenous metabolites such as reactive aldehydes.2 Unresolved interstrand crosslinks cause replication stress and p53-dependent apoptosis that results in bone marrow failure.3,4 In patients with FA, the crucial tumor-suppressive role of p53 was recently highlighted by the discovery of structural variants arising and evolving in myeloid progenitor cells in the context of enforced MDM4 activity that represses p53,5,6 thus underlying their predisposition to clonal hematopoiesis, myelodysplastic syndrome (MDS), and acute myeloid leukemia (AML).
Chromosomal instability syndromes with inherited predisposition to hematologic malignancies
| . | Fanconi anemia . | Nijmegen breakage syndrome . | Ataxia telangiectasia . | Bloom syndrome . |
|---|---|---|---|---|
| Causative mutation | FANC genes, most commonly FANCA, FANCC, and FANCG | NBN | ATM | BLM |
| Gene function | Mediates DNA interstrand cross link repair that involves cross link detection, nucleolytic processing, translesion synthesis, HRR, and DNA duplex restoration | Recognizes DSBs through its involvement in the MRN complex, thereby activating ATM | Phosphorylates proteins to regulate DDR pathways that mediate repair of DSBs through HRR and/or NHEJ | Mediates convergent branch migration and resolution of Holliday junctions, a 4-stranded intermediate DNA structure, during homologous recombination repair of DSBs |
| Participates in DNA end resection during DNA repair | ||||
| DDR defect | Interstrand crosslink repair | DSB detection and sensing | Cellular response to DSBs | HRR |
| Cell-intrinsic effects | Chromosomal breakage and accumulation of structural variants due to unrepaired ICLs from endogenous DNA damage (eg, reactive aldehydes) Hypersensitivity to ICL-inducing agents | Chromosomal translocations and genetic instability arising from failure to repair DSBs Hypersensitivity to ionizing radiation | Chromosomal translocations and genetic instability arising from failure to repair DSBs Hypersensitivity to ionizing radiation | Defective DNA end resection and the dissolution of Holliday junctions Impaired replication fork stability and increased risk of sister-chromatid exchanges, chromosomal breakage, and rearrangements |
| Hematologic features | Bone marrow failure (90%), pancytopenia, aplastic anemia, and predisposition to MDS/AML (33% prevalence by the age of 50 y) | Predisposition to lymphoid malignancies (40% prevalence by the age of 20 y), of which 45% are B-cell lymphomas, and 55% are T-cell lymphomas | Predisposition to B and T-cell lymphoid malignancies particularly in childhood; B-NHL represents 37% of all tumors observed in patients with AT | Predisposition to leukemia (AML and ALL) and lymphoma (median age of onset, 20 y) |
| Pathogenic mechanism | Damaged HSCs are genetically unstable, and undergo senescence and p53-dependent apoptosis | Unrepaired DSBs promote meiotic and somatic recombination in cells of the adaptive immune system | Unrepaired DSBs promote meiotic and somatic recombination in cells of the adaptive immune system | Genome instability in hematopoietic cells caused by defective maintenance and dissolution of double Holliday junctions |
| Prevalence | 5 per million | Very rare worldwide but higher prevalence in central and eastern Europe | 3 per million | 2 per million |
| Inheritance | Autosomal recessive (98%) | Autosomal recessive | Autosomal recessive | Autosomal recessive |
| X-linked recessive (2%) | ||||
| Recent mechanistic and therapeutic discoveries | FA genomic signature involves structural variants enriched for deletions, duplications, and unbalanced translocations, such as trisomy MDM4 that downregulates p53, promoting clonal hematopoiesis, and leukemogenesis5,6 EXO1 limits replication stress and DNA damage in FA to counteract formaldehyde-induced ICLs and genomic instability7 | MRN complex prevents genomic instability by regulating resolution of R-loops8 MRN dimers can further dimerize during response to DNA DSBs to facilitate both catalytic and tethering functions of MRN complexes9 | ATM plays a role in the regulation of centrosome clustering and resolution of R-loops10,11 New strategies for reestablishing ATM function in vivo include splice-switching as well as suppression of nonsense mutations using tRNAs with altered anticodons12,13 | BLM helicase mediates DNA end processing through formation of large single-stranded DNA loops14 BLM syndrome protein complex functions as a hetero-tetramer that involves BLM, Topo IIIα, RMI1, and RMI2. Within that complex, truncated BLM allele can exert a dominant-negative effect15 |
| . | Fanconi anemia . | Nijmegen breakage syndrome . | Ataxia telangiectasia . | Bloom syndrome . |
|---|---|---|---|---|
| Causative mutation | FANC genes, most commonly FANCA, FANCC, and FANCG | NBN | ATM | BLM |
| Gene function | Mediates DNA interstrand cross link repair that involves cross link detection, nucleolytic processing, translesion synthesis, HRR, and DNA duplex restoration | Recognizes DSBs through its involvement in the MRN complex, thereby activating ATM | Phosphorylates proteins to regulate DDR pathways that mediate repair of DSBs through HRR and/or NHEJ | Mediates convergent branch migration and resolution of Holliday junctions, a 4-stranded intermediate DNA structure, during homologous recombination repair of DSBs |
| Participates in DNA end resection during DNA repair | ||||
| DDR defect | Interstrand crosslink repair | DSB detection and sensing | Cellular response to DSBs | HRR |
| Cell-intrinsic effects | Chromosomal breakage and accumulation of structural variants due to unrepaired ICLs from endogenous DNA damage (eg, reactive aldehydes) Hypersensitivity to ICL-inducing agents | Chromosomal translocations and genetic instability arising from failure to repair DSBs Hypersensitivity to ionizing radiation | Chromosomal translocations and genetic instability arising from failure to repair DSBs Hypersensitivity to ionizing radiation | Defective DNA end resection and the dissolution of Holliday junctions Impaired replication fork stability and increased risk of sister-chromatid exchanges, chromosomal breakage, and rearrangements |
| Hematologic features | Bone marrow failure (90%), pancytopenia, aplastic anemia, and predisposition to MDS/AML (33% prevalence by the age of 50 y) | Predisposition to lymphoid malignancies (40% prevalence by the age of 20 y), of which 45% are B-cell lymphomas, and 55% are T-cell lymphomas | Predisposition to B and T-cell lymphoid malignancies particularly in childhood; B-NHL represents 37% of all tumors observed in patients with AT | Predisposition to leukemia (AML and ALL) and lymphoma (median age of onset, 20 y) |
| Pathogenic mechanism | Damaged HSCs are genetically unstable, and undergo senescence and p53-dependent apoptosis | Unrepaired DSBs promote meiotic and somatic recombination in cells of the adaptive immune system | Unrepaired DSBs promote meiotic and somatic recombination in cells of the adaptive immune system | Genome instability in hematopoietic cells caused by defective maintenance and dissolution of double Holliday junctions |
| Prevalence | 5 per million | Very rare worldwide but higher prevalence in central and eastern Europe | 3 per million | 2 per million |
| Inheritance | Autosomal recessive (98%) | Autosomal recessive | Autosomal recessive | Autosomal recessive |
| X-linked recessive (2%) | ||||
| Recent mechanistic and therapeutic discoveries | FA genomic signature involves structural variants enriched for deletions, duplications, and unbalanced translocations, such as trisomy MDM4 that downregulates p53, promoting clonal hematopoiesis, and leukemogenesis5,6 EXO1 limits replication stress and DNA damage in FA to counteract formaldehyde-induced ICLs and genomic instability7 | MRN complex prevents genomic instability by regulating resolution of R-loops8 MRN dimers can further dimerize during response to DNA DSBs to facilitate both catalytic and tethering functions of MRN complexes9 | ATM plays a role in the regulation of centrosome clustering and resolution of R-loops10,11 New strategies for reestablishing ATM function in vivo include splice-switching as well as suppression of nonsense mutations using tRNAs with altered anticodons12,13 | BLM helicase mediates DNA end processing through formation of large single-stranded DNA loops14 BLM syndrome protein complex functions as a hetero-tetramer that involves BLM, Topo IIIα, RMI1, and RMI2. Within that complex, truncated BLM allele can exert a dominant-negative effect15 |
ALL, acute lymphoblastic leukemia; BLM, Bloom; B-NHL, B-cell non-Hodgkin lymphoma; ICLs, interstrand crosslinks; MRN, MRE11-RAD50-NBS1; RMI1, RecQ–mediated genome instability 1; RMI2, RecQ–mediated genome instability 2; Topo IIIα, DNA topoisomerase-3α; tRNA, transfer RNA.
DDR defects in hematologic malignancies. The core FA complex and its downstream effectors are involved in the recognition and resolution of interstrand crosslinks (ICLs). ATM phosphorylates Chk1 and p53 as a prerequisite for induction of apoptosis in the presence of DNA damage. NBN regulates ATM–dependent DNA damage signaling and DNA DSB end resection, whereas BLM mediates replication fork stability. SAMHD1 participates in the processing of stalled replication forks that require HRR for their resolution. The spliceosome component SF3B1 regulates the level of BRCA1, a scaffold protein that facilitates the assembly of HRR effectors, whereas the cohesin complex (STAG2, RAD21, SMC1, and SMC3) regulates HRR by holding sister chromatids in proximity to facilitate strand invasion. MSH2, MSH6, PMS2, and MLH1 are components of MMR. RNase H2 has a role in RER involving the resolution of ribonucleotides erroneously embedded during DNA replication. Proteins acting upstream of HRR are presented in colored boxes if altered in hematologic malignancies, or white boxes if not.
DDR defects in hematologic malignancies. The core FA complex and its downstream effectors are involved in the recognition and resolution of interstrand crosslinks (ICLs). ATM phosphorylates Chk1 and p53 as a prerequisite for induction of apoptosis in the presence of DNA damage. NBN regulates ATM–dependent DNA damage signaling and DNA DSB end resection, whereas BLM mediates replication fork stability. SAMHD1 participates in the processing of stalled replication forks that require HRR for their resolution. The spliceosome component SF3B1 regulates the level of BRCA1, a scaffold protein that facilitates the assembly of HRR effectors, whereas the cohesin complex (STAG2, RAD21, SMC1, and SMC3) regulates HRR by holding sister chromatids in proximity to facilitate strand invasion. MSH2, MSH6, PMS2, and MLH1 are components of MMR. RNase H2 has a role in RER involving the resolution of ribonucleotides erroneously embedded during DNA replication. Proteins acting upstream of HRR are presented in colored boxes if altered in hematologic malignancies, or white boxes if not.
In contrast, patients with Nijmegen breakage syndrome or ataxia telangiectasia (AT) exhibit impaired response to DSBs attributed respectively to mutations in nibrin, a component of the MRN (MRE11-RAD50-NBS1) complex critical for DSB detection, or ATM that coordinates DSB response, resulting in an increased susceptibility to lymphoid malignancies secondary to immune system gene rearrangements. Heightened risk for both myeloid and lymphoid malignancies is characteristic of Bloom syndrome due to pathognomonic mutations in BLM, a component of HRR essential for the maintenance of replication fork stability. Recent mechanistic and therapeutic discoveries into these chromosomal instability syndromes are highlighted in Table 1.5-15 Additionally, as detailed in Table 2, germ line variants of DDR genes such as ATM, CHEK2, and mismatch repair genes are known risk alleles for sporadic hematologic cancers.16-22
Potentially important germ line variants in DDR genes across hematologic malignancies
| Gene . | Disease . | Variant . | Frequency (%) . | Study . | Strength of association . | Cohort size . | Cellular and clinical consequences . |
|---|---|---|---|---|---|---|---|
| ATM | CLL | F858L | 1.4 | Rudd et al, 200616 | OR, 2.28; P < .0001 | 992 pts; 2 707 healthy controls | Attenuated p53 response to DNA damage; selective pressure for the loss of the second ATM allele through 11q deletion; earlier disease onset. |
| P1054R | 2.8 | OR, 1.68; P = .0006 | |||||
| L2307F | 2.3 | Tiao et al, 201717 | OR, 10.1; P < .05 | 516 pts; 8 920 healthy controls | |||
| 2.8 | Lampson et al, 202318 | 2.8% vs 0% (CLL vs healthy); P = .1 | 825 pts; 143 healthy controls | ||||
| CHEK2 | CLL | I157T | 1 | Rudd et al, 200616 | OR, 14.83; P = .0008 | 992 pts; 2 707 healthy controls | Loss of G1/S cell cycle checkpoint; earlier acquisition of JAK2 V617F mutation and younger age of onset of ET. |
| ET | IVS2+1G>A | 2.8 | Janiszewska et al, 201219 | OR, 5.8; P = .02 | 106 pts; 200 healthy controls | ||
| I157T | 9.4 | OR, 2.8; P = .04 | |||||
| del5395 | 1.9 | OR, 3.8; P = .09 | |||||
| AML | c.1229delC | 2.0∗ | Yang et al, 202220 | OR, 1.21; P = .79 | 391 pts | In silico protein modeling of CHEK2 sequence variants predicts a deleterious impact on protein function. | |
| I200T | OR, 0.51; P = .3 | ||||||
| S471F | Not reported | ||||||
| R188W | |||||||
| H186R | |||||||
| R160G | |||||||
| T410M | |||||||
| BRCA2 | CLL | N372H | 2.9 | Rudd et al, 200616 | OR, 1.45; P = .0032 | 992 pts; 2 707 healthy controls | |
| MLH3 | DLBCL | C40Y | 5 | de Miranda et al, 201321,22 | 5% vs 0.002% (DLBCL vs healthy); P < .01 | 22 pts; 60 000 healthy controls | Association with MSI and genomic instability; loss of high-fidelity postreplicative DNA damage repair and protection from off-target effects of AID. |
| I988M | 5 | 5% vs 0.002% (DLBCL vs healthy); P < .01 | |||||
| L111F | 5 | 5% vs 0.0008% (DLBCL vs healthy); P < .01 | |||||
| MSH3 | DLBCL | P657S | 5 | de Miranda et al, 201321,22 | 5% vs 0.003% (DLBCL vs healthy); P < .01 | 22 pts; 60 000 healthy controls | |
| R1061G | 9 | 9% vs 0.02% (DLBCL vs healthy); P < .01 |
| Gene . | Disease . | Variant . | Frequency (%) . | Study . | Strength of association . | Cohort size . | Cellular and clinical consequences . |
|---|---|---|---|---|---|---|---|
| ATM | CLL | F858L | 1.4 | Rudd et al, 200616 | OR, 2.28; P < .0001 | 992 pts; 2 707 healthy controls | Attenuated p53 response to DNA damage; selective pressure for the loss of the second ATM allele through 11q deletion; earlier disease onset. |
| P1054R | 2.8 | OR, 1.68; P = .0006 | |||||
| L2307F | 2.3 | Tiao et al, 201717 | OR, 10.1; P < .05 | 516 pts; 8 920 healthy controls | |||
| 2.8 | Lampson et al, 202318 | 2.8% vs 0% (CLL vs healthy); P = .1 | 825 pts; 143 healthy controls | ||||
| CHEK2 | CLL | I157T | 1 | Rudd et al, 200616 | OR, 14.83; P = .0008 | 992 pts; 2 707 healthy controls | Loss of G1/S cell cycle checkpoint; earlier acquisition of JAK2 V617F mutation and younger age of onset of ET. |
| ET | IVS2+1G>A | 2.8 | Janiszewska et al, 201219 | OR, 5.8; P = .02 | 106 pts; 200 healthy controls | ||
| I157T | 9.4 | OR, 2.8; P = .04 | |||||
| del5395 | 1.9 | OR, 3.8; P = .09 | |||||
| AML | c.1229delC | 2.0∗ | Yang et al, 202220 | OR, 1.21; P = .79 | 391 pts | In silico protein modeling of CHEK2 sequence variants predicts a deleterious impact on protein function. | |
| I200T | OR, 0.51; P = .3 | ||||||
| S471F | Not reported | ||||||
| R188W | |||||||
| H186R | |||||||
| R160G | |||||||
| T410M | |||||||
| BRCA2 | CLL | N372H | 2.9 | Rudd et al, 200616 | OR, 1.45; P = .0032 | 992 pts; 2 707 healthy controls | |
| MLH3 | DLBCL | C40Y | 5 | de Miranda et al, 201321,22 | 5% vs 0.002% (DLBCL vs healthy); P < .01 | 22 pts; 60 000 healthy controls | Association with MSI and genomic instability; loss of high-fidelity postreplicative DNA damage repair and protection from off-target effects of AID. |
| I988M | 5 | 5% vs 0.002% (DLBCL vs healthy); P < .01 | |||||
| L111F | 5 | 5% vs 0.0008% (DLBCL vs healthy); P < .01 | |||||
| MSH3 | DLBCL | P657S | 5 | de Miranda et al, 201321,22 | 5% vs 0.003% (DLBCL vs healthy); P < .01 | 22 pts; 60 000 healthy controls | |
| R1061G | 9 | 9% vs 0.02% (DLBCL vs healthy); P < .01 |
AID, activation-induced cytidine deaminase; ET, essential thrombocythemia; MSI, microsatellite instability; OR, odds ratio; pts, patients.
Frequency shown represents that of all variants combined.
Acquired DDR defects in hematologic malignancies
Recent large-scale genomic studies in major hematologic tumor types have uncovered putative drivers involving somatic alterations in DDR genes. Among significantly mutated or deleted genes are those essential for DDR signaling (ATM, ATR, CHEK2, and TP53), DSB repair (BRCA1, PALB2, DYRK1A, and BRCC3; and the cohesin subunits STAG2, SMC1A, SMC3, and STAG2), replication stress response (SAMHD1 and RNASEH2B), and chromatin remodeling (ARID1A). The frequency of these genomic lesions in different tumor types, as well as their specific DDR function and pathogenic mechanism, are summarized in Table 3.23-43
Acquired DDR alterations in hematologic malignancies and their functional consequences
| Gene . | Malignancy . | Frequency . | Driver . | Gene function . | Validated or putative pathogenic mechanism . |
|---|---|---|---|---|---|
| ATM | CLL | 11% (mut); 13% (del)23 | Validated | Master regulator of DDR that recognizes DSBs and coordinates cellular response, involving cell cycle arrest, DSB repair, and/or apoptosis. | Atm deletion cooperates with mutant Sf3b1 to induce CLL.37 Loss of Atm leads to high-risk CLL in Eμ:TCL1 mice.38 ATM deficiency promotes the development of DLBCL in T-cell deficient mice.39 |
| MCL | 34% (mut); 29% (del)24 | ||||
| T-PLL | 54% (mut); 65% (del)25 | ||||
| DLBCL | 7.2% (mut or del)26 | ||||
| SMZL | 7.8% (mut)27 | ||||
| MM | 4.7% (mut or del)28 | ||||
| CHEK2 | T-PLL | 4% (mut)25 | Putative | Induces G1 cell cycle arrest and prevents entry into mitosis in response to DNA DSBs. | |
| SMZL | 1.1% (mut)27 | ||||
| ATR | MM | 1% (mut)28 | Putative | Master regulator of DDR that recognizes SSBs and replication stress, leading to cell cycle arrest and stabilization of replication forks. | ATR downregulation supports early tumor development in Myc–driven lymphoma models but can be tumor-suppressive at later stages.40 Consequences of ATR mutations have yet to be confirmed in MM. |
| TP53 | AML | 6% (mut); 5% (del)29 | Validated | In response to DNA DSBs activates the G2 cell cycle checkpoint. Activates apoptosis in the presence of excess DNA DSBs. | CRISPR drop-out screen identifies p53 as a tumor suppressor in DLBCL.26Trp53 loss cooperates with 5q and Flt3 haploinsufficiency to induce AML in mice.41 Loss of Trp53 leads to high-risk CLL in Eμ:TCL1 mice.38 |
| CML BC | 7.5% (mut), 13% (del)30 | ||||
| ALL | 2.9% (mut)31 | ||||
| CLL | 9.1% (mut); 6.7% (del)23 | ||||
| MCL | 31% (mut); 34% (del)24 | ||||
| DLBCL | 10% (mut or del)26 | ||||
| SMZL | 11% (mut)27 | ||||
| FL | 12% (mut)32 | ||||
| BRCA1 | DLBCL | 3.1% (mut)33 | Putative | Facilitates assembly of HRR proteins | BRAC1 and PALB2 mutations may induce BRCAness and genomic instability (putative). |
| PALB2 | AML | 5.2% (del)34 | Putative | Acts as a hub to bring together BRCA1, BRCA2, and RAD51, thereby facilitating HRR. | BRAC1 and PALB2 mutations may induce BRCAness and genomic instability (putative). |
| RAD21 | AML | 4.2% (mut)29 | Putative | Cohesin complex. Redistributes unfired replication origins along the chromatin loops and prevents early initiation of dormant origins. Regulates sister-chromatid cohesion, homologous recombination, and DNA looping important for organization of the genome. | Cohesin complex acts as a growth regulator of HSCs. Cohesin mutations perturb the balance between HSC self-renewal and differentiation.42 |
| SMC1A | 2.7% (mut)35 | ||||
| SMC3 | 2.9% (mut)35 | ||||
| STAG2 | 2.9% (mut)29 | ||||
| DYRK1A | CLL | 1% (mut)23 | Putative | Inhibits accumulation of 53BP1 at DSB sites, thereby promoting HRR in preference to NHEJ. | DYRK1A mutations may lead to hyperactive HRR and chromosome dysfunction (putative). |
| BRCC3 | CLL | 1.5% (mut)23 | Putative | Sequesters BRCA1 away from DSBs and promotes NHEJ in preference to HRR. | BRCC3 mutations may lead to hyperactive NHEJ and genomic instability (putative). |
| SMZL | 0.7% (mut)27 | ||||
| SAMHD1 | CLL | 1.4% (mut)23 | Putative | Regulates cellular nucleoside pools and mediates the processing of stalled replication forks. | SAMHD1 mutations may exacerbate replication stress and genomic instability (putative). |
| MCL | 4% (mut)24 | ||||
| SMZL | 1.9% (mut)27 | ||||
| RNASEH2B | CLL | 34% (monoallelic del);36 | Putative | Mediates RER and resolution of R-loops. | In the absence of functional RNase H2, ribonucleotides are removed through an error-prone, TOP1-mediated microhomology-directed repair process that results in 2-5 bp deletions and genomic instability.43 |
| 14% (biallelic del)36 | |||||
| ARID1A | ALL | 1.1% (mut)31 | Putative | Component of the chromatin remodeling complex SWI/SNF. Regulates G2/M cell cycle checkpoint, promotes DSB resection, and activates ATR. | ARID1A mutations may promote tumor initiation through deregulating gene expression and developmental programs (putative). |
| Gene . | Malignancy . | Frequency . | Driver . | Gene function . | Validated or putative pathogenic mechanism . |
|---|---|---|---|---|---|
| ATM | CLL | 11% (mut); 13% (del)23 | Validated | Master regulator of DDR that recognizes DSBs and coordinates cellular response, involving cell cycle arrest, DSB repair, and/or apoptosis. | Atm deletion cooperates with mutant Sf3b1 to induce CLL.37 Loss of Atm leads to high-risk CLL in Eμ:TCL1 mice.38 ATM deficiency promotes the development of DLBCL in T-cell deficient mice.39 |
| MCL | 34% (mut); 29% (del)24 | ||||
| T-PLL | 54% (mut); 65% (del)25 | ||||
| DLBCL | 7.2% (mut or del)26 | ||||
| SMZL | 7.8% (mut)27 | ||||
| MM | 4.7% (mut or del)28 | ||||
| CHEK2 | T-PLL | 4% (mut)25 | Putative | Induces G1 cell cycle arrest and prevents entry into mitosis in response to DNA DSBs. | |
| SMZL | 1.1% (mut)27 | ||||
| ATR | MM | 1% (mut)28 | Putative | Master regulator of DDR that recognizes SSBs and replication stress, leading to cell cycle arrest and stabilization of replication forks. | ATR downregulation supports early tumor development in Myc–driven lymphoma models but can be tumor-suppressive at later stages.40 Consequences of ATR mutations have yet to be confirmed in MM. |
| TP53 | AML | 6% (mut); 5% (del)29 | Validated | In response to DNA DSBs activates the G2 cell cycle checkpoint. Activates apoptosis in the presence of excess DNA DSBs. | CRISPR drop-out screen identifies p53 as a tumor suppressor in DLBCL.26Trp53 loss cooperates with 5q and Flt3 haploinsufficiency to induce AML in mice.41 Loss of Trp53 leads to high-risk CLL in Eμ:TCL1 mice.38 |
| CML BC | 7.5% (mut), 13% (del)30 | ||||
| ALL | 2.9% (mut)31 | ||||
| CLL | 9.1% (mut); 6.7% (del)23 | ||||
| MCL | 31% (mut); 34% (del)24 | ||||
| DLBCL | 10% (mut or del)26 | ||||
| SMZL | 11% (mut)27 | ||||
| FL | 12% (mut)32 | ||||
| BRCA1 | DLBCL | 3.1% (mut)33 | Putative | Facilitates assembly of HRR proteins | BRAC1 and PALB2 mutations may induce BRCAness and genomic instability (putative). |
| PALB2 | AML | 5.2% (del)34 | Putative | Acts as a hub to bring together BRCA1, BRCA2, and RAD51, thereby facilitating HRR. | BRAC1 and PALB2 mutations may induce BRCAness and genomic instability (putative). |
| RAD21 | AML | 4.2% (mut)29 | Putative | Cohesin complex. Redistributes unfired replication origins along the chromatin loops and prevents early initiation of dormant origins. Regulates sister-chromatid cohesion, homologous recombination, and DNA looping important for organization of the genome. | Cohesin complex acts as a growth regulator of HSCs. Cohesin mutations perturb the balance between HSC self-renewal and differentiation.42 |
| SMC1A | 2.7% (mut)35 | ||||
| SMC3 | 2.9% (mut)35 | ||||
| STAG2 | 2.9% (mut)29 | ||||
| DYRK1A | CLL | 1% (mut)23 | Putative | Inhibits accumulation of 53BP1 at DSB sites, thereby promoting HRR in preference to NHEJ. | DYRK1A mutations may lead to hyperactive HRR and chromosome dysfunction (putative). |
| BRCC3 | CLL | 1.5% (mut)23 | Putative | Sequesters BRCA1 away from DSBs and promotes NHEJ in preference to HRR. | BRCC3 mutations may lead to hyperactive NHEJ and genomic instability (putative). |
| SMZL | 0.7% (mut)27 | ||||
| SAMHD1 | CLL | 1.4% (mut)23 | Putative | Regulates cellular nucleoside pools and mediates the processing of stalled replication forks. | SAMHD1 mutations may exacerbate replication stress and genomic instability (putative). |
| MCL | 4% (mut)24 | ||||
| SMZL | 1.9% (mut)27 | ||||
| RNASEH2B | CLL | 34% (monoallelic del);36 | Putative | Mediates RER and resolution of R-loops. | In the absence of functional RNase H2, ribonucleotides are removed through an error-prone, TOP1-mediated microhomology-directed repair process that results in 2-5 bp deletions and genomic instability.43 |
| 14% (biallelic del)36 | |||||
| ARID1A | ALL | 1.1% (mut)31 | Putative | Component of the chromatin remodeling complex SWI/SNF. Regulates G2/M cell cycle checkpoint, promotes DSB resection, and activates ATR. | ARID1A mutations may promote tumor initiation through deregulating gene expression and developmental programs (putative). |
ALL, acute lymphoblastic leukemia; BC, blast crisis; bp, base pair; CML, chronic myeloid leukemia; del, deletion; FL, follicular lymphoma; MCL, mantle cell lymphoma; MM, multiple myeloma; mut, mutation; RER, ribonucleotide excision repair; SMZL, splenic marginal zone lymphoma; SSB, single-strand break; T-PLL, T-cell prolymphocytic leukemia.
Functional validation of DDR-associated genetic drivers in hematologic tumor models have yielded insight into their pathogenic mechanisms. For instance, targeted disruption of Atm in T-cell proficient mice resulted in thymic lymphomas, but similar Atm knockout in T-cell deficient mice produced diffuse large B-cell lymphoma (DLBCL)–like tumors,39 akin to that observed in patients with AT, suggesting a critical role of tumor immunosurveillance. In chronic lymphocytic leukemia (CLL), in which ATM and SF3B1 alterations commonly co-occur, conditional expression of the Sf3b1-K700E hot spot mutation in murine B cells resulted in proleukemogenic properties but simultaneously led to DDR-mediated cellular senescence, which was overcome when combined with Atm deletion,37 underlining the crucial tumor suppressor role of ATM. The role of DDR defects as cancer drivers is further substantiated by their adverse prognostic impact in many hematologic malignancies (detailed in Table 4).23,24,26,28-30,34,35,44-54 For example, in a multivariate CLL prognostic model that integrated genomic, transcriptional, epigenetic, and clinical parameters, TP53 deletion and DYRK1A mutation emerged as significant determinants of shorter time to first treatment (hazard ratio [HR], 1.98) and overall survival (HR, 2.79), respectively.23
Clinical impact of acquired alterations of DDR genes in hematologic malignancies
| Gene alteration . | Malignancy . | TTFT . | Response to treatment . | PFS and OS . |
|---|---|---|---|---|
| ATM (del/mut) | CLL | Shorter TTFT44 | Lower ORR to chemotherapy in patients with biallelic defect (P = .002).45 No impact on ORR with venetoclax-obinutuzumab treatment.46 | Reduced PFS (HR, 4.4) and OS (HR, 2.4) with chemotherapy.45 |
| TP53 (del/mut) | CLL | Shorter TTFT (HR, 1.98)23 | Lower ORR to chemotherapy (P = .001).47 No impact on ORR with targeted treatment including venetoclax-obinutuzumab,46 AVO,48 and IVO.49 | Reduced PFS (TP53 del: HR, 3.3; TP53 mut: HR, 3.8) and OS (del: HR, 2.1; mut: HR, 7.2) with chemotherapy.47,50 Reduced PFS with venetoclax-obinutuzumab (del: HR, 4.4; mut: HR, 3.1).46 Reduced PFS with IVO in patients with bialleic compared with monoalleic defect (P < .001).49 |
| MDS/AML | Shorter time to AML transformation in MDS with multihit TP53 alterations (HR, 3.0)51 | Resistance to small molecule inhibitors35 | TP53 alterations reduce OS in patients with complex karyotype29,52 or in younger patients (P < .001)53 | |
| CML BC | — | — | Reduced OS in patients with TP53 deletion or biallelic TP53 alterations (P < .001)30 | |
| MCL | — | — | Reduced PFS (HR, 3.8) and OS (HR, 4.0; P < .001)24 | |
| DLBCL | — | — | Reduced OS with TP53 alterations and high BCL2 expression (P < .001)26 | |
| MM | — | — | Reduced PFS with TP53 alterations, APOBEC signature, and HRR defect28 | |
| DYRK1A (mut) | CLL | — | — | Reduced OS (univariate HR, 4.3; multivariate HR, 2.79)23 |
| PALB2 (del) | AML | — | — | Reduced OS (2 mos vs 16.2 mos, P < .01)34 |
| SAMHD1 (expression) | AML | — | Reduced SAMHD1 expression in patients with CR after Ara-C (P < .001)54 | Reduced PFS and OS in high SAMDH1 expressors (P < .001)54 |
| Gene alteration . | Malignancy . | TTFT . | Response to treatment . | PFS and OS . |
|---|---|---|---|---|
| ATM (del/mut) | CLL | Shorter TTFT44 | Lower ORR to chemotherapy in patients with biallelic defect (P = .002).45 No impact on ORR with venetoclax-obinutuzumab treatment.46 | Reduced PFS (HR, 4.4) and OS (HR, 2.4) with chemotherapy.45 |
| TP53 (del/mut) | CLL | Shorter TTFT (HR, 1.98)23 | Lower ORR to chemotherapy (P = .001).47 No impact on ORR with targeted treatment including venetoclax-obinutuzumab,46 AVO,48 and IVO.49 | Reduced PFS (TP53 del: HR, 3.3; TP53 mut: HR, 3.8) and OS (del: HR, 2.1; mut: HR, 7.2) with chemotherapy.47,50 Reduced PFS with venetoclax-obinutuzumab (del: HR, 4.4; mut: HR, 3.1).46 Reduced PFS with IVO in patients with bialleic compared with monoalleic defect (P < .001).49 |
| MDS/AML | Shorter time to AML transformation in MDS with multihit TP53 alterations (HR, 3.0)51 | Resistance to small molecule inhibitors35 | TP53 alterations reduce OS in patients with complex karyotype29,52 or in younger patients (P < .001)53 | |
| CML BC | — | — | Reduced OS in patients with TP53 deletion or biallelic TP53 alterations (P < .001)30 | |
| MCL | — | — | Reduced PFS (HR, 3.8) and OS (HR, 4.0; P < .001)24 | |
| DLBCL | — | — | Reduced OS with TP53 alterations and high BCL2 expression (P < .001)26 | |
| MM | — | — | Reduced PFS with TP53 alterations, APOBEC signature, and HRR defect28 | |
| DYRK1A (mut) | CLL | — | — | Reduced OS (univariate HR, 4.3; multivariate HR, 2.79)23 |
| PALB2 (del) | AML | — | — | Reduced OS (2 mos vs 16.2 mos, P < .01)34 |
| SAMHD1 (expression) | AML | — | Reduced SAMHD1 expression in patients with CR after Ara-C (P < .001)54 | Reduced PFS and OS in high SAMDH1 expressors (P < .001)54 |
Ara-C, cytosine arabinoside (cytarabine); AVO, triplet therapy with acalabrutinib, venetoclax, and obinutuzumab; BC, blast crisis; BCL2. B-cell lymphoma 2; CML. chronic myeloid leukemia; CR, complete remission; del, deletion; HR, hazard ratio; IVO, triplet therapy with ibrutinib, venetoclax, and obinutuzumab; MCL, mantle cell lymphoma; MM, multiple myeloma; mut, mutation; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; TTFT, time to first treatment.
The origin and pathologic consequences of acquired DDR defects
In hematologic malignancies resulting from inherited DDR defects such as FA, DDR functional loss leads to genomic instability that typically occurs early in tumorigenesis, promoting the acquisition of pathogenic structural variants. In contrast, genomic instability arises later in sporadic tumors, typically secondary to replication stress triggered by oncogene activation or deregulated tumor proliferation that provides a selective pressure for DDR loss. The resultant DDR inactivation contributes to genomic instability that gives rise to a mutator phenotype, leading to tumor evolution, disease acceleration, and high-grade transformation. Recent studies have elucidated several important underlying mechanisms, as reviewed below.
R-loops–induced replication stress provides a selective pressure for DDR inactivation
R-loops are transcription intermediates, containing an RNA-DNA hybrid and a displaced single-stranded DNA. Enhanced R-loop formation can arise from oncogenic signaling, transcriptional alteration, RNA splicing defects, and aberrant genome editing activity. DDR defects impairing R-loop resolution also potentiate their accumulation. As transcription and replication share a common template, R-loops are obstacles to DNA replication and result in replication stress characterized by the slowing or stalling of replication fork progression.55 The pathogenic role of R-loops is best understood in MDS in which spliceosome mutations in SF3B1, SRSF2, and U2AF1 occur in 45% to 85% of patients, depending on the MDS subtype.56-58 These mutations enhance R-loop formation by impairing RNA polymerase II release from the 7SK complex at transcription start sites necessary for transcription elongation.56 The loss of R-loop homeostasis leads to transcription–replication conflict, replication fork stalling, and DSB formation. This in turn induces ATR-Chk1-p53 pathway activation, thus providing a selective pressure for TP53 loss.56-58
R-loops have also been implicated in lymphoid malignancies. Reminiscent of the effect of spliceosome mutations in MDS, SF3B1 mutations in CLL promote R-loop accumulation, resulting in chromosomal instability that is exacerbated by ATM deletion.59 In adult T-cell leukemia/lymphoma, constitutive NF-κB activation driven by the human T-cell lymphotropic virus type 1 oncoprotein Tax leads to R-loop accumulation. R-loops are processed by NER, thereby exerting selective pressure for the loss of NER endonucleases XPF and XPG that contribute to adult T-cell leukemia/lymphoma progression.60,61 Moreover, TET2 loss-of-function mutations, seen in ∼10% of DLBCL, promote R-loop and DSB accumulation at immunoglobulin switch regions that likely contribute to DLBCL pathogenesis and subsequent ATM/p53 loss.62 There is also emerging evidence that R-loops are regulated by the cytidine deaminase APOBEC3 and are particularly vulnerable to APOBEC3 mutagenesis, although this has yet to be confirmed in hematologic malignancies.63 Nevertheless, given that during antibody diversification R-loops are substrates to activation-induced cytidine deaminase, another member of the APOBEC family, one could envisage this mechanism contributing to pervasive immune system chromosomal translocations in B-cell malignancies.
Aberrant DSB repair and DDR signaling facilitate leukemic progression
Chromosome translocations arise from the erroneous relegation of DSBs. Although HRR is a high-fidelity DSB repair mechanism, its loss through inactivating mutations renders tumor cells reliant on NHEJ that mediates template-independent DSB relegation. The error-prone nature of NHEJ is genetically destabilizing, thus increasing tumor mutation burden and chromosomal aberrations.64
In chronic myeloid leukemia (CML), BCR-ABL translocation is associated with upregulation of error-prone DNA-dependent protein kinase catalytic subunit (DNA-PKCS)-independent NHEJ that may contribute to genomic instability.65 The oncogenic activity of BCR-ABL also creates replication stress through oxidative DNA damage and ATR functional repression mediated by BCR-ABL binding to ATR.66 The consequent accumulation of DSBs induces ATM-Chk2-p53 signaling that provides transient protection against tumor progression. Consistent with the notion that the loss of the ATM-Chk2-p53 checkpoint accelerates leukemic progression, loss-of-function TP53 alterations are observed in ∼20% of CML in blast crisis.30 Similarly, in AML the aberrant proliferation of myeloid progenitors are frequently driven by oncogenes such as MLL-ELN In a mouse model of MLL-ENL–induced leukemogenesis, resultant replication stress leads to ATR-Chk1-p53 and ATM-Chk2-p53 activation that induces cellular senescence in early malignant cells, providing protection against the acquisition of further stem cell–like properties and malignant progression.67 Consistent with this, loss-of-function defects of these pathways accelerate AML development (Tables 3 and 4).
DDR defects confer tumor growth advantage and evolutionary fitness
At the later stages of leukemogenesis in which DDR activity is attenuated or lost, genomic instability produces phenotypic diversity that facilitates clonal evolution. In contrast to the early stages of tumorigenesis in which DDR serves as a protective barrier to delay or prevent malignant progression, once this DDR barrier is breached (secondary to events such as TP53 loss), residual DDR activity may confer the opposite effect of supporting tumor growth acceleration and clonal diversification in the context of heightened replication stress by preventing excessive DNA damage accumulation and mitotic catastrophe,68 with studies in MYC–driven lymphoma models supporting this notion.40
Recent studies have determined the evolutionary fitness of clones harboring DDR defects across different contexts. In an analysis of clonal growth dynamics underlying progression of untreated CLL, TP53 and ATM alterations were associated with the strongest growth accelerations among driver mutations,69 which was confirmed within a CLL cell line engineered to express these different mutations.70 In contrast, in clonal hematopoiesis that precedes myeloid malignancies, TP53 mutations generally had slower growth rates (∼5% per year) relative to other drivers.71,72 However, consistent with the dominant-negative effect of missense TP53 mutations that abrogate the DDR function of p53,73 clones harboring missense TP53 variants affecting the DNA-binding domain expanded at distinctly higher rates and drove leukemogenesis.71,72
The interface between DDR defects and the immune microenvironment
The functional significance of DDR defects extends beyond their tumor-intrinsic effects. They also modulate the immune microenvironment that tumor cells interact with. We are beginning to understand the role of DDR defects as sources of tumor immunogenicity, as well as their impact upon the complex cross talk between DDR, inflammation, and antitumor immunity that influences disease trajectory and clinical outcome. Here, we review emerging concepts that have enriched our understanding of the immunopathological role of DDR defects with reference to hematologic malignancies.
DDR defects are potential sources of tumor immunogenicity
In solid tumors, higher mutation burden correlates with higher neoantigen load that translates to greater tumor immunogenicity.74 Increased mutability due to microsatellite instability, in particular, predicts for response to immune checkpoint inhibitors (ICI).75 Unrepaired DNA damage could also promote HLA class I presentation, in part through activation of the ATR-AKT-mTORC1-S6K axis that stimulates antigen production and presentation.76
In hematologic malignancies, mutation burden is generally low and microsatellite instability arising from mismatch repair defects is uncommon. However, genomic instability leading to increased mutagenesis can arise from other DDR defects such as BRCA1 and ARID1A mutations. For instance, a recent pancancer analysis demonstrated a correlation of ARID1A deficiency with microsatellite instability signatures and increased mutation burden, although this did not reach statistical significance in the subset of 48 DLBCLs investigated.77 Unresolved or aberrantly processed R-loops are also inherently immunogenic and may represent additional sources of immunogenicity.78 Further studies in each hematologic malignancy are required to confirm the relationship between specific DDR defects and neoantigen load, clonal diversity, antigen presentation capacity, and immunogenicity, as well as how these defects influence the immune microenvironment.
Immune DNA- and RNA-sensing pathways mediate DDR–immune cross talk
Replication stress and genomic instability arising from DDR defects result in the cytoplasmic accumulation of nucleus–derived DNA fragments that are detected by the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway, triggering its activation. In turn, cGAS-STING signaling leads to the transcriptional upregulation and secretion of type I interferon and other proinflammatory cytokines and chemokines. They mediate the recruitment and activation of antigen-presenting cells as well as T and natural killer (NK) cells to elicit an antitumor immune response.79 The recent discovery of the mechanism of R-loop sensing by the cGAS-STING pathway may be of particular relevance in the context of perturbed R-loop homeostasis characteristic of many hematologic malignancies. This involves the generation of RNA-DNA hybrids by the XPG-dependent processing of nuclear R-loops, the exportin-1–dependent transfer of these RNA-DNA hybrids to the cytoplasm, and their subsequent recognition in the cytoplasm by the cGAS-STING pathway.78
There is emerging evidence that unrepaired DSBs may additionally recruit RNA sensors and activate RNA-sensing pathways such as the RIG-I like receptor (RLR) pathway.80 The RLR pathway involves the pattern recognition receptors RIG-I and MDA5 that detect immunostimulatory cytosolic double-strand RNA, activating downstream IRF3 and NF-κB signaling to induce type I interferon and promote an antitumor immune response.
DDR defects affect immune signaling and immune effector cells
DDR genes mutated in hematologic malignancies may have specific consequences on antitumor immunity. Owing to the critical role of SAMHD1 for the processing of stalled replication forks, SAMHD1 loss results in cGAS-STING activation triggered by the release of DNA fragments from stalled forks.81 Evidence from AML murine models underscores the importance of cGAS-STING activation and T-cell immunity in the control of SAMHD1-deficient AML.82 Additionally, interferon response to SAMHD1 loss could be mediated through the RLR pathway.83,84 Likewise, ATM or RNASEH2 loss activates cGAS/STING signaling, the former through the cytoplasmic leakage of mitochondrial DNA associated with ATM loss.85,86 However, the latter has hitherto been demonstrated only in cell line and murine models of solid tumors, and therefore requires confirmation in hematologic malignancies. In contrast, ARID1A alterations were shown to curtail chromatin accessibility to interferon-responsive genes and downregulate interferon gene expression,87 which could be overcome by cGAS/STING activation through ATM inhibition.88
Mutant p53 suppresses cGAS/STING activity by interfering with downstream STING signaling,89 potentially contributing to the inferior response of TP53-mutated DLBCL to CD19 chimeric antigen receptor T-cell therapy that is associated with subdued interferon response and reduced CD8+ T-cell infiltration within the lymphoma microenvironment.90 In lymphoma, programmed death-ligand 1 (PD-L1) expression and macrophage phagocytic capacity also decrease upon TP53 loss.91 In contrast, TP53 alterations correlate with enhanced interferon gene expression and immunotherapeutic response in AML,92,93 highlighting the context specific effect of TP53 loss.
cGAS-STING activation could upregulate tumor PD-L1, which could explain the predictive role of ATM mutations for ICI response in solid tumors. However, the existence of an ATM-dependent, cGAS-independent noncanonical STING signaling pathway has been reported,94 and the extent to which cGAS-STING signaling results in effective antitumor immunity might be context dependent. It therefore remains to be determined whether DDR alterations could identify exceptional responders to ICI and other immunotherapies in hematologic malignancies.
Importantly, a recent study highlighted an additional function for ATM in lymphoma immunosurveillance, wherein ATM-deficient T cells displayed replication stress and impaired proliferation, whereas restoring ATM function led to lymphoma regression via ATM-dependent, T-cell–mediated antitumor activity.95 These findings may support a potential role of immune reconstitution, such as through allogeneic hematopoietic stem cell transplantation, for the immunoprevention and early treatment of lymphoma developing in patients with AT harboring constitutional ATM loss.
DDR defects and chronic inflammation synergize to drive leukemic evolution
Acute inflammation mediated by cGAS-STING signaling facilitates antitumor immune response. On the contrary, DNA damage–induced chronic inflammation can be tumor supportive. This is underscored by a recent study that tracked the transformation of myeloproliferative neoplasms to AML, demonstrating that chronic inflammation enhances the evolutionary fitness of TP53-mutant hematopoietic stem cells (HSCs) and promotes the selection of TP53-mutant over wild-type TP53 HSCs.96 Relative to their TP53 wild-type counterpart, TP53-mutant HSCs demonstrated resistance to inflammation-associated, DNA damage–inducing proliferative stress, suggesting that TP53 mutation promotes leukemic evolution by rescuing HSCs from DNA damage–induced attrition. Although this study illustrates the power of integrating multidimensional single-cell analysis with functional interrogation, it remains to be determined how other subclonal genetic events affecting DDR shape evolutionary fitness at a transcriptional and phenotypic level within distinct immune microenvironments, and how this, in turn, influences disease trajectory and treatment response in different hematologic malignancies.
DDR defects as therapeutic targets: current and emergent strategies
The characterization of DDR defects and their functional dependencies provides opportunities for therapeutic targeting. There exist functional redundancies across DDR pathways such that defects in 1 pathway may be partially compensated by collateral pathways. Hence, tumor cell survival may be unaffected by the loss of individual pathways but their concurrent loss may result in tumor lethality. Tumor cells harboring DDR defects could, therefore, exhibit functional addiction to specific compensatory mechanisms and be exquisitely sensitive to their inhibition, thus providing the basis for “synthetic lethality” as a novel concept for targeting DDR defects. Moreover, the cross talk between DDR and antitumor immunity underscores the therapeutic potential of combining DDR modulation with immune modulation. These emerging therapeutic strategies as applied to hematologic malignancies are discussed below.
PARP inhibition exerts synthetic lethality in DDR-defective tumors
The most studied synthetic lethal therapeutic target is poly-(adenosine 5′-diphosphate ribose) polymerase (PARP). In the context of impaired HRR arising from BRCA1/BRCA2 mutation, PARP mediates base-excision repair and alternative NHEJ as compensatory mechanisms to prevent DNA damage accumulation in HRR-defective cells.97,98 Other genetic or epigenetic defects can phenocopy BRCA1/2 mutations, causing BRCAness that is characterized by compromised replication fork protection and sensitivity to replication fork–damaging agents.99
Our understanding of replication stress response mechanisms is evolving; it is not completely understood why some tumors respond to PARP inhibitors and other replication stress–inducing agents whereas others do not. In hematologic malignancies, BRCA1/2 mutations are rare, with PARP dependence arising from oncogenes such as mutant IDH1 and AML1-ETO in AML, PML-RARA in acute promyelocytic leukemia, and BCR-ABL in CML that confer BRCAness, as well as other HRR defects such as ATM mutation.100-104 Accordingly, PARP inhibitors demonstrated preclinical efficacy in hematologic malignancies harboring these genetic alterations (Table 5),36,101,104-111 but their clinical applicability currently remains limited. Major obstacles include a lack of genetic stratification in clinical trials, hampering their evaluation with many studies showing predictably modest activity in genetically unselected patients (Table 6),112-116 and the absence of robust, validated assays for BRCAness detection. Previous methods to define BRCAness include homologous recombination deficiency scores (ie, loss of heterozygosity, telomeric allelic imbalance, and large-scale state transitions scores) and assessment of Rad51 function and transcriptional adaptations that compensate for HRR loss.117 However, recent evidence suggests that BRCAness involves exclusively HRR defects that impede fork protection. Moreover, certain hot spot mutations such as SF3B1 K700E give rise to BRCAness, as demonstrated by PARP inhibitor sensitivity, but do not affect Rad51 foci formation.118 Redefining BRCAness will therefore likely improve the clinical translation of PARP inhibitors in hematologic malignancies.
Preclinical studies of PARP inhibitors in DDR-defective hematologic malignancies
| Malignancy . | Study . | PARP inhibitor . | Drug combination . | Preclinical models . | Findings . |
|---|---|---|---|---|---|
| AML | Molenaar et al, 2018101 | Olaparib, talazoparib | Daunorubicin | Primary tumor cells | IDH1/2 mutation sensitizes AML cells to daunorubicin and PARP inhibition. Combined treatment with PARP inhibitor and daunorubicin has additive effect. |
| AML | Esposito et al, 2015105 | Olaparib, veliparib | GSK3 inhibitor | Cell lines, AML cell line–derived xenograft model | AML cells driven by AML1-ETO and PML-RARA are hypersensitive to PARP inhibition. Combined PARP/GSK3 inhibition can overcome PARP inhibitor resistance in MLL-driven leukemia. |
| AML, MDS | Tothova et al, 2021106 | Talazoparib | — | Cell lines, transgenic murine model | Cohesin-mutant AML/MDS displays enhanced sensitivity to PARP inhibition in vitro and in vivo |
| AML | Maifrede et al, 2018107 | Talazoparib, olaparib | FLT3 inhibitors: AC220, gilteritinib, and crenolanib | Cell lines, primary tumor cells, transgenic murine model, and PDX models | FLT3 inhibition suppresses DNA DSB repair and sensitizes FLT3-ITD–positive leukemia to PARP inhibition. FLT3 and PARP inhibition delays AML onset in a FLT3-ITD–positive murine model. |
| CML | Tobin et al, 2013104 | NU1025 | DNA ligase inhibitor | Cell lines and primary tumor cells | Imatinib-resistant CML cells are sensitive to combined DNA ligase and PARP inhibition. In patients with CML, this sensitivity correlates with the expression in CML cells of PARP1 and DNA ligase IIIα, and with alternative NHEJ activity. |
| Myeloid malignancies | Poh et al, 2019108 | Veliparib | — | Cell lines and primary tumor cells | Myeloid neoplasms exhibit homologous recombination defects caused by the epigenetic silencing of BRCA1. BRCA1-repressed leukemic cells show increased miR-155 expression and sensitivity to PARP inhibition. |
| T-ALL | Bamezai et al, 2021109 | Olaparib | — | Cell lines, primary tumor cells, and T-ALL cell line–derived xenograft model | TET1 is highly expressed in T-ALL cells, interacts with PARP, and regulates cell cycle and DNA repair genes. PARP inhibitor abrogates TET1 expression and antagonizes the growth of T-ALL cells. |
| CLL, MCL | Weston et al, 2010110 | Olaparib | Bendamustine, fludarabine, and cyclophosphamide | Cell lines, primary tumor cells, and MCL cell line–derived xenograft model | ATM-deficient CLL and MCL are sensitive to PARP inhibition in vitro and in vivo. PARP inhibitor sensitizes ATM-mutant CLL/MCL to chemotherapy. |
| CLL | Quijada-Alamo et al, 2020111 | Olaparib | BCR inhibitors including ibrutinib | Cell lines and primary tumor cells | Del(11q)/ATM-KO in CLL cells results in increased sensitivity to PARP inhibition. PARP inhibitor olaparib synergizes with BCR inhibition in del(11q) CLL cells. |
| CLL | Zimmermann et al, 201836 | Olaparib, talazoparib | — | Primary tumor cells | RNASEH2B-deficient CLL cells display enhanced sensitive to PARP inhibitors, especially to talazoparib, with the degree of sensitivity correlating with the number of RNASEH2B alleles lost |
| Malignancy . | Study . | PARP inhibitor . | Drug combination . | Preclinical models . | Findings . |
|---|---|---|---|---|---|
| AML | Molenaar et al, 2018101 | Olaparib, talazoparib | Daunorubicin | Primary tumor cells | IDH1/2 mutation sensitizes AML cells to daunorubicin and PARP inhibition. Combined treatment with PARP inhibitor and daunorubicin has additive effect. |
| AML | Esposito et al, 2015105 | Olaparib, veliparib | GSK3 inhibitor | Cell lines, AML cell line–derived xenograft model | AML cells driven by AML1-ETO and PML-RARA are hypersensitive to PARP inhibition. Combined PARP/GSK3 inhibition can overcome PARP inhibitor resistance in MLL-driven leukemia. |
| AML, MDS | Tothova et al, 2021106 | Talazoparib | — | Cell lines, transgenic murine model | Cohesin-mutant AML/MDS displays enhanced sensitivity to PARP inhibition in vitro and in vivo |
| AML | Maifrede et al, 2018107 | Talazoparib, olaparib | FLT3 inhibitors: AC220, gilteritinib, and crenolanib | Cell lines, primary tumor cells, transgenic murine model, and PDX models | FLT3 inhibition suppresses DNA DSB repair and sensitizes FLT3-ITD–positive leukemia to PARP inhibition. FLT3 and PARP inhibition delays AML onset in a FLT3-ITD–positive murine model. |
| CML | Tobin et al, 2013104 | NU1025 | DNA ligase inhibitor | Cell lines and primary tumor cells | Imatinib-resistant CML cells are sensitive to combined DNA ligase and PARP inhibition. In patients with CML, this sensitivity correlates with the expression in CML cells of PARP1 and DNA ligase IIIα, and with alternative NHEJ activity. |
| Myeloid malignancies | Poh et al, 2019108 | Veliparib | — | Cell lines and primary tumor cells | Myeloid neoplasms exhibit homologous recombination defects caused by the epigenetic silencing of BRCA1. BRCA1-repressed leukemic cells show increased miR-155 expression and sensitivity to PARP inhibition. |
| T-ALL | Bamezai et al, 2021109 | Olaparib | — | Cell lines, primary tumor cells, and T-ALL cell line–derived xenograft model | TET1 is highly expressed in T-ALL cells, interacts with PARP, and regulates cell cycle and DNA repair genes. PARP inhibitor abrogates TET1 expression and antagonizes the growth of T-ALL cells. |
| CLL, MCL | Weston et al, 2010110 | Olaparib | Bendamustine, fludarabine, and cyclophosphamide | Cell lines, primary tumor cells, and MCL cell line–derived xenograft model | ATM-deficient CLL and MCL are sensitive to PARP inhibition in vitro and in vivo. PARP inhibitor sensitizes ATM-mutant CLL/MCL to chemotherapy. |
| CLL | Quijada-Alamo et al, 2020111 | Olaparib | BCR inhibitors including ibrutinib | Cell lines and primary tumor cells | Del(11q)/ATM-KO in CLL cells results in increased sensitivity to PARP inhibition. PARP inhibitor olaparib synergizes with BCR inhibition in del(11q) CLL cells. |
| CLL | Zimmermann et al, 201836 | Olaparib, talazoparib | — | Primary tumor cells | RNASEH2B-deficient CLL cells display enhanced sensitive to PARP inhibitors, especially to talazoparib, with the degree of sensitivity correlating with the number of RNASEH2B alleles lost |
ALL, acute lymphoblastic leukemia; BCR, B-cell receptor signaling; KO, knockout; MCL, mantle cell lymphoma; MLL, mixed lineage leukemia; miR, microRNA; PDX, patient–derived xenograft; T-ALL, T-cell acute lymphoblastic leukemia.
Clinical studies of PARP inhibitors in hematologic malignancies
| PARP inhibitor . | Phase . | Study . | Treatment . | Malignancy . | Number of patients . | ClinicalTrials.gov identifier . | Findings . |
|---|---|---|---|---|---|---|---|
| Talazoparib | 1 | Gopal et al, 2021112 | Monotherapy | Refractory AML, MDS, CLL, and MCL | 33 | NCT01399840 | Well tolerated. The MTD of 2.0 mg per day in the AML/MDS cohort and 0.9 mg per day in the CLL/MCL cohort was comparable to that for solid tumors. Stable disease was observed in 18 patients. |
| Olaparib | 1 | Pratt et al 2018113 | Monotherapy | Refractory CLL, T-PLL, and MCL | 15 | ISRCTN34386131 | Well tolerated. Most common dose-limiting toxicities were hematologic. Patients harboring defects in the ATM pathway displayed a trend toward more durable response. |
| Veliparib | 1 | Pratz et al, 2017114 | Combination with topotecan and carboplatin | Refractory AML, MPN, and CMML | 99 | NCT00588991 | Acceptable safety and tolerability. Objective response was observed in 33 patients and complete response in 25 of 99 patients. Leukemic cells from responders displayed increased H2AX phosphorylation. |
| Veliparib | 1 | Gojo et al, 2017115 | Combination with temozolomide | High-risk AML | 48 | NCT01139970 | No dose-limiting toxicity. Complete response was observed in 8 of 48 patients. Clinical response correlated with MGMT promoter hypermethylation and treatment-induced H2AX phosphorylation. |
| Talazoparib | 1 | Baer et al, 2022116 | Combination with decitabine | Refractory AML | 22 | NCT02878785 | Well tolerated. Complete remission was observed in 2 patients and hematologic improvement in 3 patients. Responding patients displayed DNA demethylation, increased PARP-trapping in chromatin, increased γH2AX foci, and decreased HRR activity. |
| PARP inhibitor . | Phase . | Study . | Treatment . | Malignancy . | Number of patients . | ClinicalTrials.gov identifier . | Findings . |
|---|---|---|---|---|---|---|---|
| Talazoparib | 1 | Gopal et al, 2021112 | Monotherapy | Refractory AML, MDS, CLL, and MCL | 33 | NCT01399840 | Well tolerated. The MTD of 2.0 mg per day in the AML/MDS cohort and 0.9 mg per day in the CLL/MCL cohort was comparable to that for solid tumors. Stable disease was observed in 18 patients. |
| Olaparib | 1 | Pratt et al 2018113 | Monotherapy | Refractory CLL, T-PLL, and MCL | 15 | ISRCTN34386131 | Well tolerated. Most common dose-limiting toxicities were hematologic. Patients harboring defects in the ATM pathway displayed a trend toward more durable response. |
| Veliparib | 1 | Pratz et al, 2017114 | Combination with topotecan and carboplatin | Refractory AML, MPN, and CMML | 99 | NCT00588991 | Acceptable safety and tolerability. Objective response was observed in 33 patients and complete response in 25 of 99 patients. Leukemic cells from responders displayed increased H2AX phosphorylation. |
| Veliparib | 1 | Gojo et al, 2017115 | Combination with temozolomide | High-risk AML | 48 | NCT01139970 | No dose-limiting toxicity. Complete response was observed in 8 of 48 patients. Clinical response correlated with MGMT promoter hypermethylation and treatment-induced H2AX phosphorylation. |
| Talazoparib | 1 | Baer et al, 2022116 | Combination with decitabine | Refractory AML | 22 | NCT02878785 | Well tolerated. Complete remission was observed in 2 patients and hematologic improvement in 3 patients. Responding patients displayed DNA demethylation, increased PARP-trapping in chromatin, increased γH2AX foci, and decreased HRR activity. |
CMML, chronic myelomonocytic leukemia; MCL, mantle cell lymphoma; MPN, myeloproliferative neoplasms; MTD, maximum tolerable drug dose; T-PLL, T-cell prolymphocytic leukemia.
Importantly, recent findings suggest that the therapeutic use of PARP inhibitors can be extended to some HRR-proficient hematologic cancers. For example, CLL harboring RNASEH2 loss have functional HRR but defective ribonucleotide excision repair (Table 2);36 their sensitivity to PARP inhibition could be attributed to the compensatory activity of the TOP1 helicase on ribonucleotides that creates PARP-trapping lesions upon which PARP inhibition leads to DNA damage accumulation and cell death. Moreover, demethylation agents, as well as histone deacetylase, BET, and proteasome inhibitors modify HRR and induce BRCAness, thus providing rationale for their use in combination with PARP inhibitors.116,119-121
Replication stress can be targeted through ATR/CHK1/WEE1 inhibition
The kinases ATR, CHK1, and WEE1 resolve replication stress and promote cell survival through stabilization of stalled replication forks, activation of dormant origins to complete DNA replication, and induction of cell cycle arrest to facilitate DNA repair and prevent the propagation of damaged or underreplicated DNA into daughter cells. Inhibition of ATR, CHK1, or WEE1 permits unbridled cell cycle progression during replication stress, leading to cell death by apoptosis and mitotic catastrophe (Figure 3). Predictive biomarkers of sensitivity to ATR/CHK1 inhibition involve genetic defects associated with increased replication stress that confer cellular dependence on the ATR/CHK1 pathway. These include BRCAness with compromised HRR–dependent fork stability, inactivation of ATM/p53-dependent DDR response, as well as KRAS and MYC oncogene activation.
Induction of synthetic lethality in hematologic malignancies with DDR inhibitors. DDR defects and oncogene activation associated with the BRCAness phenotype confer sensitivity to the inhibition of PARP proteins that participate in alternative DNA repair pathways. DDR defects and the BRCAness phenotype also compromise DNA replication, leading to replication stress. ATR, a principal regulator of replication stress response, is activated by the single-stranded (ss) DNA-RPA complex, which forms rapidly after the exposure of ssDNA at stalled replication forks. The ATR effector kinase CHK1 induces the S-phase cell cycle checkpoint and activates WEE1 kinase to induce the G2-M cell cycle checkpoints. CHK1 also regulates replication origin firing and timely S to G2 transition. Normal ATR/CHK1 function (in green) prevents replication fork collapse, exacerbation of replication stress (in purple), and early S/G2 transition before replication is successfully completed. ATR, CHK1, or WEE1 inhibition therefore results in uncontrolled cell cycle progression despite replication stress, leading to mitotic catastrophe and apoptosis.
Induction of synthetic lethality in hematologic malignancies with DDR inhibitors. DDR defects and oncogene activation associated with the BRCAness phenotype confer sensitivity to the inhibition of PARP proteins that participate in alternative DNA repair pathways. DDR defects and the BRCAness phenotype also compromise DNA replication, leading to replication stress. ATR, a principal regulator of replication stress response, is activated by the single-stranded (ss) DNA-RPA complex, which forms rapidly after the exposure of ssDNA at stalled replication forks. The ATR effector kinase CHK1 induces the S-phase cell cycle checkpoint and activates WEE1 kinase to induce the G2-M cell cycle checkpoints. CHK1 also regulates replication origin firing and timely S to G2 transition. Normal ATR/CHK1 function (in green) prevents replication fork collapse, exacerbation of replication stress (in purple), and early S/G2 transition before replication is successfully completed. ATR, CHK1, or WEE1 inhibition therefore results in uncontrolled cell cycle progression despite replication stress, leading to mitotic catastrophe and apoptosis.
Despite robust preclinical data (Table 7),40,122-139 clinical studies demonstrating unequivocal efficacy in hematologic malignancies currently remain limited (Table 8).140-144 Many synthetic lethal interactions between replication stress response and DDR effectors have been discovered through CRISPR-CRISPR–associated protein 9 screens in cells of nonhematologic origins. Given that these interactions can be context specific, they may not necessarily operate in hematopoietic cells.145-147 Furthermore, not all synthetically lethal interactions can be experimentally predicted. For example, mutation-associated DDR inactivation arising in primary tumor cells may be more detrimental to cellular survival compared with the loss of DDR gene expression represented in CRISPR screens. Intratumoral heterogeneity also means that although the majority of tumor cells may harbor DDR defects that predict sensitivity to ATR/CHK1/WEE1 inhibitors, those tumor subpopulations with an intact DDR treated with sublethal inhibitor doses that increase genomic instability may constitute a cellular reservoir from which eventual therapeutic resistance could arise. Finally, genomic instability associated with DDR defects frequently leads to DDR inhibitor resistance. In that respect, several studies have demonstrated the ability of ATR inhibitor to reverse PARP inhibitor resistance by disrupting HRR rewiring and fork protection in BRCA-deficient tumor cells.148 In addition, ATR inhibition exacerbates DNA damage through abrogating the ATR-dependent S/G2 checkpoint that leads to accelerated mitotic entry of cells harboring unresolved postreplicative DNA damage.149 Combining PARP inhibition with ATR inhibition therefore holds promise but is yet to be substantively investigated in hematologic malignancies.
Preclinical studies of replication stress response inhibitors in DDR-defective hematologic malignancies
| Disease . | Phenotype . | Study . | Mode of targeting . | Drug combination . | Models . | Findings . |
|---|---|---|---|---|---|---|
| AML | FLT3-ITD | Yuan et al, 2014122 | CHK1 inhibition with SCH900776, UCN01, and CHIR124 | FLT3 inhibitor | Cell lines | AML proliferation is dependent on functional CHK1. CHK1 inhibitors reduce FLT3 activation |
| AML | Cytarabine resistance | Qi et al, 2014123 | CHK1 inhibition with LY2603618; WEE1 inhibition with MK-1775 | Cytarabine and roscovitine | Cell lines, primary tumor cells | AML cells exhibit dose-dependent sensitivity to CHK1 inhibitor that is CDK-dependent. CHK1 inhibitor synergizes with WEE1 inhibition. |
| AML | Cytarabine resistance | Di Tullio et al, 2017124 | CHK1 inhibition with GDC-0575 | Cytarabine | Cell lines, primary tumor cells, and PDX models | CHK1 inhibition increases in vitro and in vivo AML sensitivity to cytarabine, but does not affect hematopoiesis |
| AML | — | Ma et al, 2017125 | ATR inhibition with AZ20 and AZD6738 | Cytarabine | Cell lines, primary tumor cells | ATR inhibition abrogates the S and G2/M checkpoints and synergizes with cytarabine against AML cells |
| AML | — | Fordham et al, 2018126 | ATR inhibition with VX-970 | Gemcitabine and hydroxyurea | Orthotopic murine model | Antileukemic activity of hydroxyurea and gemcitabine is potentiated by ATR inhibition through abrogation of replication fork progression |
| AML | — | Qi et al, 2019127 | ATR inhibition with VE-821; Wee1 inhibition with AZD1775 | — | Cell lines | Combined ATR and WEE1 inhibition synergistically increases replication stress and DNA damage and induces apoptosis in AML cells |
| AML | MLL-ENL | Morgado-Palacin et al, 2016128 | ATM inhibition with AZD0156 ATR inhibition with AZ20 | — | N-RAS–driven MLL-ENL mouse model | ATR and ATM inhibition suppresses MLL-driven leukemias independently of p53 function |
| CML | BCR-ABL T315I | Lei et al, 2018129 | CHK1 inhibition with AZD7762 and MK-8776 | Imatinib | Cell lines, primary tumor cells, and cell line xenograft models | CHK1 inhibitors can overcome imatinib resistance in BCR-ABL T315I-mutant CML cells through CHIP–dependent degradation of BCR-ABL |
| ALL | — | Ghelli Luserni Di Rorà et al, 2021130 | CHK1 inhibition with prexasertib; ATR inhibition with VE-821 | Doxorubicin | Cell lines, primary tumor cells | ATR/CHK1 inhibitors potentiates doxorubicin–induced cytotoxicity in ALL |
| B-ALL | Mll-Af4/N-RasG12D | Chu et al, 2018131 | ATR inhibition with AZ20 | MEK inhibitors PD901 and trametinib | Transgenic and PDX mouse models | Combined MEK/ATR inhibition is effective against Mll-Af4/N-RasG12D B-ALL |
| T-ALL | — | Le et al, 2017132 | ATR inhibition with VE-822 | CDK inhibitor palbociclib | Cell lines, transgenic murine model | ATR inhibition abrogates nucleotide synthesis in T-ALL by suppressing ribonucleotide reductase and deoxycytidine kinase activity |
| CLL | ATM/TP53 mut/del | Kwok et al, 2016133 | ATR inhibition with ADZ6738 | Chemotherapy, ibrutinib | Cell lines, primary tumor cells | Defective ATM or p53 increases reliance on ATR-dependent regulation of replication stress in CLL |
| DLBCL | — | De Jong et al, 2020134 | WEE1 inhibition with AZD1775 | CHOP, radiotherapy | Cell lines | Combination of AZD1775 with radiotherapy or CHOP enhances sensitivity of DLBCL cells to WEE1 inhibition through unscheduled G2/M progression and increased DNA damage |
| Myc-driven lymphoma | Eμ-Myc+; ARF−/− | Murga et al, 201140 | ATR inhibition with ETP46464; CHK1 inhibition with SB-218078 | — | Murine models with defined levels of ATR signaling | The threshold of ATR signaling determines tumor outcome. ATR inhibition is tumor-suppressive in early tumorigenesis but is tumor promoting at later stages. |
| MM | Chromosome instability | Cottini et al, 2015135 | ATR inhibition with VE-821 | ROS inducer piperlongumine | Cell lines, primary tumor cells | MYC drives replicative and oxidative stress in MM. ATR inhibitor synergizes with piperlongumine. |
| MM | — | Xing et al, 2020136 | ATR inhibition with AZD6738; WEE1 inhibition with AZD1775; ATM inhibition with AZD0156 | Antibody-drug conjugate with DNA crosslinker | Cell lines, primary tumor cells, cell line xenograft models | Antibody-drug conjugate-induced DNA damage is synergistic with replication stress response inhibitors in MM |
| MM | Upregulation of p38/MK2 | Guo, 2019;137 Gu, 2021;138 Dietlein et al, 2016139 | MK2 knock out; MK2 inhibition with PF3644022 | Bortezomib, doxorubicin, dexamethasone, and Chk1 inhibitor | Cell lines, Transgenic models | MK2 is upregulated in high-risk MM and confers chemoresistance. Targeting MK2 induces MM killing in vitro and in vivo. MK2 and Chk1 inhibition are synthetically lethal in KRAS-mutant cancer cells. |
| Disease . | Phenotype . | Study . | Mode of targeting . | Drug combination . | Models . | Findings . |
|---|---|---|---|---|---|---|
| AML | FLT3-ITD | Yuan et al, 2014122 | CHK1 inhibition with SCH900776, UCN01, and CHIR124 | FLT3 inhibitor | Cell lines | AML proliferation is dependent on functional CHK1. CHK1 inhibitors reduce FLT3 activation |
| AML | Cytarabine resistance | Qi et al, 2014123 | CHK1 inhibition with LY2603618; WEE1 inhibition with MK-1775 | Cytarabine and roscovitine | Cell lines, primary tumor cells | AML cells exhibit dose-dependent sensitivity to CHK1 inhibitor that is CDK-dependent. CHK1 inhibitor synergizes with WEE1 inhibition. |
| AML | Cytarabine resistance | Di Tullio et al, 2017124 | CHK1 inhibition with GDC-0575 | Cytarabine | Cell lines, primary tumor cells, and PDX models | CHK1 inhibition increases in vitro and in vivo AML sensitivity to cytarabine, but does not affect hematopoiesis |
| AML | — | Ma et al, 2017125 | ATR inhibition with AZ20 and AZD6738 | Cytarabine | Cell lines, primary tumor cells | ATR inhibition abrogates the S and G2/M checkpoints and synergizes with cytarabine against AML cells |
| AML | — | Fordham et al, 2018126 | ATR inhibition with VX-970 | Gemcitabine and hydroxyurea | Orthotopic murine model | Antileukemic activity of hydroxyurea and gemcitabine is potentiated by ATR inhibition through abrogation of replication fork progression |
| AML | — | Qi et al, 2019127 | ATR inhibition with VE-821; Wee1 inhibition with AZD1775 | — | Cell lines | Combined ATR and WEE1 inhibition synergistically increases replication stress and DNA damage and induces apoptosis in AML cells |
| AML | MLL-ENL | Morgado-Palacin et al, 2016128 | ATM inhibition with AZD0156 ATR inhibition with AZ20 | — | N-RAS–driven MLL-ENL mouse model | ATR and ATM inhibition suppresses MLL-driven leukemias independently of p53 function |
| CML | BCR-ABL T315I | Lei et al, 2018129 | CHK1 inhibition with AZD7762 and MK-8776 | Imatinib | Cell lines, primary tumor cells, and cell line xenograft models | CHK1 inhibitors can overcome imatinib resistance in BCR-ABL T315I-mutant CML cells through CHIP–dependent degradation of BCR-ABL |
| ALL | — | Ghelli Luserni Di Rorà et al, 2021130 | CHK1 inhibition with prexasertib; ATR inhibition with VE-821 | Doxorubicin | Cell lines, primary tumor cells | ATR/CHK1 inhibitors potentiates doxorubicin–induced cytotoxicity in ALL |
| B-ALL | Mll-Af4/N-RasG12D | Chu et al, 2018131 | ATR inhibition with AZ20 | MEK inhibitors PD901 and trametinib | Transgenic and PDX mouse models | Combined MEK/ATR inhibition is effective against Mll-Af4/N-RasG12D B-ALL |
| T-ALL | — | Le et al, 2017132 | ATR inhibition with VE-822 | CDK inhibitor palbociclib | Cell lines, transgenic murine model | ATR inhibition abrogates nucleotide synthesis in T-ALL by suppressing ribonucleotide reductase and deoxycytidine kinase activity |
| CLL | ATM/TP53 mut/del | Kwok et al, 2016133 | ATR inhibition with ADZ6738 | Chemotherapy, ibrutinib | Cell lines, primary tumor cells | Defective ATM or p53 increases reliance on ATR-dependent regulation of replication stress in CLL |
| DLBCL | — | De Jong et al, 2020134 | WEE1 inhibition with AZD1775 | CHOP, radiotherapy | Cell lines | Combination of AZD1775 with radiotherapy or CHOP enhances sensitivity of DLBCL cells to WEE1 inhibition through unscheduled G2/M progression and increased DNA damage |
| Myc-driven lymphoma | Eμ-Myc+; ARF−/− | Murga et al, 201140 | ATR inhibition with ETP46464; CHK1 inhibition with SB-218078 | — | Murine models with defined levels of ATR signaling | The threshold of ATR signaling determines tumor outcome. ATR inhibition is tumor-suppressive in early tumorigenesis but is tumor promoting at later stages. |
| MM | Chromosome instability | Cottini et al, 2015135 | ATR inhibition with VE-821 | ROS inducer piperlongumine | Cell lines, primary tumor cells | MYC drives replicative and oxidative stress in MM. ATR inhibitor synergizes with piperlongumine. |
| MM | — | Xing et al, 2020136 | ATR inhibition with AZD6738; WEE1 inhibition with AZD1775; ATM inhibition with AZD0156 | Antibody-drug conjugate with DNA crosslinker | Cell lines, primary tumor cells, cell line xenograft models | Antibody-drug conjugate-induced DNA damage is synergistic with replication stress response inhibitors in MM |
| MM | Upregulation of p38/MK2 | Guo, 2019;137 Gu, 2021;138 Dietlein et al, 2016139 | MK2 knock out; MK2 inhibition with PF3644022 | Bortezomib, doxorubicin, dexamethasone, and Chk1 inhibitor | Cell lines, Transgenic models | MK2 is upregulated in high-risk MM and confers chemoresistance. Targeting MK2 induces MM killing in vitro and in vivo. MK2 and Chk1 inhibition are synthetically lethal in KRAS-mutant cancer cells. |
ALL, acute lymphoblastic leukemia; B-ALL; B-cell acute lymphoblastic leukemia; CDK, cyclin-dependent kinase; CHIP, carboxyl terminus of the Hsc70-interacting protein; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisolone; MEK, mitogen-activated protein kinase; MM, multiple myeloma; ROS, reactive oxygen species; PDX, patient–derived xenograft; T-ALL, T-cell acute lymphoblastic leukemia.
Clinical studies of replication stress response inhibitors in hematologic malignancies
| Inhibitor . | Phase . | Study . | Target . | Treatment . | Malignancy . | Number of patients . | ClinicalTrials.gov identifier . | Findings . |
|---|---|---|---|---|---|---|---|---|
| SRA737 | 1/2 | Sponsored by Sierra Oncology | CHK1 | Monotherapy | Advanced solid tumors or NHL | 107 | NCT02797964 | Completed in 2019, not reported |
| PEP07 | 1 | Sponsored by Pharmaengine | CHK1 | Monotherapy | Refractory AML and MCL | 32 | NCT05659732 | Ongoing |
| Prexasertib | 1 | Sponsored by M.D. Anderson Cancer Center | CHK1 | Combined with cytarabine and fludarabine | Refractory CML, AML, and MDS | 15 | NCT02649764 | Completed in 2022, not reported |
| Prexasertib | 1 | Sponsored by Dana-Farber Cancer Institute | CHK1 | Combined with mitoxantrone, etoposide, and cytarabine | Refractory AML and MDS | 2 | NCT03735446 | Terminated in 2019, not reported |
| Ceralasertib | 1/2 | Jurczak et al, 2023140 | ATR | Monotherapy; combined with acalabrutinib | Relapsed/refractory CLL | 11 | NCT03328273 | Ceralasertib alone showed limited clinical benefit. Acalabrutinib + ceralasertib was tolerable with limited preliminary clinical activity observed in 2 patients with BTK inhibitor-naïve, del(11q) CLL |
| Ceralasertib | 1 | Sponsored by AstraZeneca | ATR | Monotherapy | Refractory CLL, PLL, and B-cell NHL | 2 | NCT01955668 | Terminated in 2013, not reported |
| Ceralasertib | 1 | Sponsored by AstraZeneca | ATR | Monotherapy | Progressive MDS, CMML | 52 | NCT03770429 | Ongoing |
| Camonsertib | 1/2 | Hu et al, 2023141 | ATR | Combined with olaparib | Relapsed/refractory CLL | 45 | NCT05405309 | Ongoing |
| AZD1775 | 2 | Sponsored by Mayo Clinic | WEE1 | Monotherapy; combination with cytarabine | Refractory AML amd MDS | 3 | NCT02666950 | Terminated in 2019, not reported |
| MK-8776 | 2 | Webster et al, 2017142 | WEE1 | Combination with cytarabine | Refractory AML | 32 | NCT01870596 | Well tolerated. Combination therapy did not improve complete response rates or 1-year overall survival when compared with cytarabine alone, despite transiently increasing DNA damage in vivo |
| MK-8776 | 1 | Karp et al, 2012143 | WEE1 | Combination with cytarabine | Refractory AML, ALL, and CML | 24 | NCT00907517 | Cardiac and neurotoxic effects occurred at the WEE1 inhibitor dose of 80 mg/m2; 8 of 24 patients, including 2 with complex karyotype, treated with ≥80 mg/m2 WEE1 inhibitor doses attained complete remission. WEE1 inhibitor at 40 mg/m2 induced H2AX phosphorylation, a marker of DNA damage, in leukemic cells. |
| Adavosertib | 1 | Shafer et al, 2023144 | WEE1 | Combination with belinostat | Relapsed and refractory AML and MDS | 20 | NCT02381548 | Grade 4 CRS occurred at 225 mg per day adavosertib and 1000 mg per m2 belinostat, and was dose limiting. No clinical benefit was observed. |
| Inhibitor . | Phase . | Study . | Target . | Treatment . | Malignancy . | Number of patients . | ClinicalTrials.gov identifier . | Findings . |
|---|---|---|---|---|---|---|---|---|
| SRA737 | 1/2 | Sponsored by Sierra Oncology | CHK1 | Monotherapy | Advanced solid tumors or NHL | 107 | NCT02797964 | Completed in 2019, not reported |
| PEP07 | 1 | Sponsored by Pharmaengine | CHK1 | Monotherapy | Refractory AML and MCL | 32 | NCT05659732 | Ongoing |
| Prexasertib | 1 | Sponsored by M.D. Anderson Cancer Center | CHK1 | Combined with cytarabine and fludarabine | Refractory CML, AML, and MDS | 15 | NCT02649764 | Completed in 2022, not reported |
| Prexasertib | 1 | Sponsored by Dana-Farber Cancer Institute | CHK1 | Combined with mitoxantrone, etoposide, and cytarabine | Refractory AML and MDS | 2 | NCT03735446 | Terminated in 2019, not reported |
| Ceralasertib | 1/2 | Jurczak et al, 2023140 | ATR | Monotherapy; combined with acalabrutinib | Relapsed/refractory CLL | 11 | NCT03328273 | Ceralasertib alone showed limited clinical benefit. Acalabrutinib + ceralasertib was tolerable with limited preliminary clinical activity observed in 2 patients with BTK inhibitor-naïve, del(11q) CLL |
| Ceralasertib | 1 | Sponsored by AstraZeneca | ATR | Monotherapy | Refractory CLL, PLL, and B-cell NHL | 2 | NCT01955668 | Terminated in 2013, not reported |
| Ceralasertib | 1 | Sponsored by AstraZeneca | ATR | Monotherapy | Progressive MDS, CMML | 52 | NCT03770429 | Ongoing |
| Camonsertib | 1/2 | Hu et al, 2023141 | ATR | Combined with olaparib | Relapsed/refractory CLL | 45 | NCT05405309 | Ongoing |
| AZD1775 | 2 | Sponsored by Mayo Clinic | WEE1 | Monotherapy; combination with cytarabine | Refractory AML amd MDS | 3 | NCT02666950 | Terminated in 2019, not reported |
| MK-8776 | 2 | Webster et al, 2017142 | WEE1 | Combination with cytarabine | Refractory AML | 32 | NCT01870596 | Well tolerated. Combination therapy did not improve complete response rates or 1-year overall survival when compared with cytarabine alone, despite transiently increasing DNA damage in vivo |
| MK-8776 | 1 | Karp et al, 2012143 | WEE1 | Combination with cytarabine | Refractory AML, ALL, and CML | 24 | NCT00907517 | Cardiac and neurotoxic effects occurred at the WEE1 inhibitor dose of 80 mg/m2; 8 of 24 patients, including 2 with complex karyotype, treated with ≥80 mg/m2 WEE1 inhibitor doses attained complete remission. WEE1 inhibitor at 40 mg/m2 induced H2AX phosphorylation, a marker of DNA damage, in leukemic cells. |
| Adavosertib | 1 | Shafer et al, 2023144 | WEE1 | Combination with belinostat | Relapsed and refractory AML and MDS | 20 | NCT02381548 | Grade 4 CRS occurred at 225 mg per day adavosertib and 1000 mg per m2 belinostat, and was dose limiting. No clinical benefit was observed. |
BTK, Bruton tyrosine kinase; CMML, chronic myelomonocytic leukemia; CRS, cytokine release syndrome; MCL, mantle cell lymphoma; NHL, non-Hodgkin lymphoma.
Combining DDR targeting with immunotherapy may have therapeutic potential
Recently, it was discovered that PARP inhibitors and ATR inhibitors have immunomodulatory properties that could support their potential use with immunotherapy (Figure 4). In AML, PARP promotes immune escape by repressing the expression of ligands for NKG2D, an NK-cell activating receptor, on leukemic stem cells.150 Consequently, reexpression of NKG2D ligands by PARP inhibition, combined with the adoptive transfer of allogeneic NK cells, suppressed leukemogenesis in patient–derived xenotransplantation models and is currently being evaluated within a phase 1/2 trial (ClinicalTrials.gov identifier: NCT05319249). PARP inhibitors and ATR inhibitors could also promote antitumor immunity by inducing cGAS-STING activity, as demonstrated across solid tumors,151-154 although this requires validation in hematologic malignancies. In the context of TP53 loss, ATR inhibition could additionally exert immunogenic activity through activation of the RLR pathway resulting from the concomitant loss of the G1/S and G2/M cell cycle checkpoints.155
Interaction between DDR defects and antitumor immunity. DDR defects may promote antitumor immune responses through increased mutability and enhanced tumor antigenicity, as well as by inducing cGAS/STING signaling and interferon-stimulated gene (ISG) response that result in the recruitment of immune effector cells. Tumor cells evade antitumor immunity, for example by upregulating the programmed death-ligand 1 (PD-L1)/programmed cell death protein 1 (PD-1) immune checkpoint. DDR–targeting therapies such as MDM2 inhibitors, PARP inhibitors, and ATR inhibitors (indicated by red arrows) could augment antitumor immunity and be used in conjunction with ICIs. Professional illustration by Patrick Lane, ScEYEnce Studios.
Interaction between DDR defects and antitumor immunity. DDR defects may promote antitumor immune responses through increased mutability and enhanced tumor antigenicity, as well as by inducing cGAS/STING signaling and interferon-stimulated gene (ISG) response that result in the recruitment of immune effector cells. Tumor cells evade antitumor immunity, for example by upregulating the programmed death-ligand 1 (PD-L1)/programmed cell death protein 1 (PD-1) immune checkpoint. DDR–targeting therapies such as MDM2 inhibitors, PARP inhibitors, and ATR inhibitors (indicated by red arrows) could augment antitumor immunity and be used in conjunction with ICIs. Professional illustration by Patrick Lane, ScEYEnce Studios.
Restoration of p53 function could also be achieved in TP53-aberrant tumors with residual wild-type TP53 by inhibition of murine double minute 2 (MDM2), a negative regulator of p53. In AML, MDM2 inhibitors simultaneously increase leukemic TNF-related apoptosis-inducing ligand (TRAIL) receptor and HLA class II expression, resulting in enhanced sensitivity to allogeneic T-cell–mediated cytotoxicity.156 Moreover, variable expression of human endogenous retroviruses has been reported in hematologic malignancies.157,158 Hence, the recent demonstration that MDM2 inhibition promotes interferon-dependent immunogenicity, via human endogenous retrovirus induction, could be of relevance.159 Furthermore, MDM2 inhibitors act on T cells to potentiate antitumor activity by enhancing STAT5 stability.160 Together, these observations support therapeutic strategies that combine MDM2 inhibitors with immunotherapy.
Unresolved questions and future perspectives
Novel mechanistic insights from recent research, as reviewed herein, have advanced our understanding of the role of DDR defects in driving hematologic malignancies and potential approaches in which these defects could be therapeutically targeted. These fundamental advances, however, have yet to result in major paradigm shifts toward precision medicine in the clinical management of such malignancies. In our view, several important questions remain to be addressed for precision targeting of DDR defects to be realized.
First, how do cellular addictions resulting from a specific DDR defect differ across tumor types?
Functional perturbation screens are key to identifying synthetically lethal partners with specific DDR defects but are lacking in many hematologic malignancies. The effect of DDR defects are likely context dependent, and the landscape of functional addictions conferred by a specific DDR defect may vary according to tumor type, the timing of its acquisition during tumor development (early vs late), as well as clonal and microenvironmental features. Understanding the nature of such heterogeneity through functional interrogation of robust models of hematologic malignancies and analysis of responders vs nonresponders to specific DDR inhibitors across diverse tumor types will facilitate therapeutic stratification and optimization of synthetic lethal strategies.
Second, how can DDR–immune interactions be effectively harnessed for cancer treatment?
Emerging evidence suggests that immune microenvironments in hematologic malignancies could be shaped by DDR defects, but the exact mechanisms remain unclear. In particular, the impact of specific DDR defects on cancer cell state, neoantigen load, and tumor immunogenicity remains to be clarified. Likewise, the functional integrity of antigen-agnostic DNA/RNA-sensing pathways and immune effector responses to DDR defects are yet to be characterized fully across different hematologic malignancies. Deciphering the characteristics and dynamics of the DDR-immune cross talk will be critical for harnessing these interactions and overcoming immune evasion. In this regard, strategies to redirect tumor-supporting inflammatory response to more specific antitumor response may hold promise.
Finally, how do intratumoral heterogeneity and clonal evolution shape tumor response to DDR inhibitors?
In addition to intertumoral heterogeneity, intratumoral heterogeneity inherent across hematologic malignancies may complicate therapeutic targeting of DDR defects. Each subclonal population within a tumor harboring a different constellation of DDR alterations (or a lack thereof) will possess residual DDR activity of a different nature and magnitude. A threshold of residual DDR signaling activity has been postulated to dictate the nature of response to DDR inhibitors68; consequently, each clonal subpopulation will exhibit differential response to different DDR inhibitors and to different doses of the same inhibitor. Given such intratumoral heterogeneity and the vast network of backup DDR pathways that tumor cells could use, targeting multiple cellular dependencies simultaneously would likely be necessary to reduce the likelihood of therapeutic resistance. Hence, the evaluation and optimization of therapeutic combinations will be important. Moreover, the careful calibration of DDR inhibitor drug doses in relation to tumor subclonal composition could prevent underdosing that may lead to potentiation of genomic instability and clonal escape, while avoiding toxic effects from overdosing. Single-agent and combinatorial testing of DDR inhibitors and immune modulators in the preclinical and clinical settings, guided by longitudinal monitoring of clonal dynamics and DDR function at subclonal or single-cell resolution, represents the way forward toward this goal.
Acknowledgments
This work was supported by a Cancer Research UK Clinician Scientist Fellowship (RCCFELCSF-May21∖100002) (M.K.), and by a grant from the Cancer Research UK Programme (grant C20807/A2864) (T.S., A.A.).
Authorship
Contribution: M.K., A.A., and T.S. wrote and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tatjana Stankovic, Institute of Cancer and Genomic Sciences, University of Birmingham, Vincent Drive, Edgbaston, Birmingham B15 2TT, United Kingdom; email: t.stankovic@bham.ac.uk; and Marwan Kwok, Institute of Cancer and Genomic Sciences, University of Birmingham, Vincent Drive, Edgbaston, Birmingham B15 2TT, United Kingdom; email: m.kwok@bham.ac.uk.
References
Author notes
Data are available upon reasonable request from the corresponding authors, Tatjana Stankovic (t.stankovic@bham.ac.uk) and Marwan Kwok (m.kwok@bham.ac.uk).


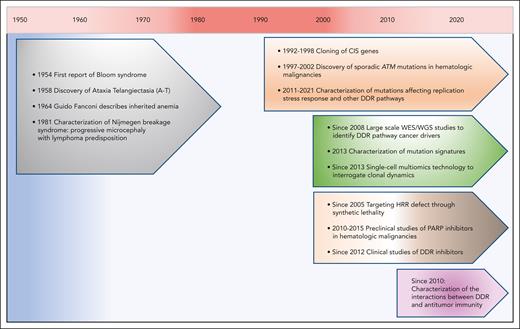



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal