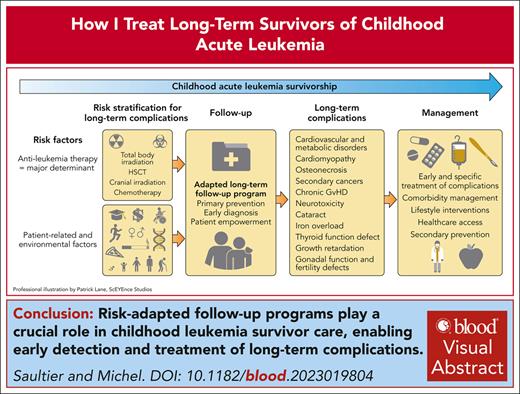
Visual Abstract
The population of survivors of childhood leukemia who reach adulthood is growing due to improved therapy. However, survivors are at risk of long-term complications. Comprehensive follow-up programs play a key role in childhood leukemia survivor care. The major determinant of long-term complications is the therapeutic burden accumulated over time. Relapse chemotherapy, central nervous system irradiation, hematopoietic stem cell transplantation, and total body irradiation are associated with greater risk of long-term complications. Other parameters include clinical characteristics such as age and sex as well as environmental, genetic, and socioeconomic factors, which can help stratify the risk of long-term complications and organize follow-up program. Early diagnosis improves the management of several late complications such as anthracycline-related cardiomyopathy, secondary cancers, metabolic syndrome, development defects, and infertility. Total body irradiation is the treatment associated with worse long-term toxicity profile with a wide range of complications. Patients treated with chemotherapy alone are at a lower risk of long-term complications, although the optimal long-term follow-up remains unclear. Novel immunotherapies and targeted therapy are generally associated with a better short-term safety profile but still require careful long-term toxicity monitoring. Advances in understanding genetic susceptibility to long-term complications could enable tailored therapeutic strategies for leukemia treatment and optimized follow-up programs.
Introduction
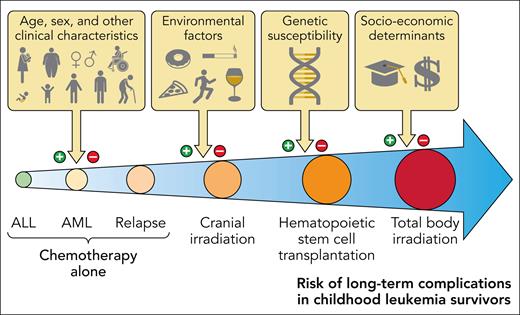
Improved antileukemia therapy and supportive care has led to a significant improvement in the prognosis of childhood acute leukemias. The cure rate now exceeds 90% for acute lymphoblastic leukemia (ALL), which is the most common type of pediatric leukemia. The population of survivors of childhood leukemia who reach adulthood is thus rapidly growing. However, survivors are at risk of long-term chronic health conditions due to the disease and therapy.1 Minimizing the long-term impacts of treatment is a significant challenge. The major determinant of long-term complications is therapeutic exposure. Other parameters include some clinical characteristics (eg, age and sex) as well as environmental, genetic, and socioeconomic factors (Figure 1). The objective of this document is to provide guidance on the risk-stratification, diagnosis, and clinical management principles of these long-term complications.
Risk of long-term complications in survivors of childhood acute leukemia. Professional illustration by Patrick Lane, ScEYEnce Studios.
Risk of long-term complications in survivors of childhood acute leukemia. Professional illustration by Patrick Lane, ScEYEnce Studios.
Early detection improves the management of several long-term complications
Clinical case
A 19-year-old female patient consulted with the childhood leukemia survivorship program. She had a history of acute myeloid leukemia (AML) diagnosed at age 16 years and was treated with intensive chemotherapy without hematopoietic stem cell transplantation (HSCT). She has never relapsed from the disease. The patient weighed 89 kg and had a height of 163 cm and a body mass index of 33.5 kg/m2, vs 49 kg, 158 cm, and 19.6 kg/m2 at AML diagnosis. Her blood pressure was 147/85 mm Hg, and her abdominal circumference was 112 cm. Her fasting blood test revealed elevated levels of fasting triglycerides (3.4 mmol/L) and hyperglycemia (120 mg/dL; 6.7 mmol/L). The patient was diagnosed with metabolic syndrome. An ultrasound with transient elastography was performed and revealed hepatic steatosis without fibrosis. This patient was referred to an endocrinologist and was started on a nutritional and physical activity intervention to improve the metabolic parameters. The patient did not require pharmaceutical intervention for the metabolic parameters. Those parameters improved, and no overt cardiovascular complication was observed at the last evaluation.
This clinical case illustrates the importance of long-term follow-up programs in the early diagnosis of certain complications. Early diagnosis of long-term side effects is likely key to increase the chances of curing or preventing the complications of the side effects and can help preserve quality of life (Table 1). The management of metabolic syndrome, secondary malignant neoplasms and meningiomas, and anthracycline-related cardiomyopathy is significantly improved by early detection. Because the latter complication is specifically due to anthracycline exposure, it will be discussed in the dedicated section of the manuscript. Early diagnosis of fertility and developmental defects (growth retardation, neurodevelopmental defects, and pubertal abnormalities) are also essential. Because HSCT recipients are particularly exposed to these complications, they are discussed in the dedicated section of the manuscript.
Long-term complications of childhood leukemia that benefit from early diagnosis
| Long-term complications . | Screening and diagnostic tools . | Early intervention . |
|---|---|---|
| Secondary tumors Thyroid cancer Breast cancer Skin cancer Meningioma and other brain tumors | Thyroid US Mammography, US, MRI Clinical screening Brain MRI/CT as clinically indicated | Early oncological management |
| Cardiac and vascular risk Anthracycline-related cardiomyopathy Metabolic syndrome | Calculation of anthracyclines cumulative dose (doxorubicin-equivalent dose), echocardiography (with global longitudinal strain) Metabolic parameters (weight, abdominal circumference, fasting glycemia, insulin level and lipid profiles, HbA1c) | Cardioprotective drugs (eg, angiotensin-converting enzyme inhibitors), cardiovascular risk Nutritional and physical activity intervention, lipid-lowering and antidiabetic medications |
| Development Delayed puberty Growth retardation Neurodevelopment | Puberty assessment (Tanner) Growth chart analysis and follow-up Endocrine evaluation Focused clinical evaluation of neurodevelopment | Induction of puberty Hormone therapy Educational support and interventions |
| Iron overload | Number of RBC transfusions, posttreatment ferritin levels, liver function tests, MRI liver iron quantification | Phlebotomy, iron chelation |
| Infertility | Calculation of alkylating agent cumulative dose (cyclophosphamide-equivalent dose), AMH levels | Early postcancer fertility counseling |
| Long-term complications . | Screening and diagnostic tools . | Early intervention . |
|---|---|---|
| Secondary tumors Thyroid cancer Breast cancer Skin cancer Meningioma and other brain tumors | Thyroid US Mammography, US, MRI Clinical screening Brain MRI/CT as clinically indicated | Early oncological management |
| Cardiac and vascular risk Anthracycline-related cardiomyopathy Metabolic syndrome | Calculation of anthracyclines cumulative dose (doxorubicin-equivalent dose), echocardiography (with global longitudinal strain) Metabolic parameters (weight, abdominal circumference, fasting glycemia, insulin level and lipid profiles, HbA1c) | Cardioprotective drugs (eg, angiotensin-converting enzyme inhibitors), cardiovascular risk Nutritional and physical activity intervention, lipid-lowering and antidiabetic medications |
| Development Delayed puberty Growth retardation Neurodevelopment | Puberty assessment (Tanner) Growth chart analysis and follow-up Endocrine evaluation Focused clinical evaluation of neurodevelopment | Induction of puberty Hormone therapy Educational support and interventions |
| Iron overload | Number of RBC transfusions, posttreatment ferritin levels, liver function tests, MRI liver iron quantification | Phlebotomy, iron chelation |
| Infertility | Calculation of alkylating agent cumulative dose (cyclophosphamide-equivalent dose), AMH levels | Early postcancer fertility counseling |
AMH, anti-Mullerian hormone; CT, computed tomography; Hb, hemoglobin; MRI, magnetic resonance imaging; RBC, red blood cell; US, ultrasound.
Metabolic syndrome
Survivors of childhood acute leukemia have a cardiovascular mortality rate >4 times higher than their siblings or the general population.2,3 This excess mortality is partly associated with ischemic heart disease and stroke. These patients show early signs of atherosclerotic lesions.4 The natural history of atheromatous disease begins years before the onset of clinically relevant lesions. Metabolic syndrome, also called insulin resistance syndrome, is a cluster of conditions (abdominal obesity, dyslipidemia, glucose intolerance, and hypertension) associated with a high risk of cardiovascular events, including coronary heart disease, diabetes, and stroke.5 Hence, monitoring the metabolic syndrome in survivors of childhood leukemia is important, although recent data show a trend in declining cardiovascular toxicity among more recently treated patients with ALL.6
Metabolic syndrome is more prevalent in young adult survivors of childhood leukemia than in the age- and sex-matched general population. Central nervous system (CNS) irradiation, HSCT, and total body irradiation (TBI) are key risk factors,7 although an increased risk has also been demonstrated for those who received chemotherapy alone (Table 2).8,9 Obesity, hyperlipidemia, and insulin resistance have been associated with constitutional variants in the glucocorticoid receptor gene NR3C1, the cadherin family gene CDH19, and genes involved in brain development (NALF1, SOX11, and GLRA3).14,15 Other studies have shown that genetic risk scores of the general population can be used to predict severe obesity in survivors of childhood leukemia.16
Metabolic syndrome according to therapeutic exposure
| Therapeutic exposure . | Relative risk8,∗ . | Specific characteristics7,9 . | Pathophysiology10-12 . | Early detection12,13 . | Treatment specificities . | |
|---|---|---|---|---|---|---|
| Treatment-specific . | All survivors . | |||||
| HSCT with TBI | ×6.3 (×9.2 in females) | Increased severity of metabolic syndrome Lower incidence of obesity and lower abdominal circumference, higher triglycerides, and glucose level | Radiation induced alteration of subcutaneous adipose tissue (preadipocyte differentiation) Additional role of pancreatic radiation, testosterone, and growth hormone deficiency | Low-grade chronic inflammation Poor eating habits and reduced activity during prolonged periods Genetic predisposition | Regular monitoring: Blood pressure Abdominal circumference Fasting glucose, triglyceride, HDL- and LDL-cholesterol Potential interest of early biomarkers (adipokines)? | Few specific data Moderate effect of lifestyle modifications in the LEA experience |
| HSCT without TBI | ×2.2 | Usually less severe than after HSCT with TBI | Largely unknown Role of testosterone deficiency | |||
| Chemotherapy and CNS irradiation | ×2.3 | More frequent abdominal obesity Low incidence of hypertension | Important role of obesity Leptin resistance and overproduction (damaged hypothalamic receptors) Growth hormone deficiency | |||
| Chemotherapy without CNS irradiation | ×1.7 | More frequent hypertension (compared to CNS irradiation) | Largely unknown Uncertain long-term role of steroid and asparaginase | |||
| Therapeutic exposure . | Relative risk8,∗ . | Specific characteristics7,9 . | Pathophysiology10-12 . | Early detection12,13 . | Treatment specificities . | |
|---|---|---|---|---|---|---|
| Treatment-specific . | All survivors . | |||||
| HSCT with TBI | ×6.3 (×9.2 in females) | Increased severity of metabolic syndrome Lower incidence of obesity and lower abdominal circumference, higher triglycerides, and glucose level | Radiation induced alteration of subcutaneous adipose tissue (preadipocyte differentiation) Additional role of pancreatic radiation, testosterone, and growth hormone deficiency | Low-grade chronic inflammation Poor eating habits and reduced activity during prolonged periods Genetic predisposition | Regular monitoring: Blood pressure Abdominal circumference Fasting glucose, triglyceride, HDL- and LDL-cholesterol Potential interest of early biomarkers (adipokines)? | Few specific data Moderate effect of lifestyle modifications in the LEA experience |
| HSCT without TBI | ×2.2 | Usually less severe than after HSCT with TBI | Largely unknown Role of testosterone deficiency | |||
| Chemotherapy and CNS irradiation | ×2.3 | More frequent abdominal obesity Low incidence of hypertension | Important role of obesity Leptin resistance and overproduction (damaged hypothalamic receptors) Growth hormone deficiency | |||
| Chemotherapy without CNS irradiation | ×1.7 | More frequent hypertension (compared to CNS irradiation) | Largely unknown Uncertain long-term role of steroid and asparaginase | |||
CNS, central nervous system; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Compared with age- and sex-matched controls.
Regular monitoring of blood pressure, abdominal circumference, body mass index, as well as glycemic and lipid parameters should be included in long-term survivorship evaluations. In our experience, lifestyle modifications have a moderate effect on metabolic parameters in survivors of childhood leukemia diagnosed with metabolic syndrome. In these patients, endocrinology referral is required for evaluation and management. If nutritional and physical activity interventions do not correct the metabolic syndrome, appropriate medications should be prescribed to mitigate the cardiovascular and metabolic risks. Lipid-lowering medications include statins to prevent cardiovascular events. Antidiabetic medications primarily include metformin and glucagon-like-peptide 1 agonists, which are efficient therapies to treat diabetes associated with severe insulin resistance.
Secondary malignant neoplasm and meningioma
Secondary tumors are relatively rare (∼1%-2%) but severe long-term complications of childhood leukemia. These complications may occur rapidly after treatment (eg, secondary leukemia related to epipodophyllotoxin treatment associated with KMT2A rearrangement)17 or very late after the initial diagnosis. Continued patient follow-up suggests that the cumulative incidence of some secondary cancers such as meningioma, breast cancer, and other carcinomas does not plateau.18 The initial characteristics of primary leukemia are not related to the occurrence of secondary tumors. By contrast, therapeutic exposure is highly correlated with these complications. Patients treated with radiotherapy (TBI or CNS irradiation) have an increased risk of secondary tumors as described in the dedicated sections of this document. Chemotherapy with alkylating agents and epipodophyllotoxins are also risk factors of secondary cancers, primarily secondary leukemia. The prognosis of these secondary tumors varies. Some are associated with an adverse prognosis (eg, secondary myelodysplastic syndrome, AML, and nonmeningioma brain tumor). Other secondary tumors have a more favorable prognosis (eg, meningioma, Hodgkin lymphoma, thyroid carcinoma, and basal cell carcinoma), provided that the disease is diagnosed early. Long-term follow-up visits with clinical evaluation and screening tests for patients at high risk (eg, complete blood count monitoring, breast cancer screening, and thyroid ultrasound) may enable the detection of secondary tumors at an earlier stage. Other observations suggest that early oncological management may have a positive impact on the prognosis.19 Further research is needed to identify the molecular mechanisms and potential interventions to prevent or treat secondary cancers in survivors of pediatric leukemia.
Risk of long-term complications after specific therapeutic exposure
Clinical case
The patient is a 44-year-old woman. She was treated for relapsed ALL at age 6 years and received combination chemotherapy and HSCT. The preparation included a 12 Gy TBI in 6 fractions over 3 days with 8 Gy lung shielding. This treatment cured the leukemia. Several long-term complications affecting her quality of life were diagnosed during the follow-up program. She had normal puberty and menstruation. However, she had a miscarriage after her first pregnancy at age 20 years, which may have been associated with radiation-induced uterus damage. She was diagnosed with bilateral posterior subcapsular cataracts that did not require surgery. She also had a growth hormone deficiency with growth retardation. Despite hormone replacement, she stopped growing at a height of 153 cm. The neck palpation was normal. However, a systematic thyroid ultrasound identified several suspect thyroid nodules at age 26 years. A secondary thyroid cancer was diagnosed and treated using thyroidectomy and radioactive iodine treatment.
This patient had several long-term complications directly associated with HSCT and TBI. This case illustrates that therapeutic exposure is the key determinant of long-term complications in survivors of childhood leukemia (Table 3).
Major complications according to therapeutic exposure
| Therapeutic exposure . | Long-term complications . |
|---|---|
| TBI | Metabolic syndrome (lipodystrophy-like features), and overt metabolic and cardiovascular complications (myocardial infarction, stroke, diabetes), osteonecrosis, low bone mineral density, severe growth retardation, hypothyroidism, secondary malignant neoplasms and meningioma, ovarian/testicular hormone deficiency, reduced ovarian follicular pool, impaired spermatogenesis, uterine toxicity, cataract, chronic renal insufficiency |
| High-dose alkylating agents (primarily busulfan-based conditioning) | Complications overlap with TBI (notably fertility defects); less frequent metabolic syndrome, secondary malignant neoplasms and meningioma, growth retardation and cataracts; increased risk of persistent partial alopecia |
| HSCT (any conditioning regimen) | Bronchiolitis obliterans, chronic graft-versus-host disease |
| CNS irradiation | Metabolic syndrome, obesity, endocrine complications (central hypothyroidism, gonadotropin deficiency, precocious puberty), growth retardation, neurocognitive defects, secondary malignant neoplasms and meningioma, cataracts |
| Anthracyclines and anthraquinones | Anthracycline-related cardiomyopathy |
| High-dose corticosteroids | Osteonecrosis, cataracts |
| Therapeutic exposure . | Long-term complications . |
|---|---|
| TBI | Metabolic syndrome (lipodystrophy-like features), and overt metabolic and cardiovascular complications (myocardial infarction, stroke, diabetes), osteonecrosis, low bone mineral density, severe growth retardation, hypothyroidism, secondary malignant neoplasms and meningioma, ovarian/testicular hormone deficiency, reduced ovarian follicular pool, impaired spermatogenesis, uterine toxicity, cataract, chronic renal insufficiency |
| High-dose alkylating agents (primarily busulfan-based conditioning) | Complications overlap with TBI (notably fertility defects); less frequent metabolic syndrome, secondary malignant neoplasms and meningioma, growth retardation and cataracts; increased risk of persistent partial alopecia |
| HSCT (any conditioning regimen) | Bronchiolitis obliterans, chronic graft-versus-host disease |
| CNS irradiation | Metabolic syndrome, obesity, endocrine complications (central hypothyroidism, gonadotropin deficiency, precocious puberty), growth retardation, neurocognitive defects, secondary malignant neoplasms and meningioma, cataracts |
| Anthracyclines and anthraquinones | Anthracycline-related cardiomyopathy |
| High-dose corticosteroids | Osteonecrosis, cataracts |
TBI
TBI has the most toxic profile of all antileukemia therapies. However, in children aged >4 years with ALL, TBI has been associated with improved overall survival and lower relapse risk than the chemotherapy-only conditioning.20 TBI is generally not used in children aged <2 to 4 years due to fear of major toxicity. Most patients now receive a fractionated schedule of 12 Gy over 3 days and 6 fractions with lung shielding at 8 Gy, but some patients treated during the 1980s may have received less fractionated schedules associated with increased toxicity. The long-term survivors of childhood leukemia treated with TBI require life-long follow-up, because they are at risk of various complications. Our recommendations for the surveillance of patients with childhood leukemia treated with TBI are described in Table 4. These recommendations are based on the data from the French LEA cohort and largely overlap with the Children Oncology Group and International Guideline Harmonization Group/PanCare guidelines.
Surveillance of patients treated with TBI
| System or organ involved . | Complications and screening method . |
|---|---|
| Growth | Failure to thrive: weight and height measurements compared against previous values and values of children of the same age using growth charts (yearly), pediatric endocrinology referral as clinically indicated |
| Puberty, fertility | Pubertal delay: Tanner stages including testicular volume evaluation in boys (yearly until sexually mature); ovarian/testicular hormone deficiency: FSH, LH, estradiol/testosterone levels at adolescence |
| Brain | Meningiomas and other CNS tumors: brain MRI (or CT) as clinically indicated; neurodevelopmental defects: referral for neurodevelopmental evaluation as clinically indicated |
| Skin | Skin cancer (basal cell carcinomas, melanomas): dermatological examination (yearly) |
| Breast | Breast cancer: education to breast autopalpation, mammogram and breast MRI (yearly after age 25 y) |
| Heart | Anthracycline-related cardiomyopathy: echocardiography with global longitudinal strain (every 2 y) |
| Thyroid | Thyroid cancer: neck palpation (yearly) and thyroid US (every 2 y), oncological referral in case of thyroid nodule; thyroid function: TSH, FT4 (yearly) |
| Lungs | Pulmonary function defects: pulmonary function tests including DLCO and spirometry (at entry into long-term follow-up, repeat as clinically indicated); lung cancer: pulmonary examination, consider lung spiral CT in high-risk patients (other risk factors such as smoking) |
| Metabolic and cardiovascular risk | Metabolic syndrome: blood pressure, waist circumference, body mass index, fasting triglyceride, total, LDL- and HDL-cholesterol, glucose, and insulin levels, HbA1c level (yearly from adulthood) |
| Eyes | Cataracts: ophthalmology referral for slit-lamp examination (every 2 y) |
| Oral health | Dental defects: dental care (yearly) |
| Liver | Liver toxicity: physical examination, AST, ALT, GGT, bilirubin (at entry into long-term follow-up, repeat as clinically indicated) |
| Kidney | Renal toxicity: creatinine, electrolytes including Ca, P, and Mg, urinary tests (at entry into long-term follow-up, repeat as clinically indicated) |
| System or organ involved . | Complications and screening method . |
|---|---|
| Growth | Failure to thrive: weight and height measurements compared against previous values and values of children of the same age using growth charts (yearly), pediatric endocrinology referral as clinically indicated |
| Puberty, fertility | Pubertal delay: Tanner stages including testicular volume evaluation in boys (yearly until sexually mature); ovarian/testicular hormone deficiency: FSH, LH, estradiol/testosterone levels at adolescence |
| Brain | Meningiomas and other CNS tumors: brain MRI (or CT) as clinically indicated; neurodevelopmental defects: referral for neurodevelopmental evaluation as clinically indicated |
| Skin | Skin cancer (basal cell carcinomas, melanomas): dermatological examination (yearly) |
| Breast | Breast cancer: education to breast autopalpation, mammogram and breast MRI (yearly after age 25 y) |
| Heart | Anthracycline-related cardiomyopathy: echocardiography with global longitudinal strain (every 2 y) |
| Thyroid | Thyroid cancer: neck palpation (yearly) and thyroid US (every 2 y), oncological referral in case of thyroid nodule; thyroid function: TSH, FT4 (yearly) |
| Lungs | Pulmonary function defects: pulmonary function tests including DLCO and spirometry (at entry into long-term follow-up, repeat as clinically indicated); lung cancer: pulmonary examination, consider lung spiral CT in high-risk patients (other risk factors such as smoking) |
| Metabolic and cardiovascular risk | Metabolic syndrome: blood pressure, waist circumference, body mass index, fasting triglyceride, total, LDL- and HDL-cholesterol, glucose, and insulin levels, HbA1c level (yearly from adulthood) |
| Eyes | Cataracts: ophthalmology referral for slit-lamp examination (every 2 y) |
| Oral health | Dental defects: dental care (yearly) |
| Liver | Liver toxicity: physical examination, AST, ALT, GGT, bilirubin (at entry into long-term follow-up, repeat as clinically indicated) |
| Kidney | Renal toxicity: creatinine, electrolytes including Ca, P, and Mg, urinary tests (at entry into long-term follow-up, repeat as clinically indicated) |
AMH, anti-Mullerian hormone; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CT, computed tomography; DLCO, carbon monoxide diffusing capacity; FSH, follicle-stimulating hormone; FT4, thyroxin; GGT, gamma-glutamyl transferase; Hb, hemoglobin; HDL, high-density lipoprotein; LH, luteinizing hormone; TSH, thyroid-stimulating hormone; US, ultrasound.
Patients treated with TBI are at high risk of metabolic syndrome. Notably, many adults who have received TBI have dyslipidemia, insulin resistance, and ectopic fat accumulation including nonalcoholic fatty liver disease.8 These lipodystrophy-like features are associated with altered gene expression profiles of the subcutaneous adipose tissue and the alteration of preadipocyte differentiation.10
The risk of secondary cancer (including thyroid papillary carcinomas, breast cancer, skin cancer, sarcoma, meningioma, and other brain tumors) is increased in patients who received TBI. The risk of breast cancer should be particularly considered in women.21 Breast cancer usually occurs very late (>20 years) after initial diagnosis with a relative risk of ∼20 compared with the general population.22
Patients treated with TBI are at high risk of premature cataracts.23 High-dose corticosteroids and CNS irradiation are other demonstrated risk factors.24 Posterior subcapsular cataracts without clinically significant visual symptoms are the most frequent forms. However, cataracts can rarely manifest with decreased visual acuity, halos, or diplopia. Ophthalmologic consultation with slit-lamp examination is required for the diagnosis. Patients with severe visual discomfort should be treated with surgical replacement of the opaque lens with an intraocular implant.
Patients treated with TBI are at risk of hypothyroidism, especially when treated at a young age.25 Patients with hypothyroidism should be referred to endocrinology and appropriately supplemented.
Height growth after HSCT requires careful assessment. The risk of decreased growth velocity mainly depends on age, sex, and conditioning regimen. The median final height of adults included in the French LEA program who received TBI for ALL before age 9 years for girls and before 11 years for boys was 153 cm and 163 cm, respectively. After busulfan-based conditioning regimen, the median final height was 162 cm and 170 cm, respectively. Appropriate hormonal replacement therapy may improve the final height of patients with documented growth hormone deficiency after HSCT.26 Height growth and final height are usually little to unaffected in patients treated with chemotherapy alone.27
Gonadal toxicity is very frequent after a myeloablative conditioning regimen and is influenced by age at HSCT and sex (Table 5). Infertility is clearly associated with premature ovarian insufficiency, with most risk factors shared between these 2 complications. However, some women who required puberty induction for apparent immediate premature ovarian insufficiency may experience spontaneous and sometimes undesired pregnancies.28 In addition to ovarian function, uterine volume and function are also determinants of fertility. Adult women who received HSCT during childhood often have a reduced uterus volume.29 The reduced uterus volume may be associated with significant pregnancy and delivery issues. Data from our group showed that less than half of spontaneous pregnancies result in live births due to a high rate of miscarriages and abortions.28 All pregnancies after HSCT should be considered at risk of unfavorable obstetric outcome and require adapted follow-up. Pre-HSCT ovarian cryopreservation should be discussed with patients according to age and after considering the potential increased risk of premature ovarian failure associated with ovariectomy.28 Two-thirds of men who received a 12 Gy fractionated TBI during childhood will have total or partial testosterone deficiency, with a higher risk for older boys at the time of HSCT. After a busulfan-based conditioning, more than half will retain normal testosterone production without any effect of age at the time of therapy.11
Gonadal hormone function and uterine volume after HSCT according to the conditioning regimen
| Sex/age at HSCT . | Parameter . | Outcome . | Busulfan–based conditioning regimen . | TBI–based conditioning regimen . |
|---|---|---|---|---|
| Adult women who underwent transplantation before puberty | Ovarian hormone function28 | Immediate POI∗ (pubertal induction required) | 60% | |
| Spontaneous menarche with secondary POI during the 10 y after puberty | 20% | |||
| Spontaneous menarche without POI at 10 y after puberty | 20% | |||
| Favorable risk factor | Younger age at HSCT | |||
| Uterus volume29 | Uterine volume† (vs general population) | 45% volume reduction | 75% volume reduction | |
| Favorable risk factor | Hormone level‡ and younger age at HSCT | None | ||
| Adult men | Leydig cell function11 | Total testosterone deficiency§ | 25% | 45% |
| Partial testosterone deficiency | 15% | 15% | ||
| No testosterone deficiency | 60% | 35% | ||
| Favorable risk factor | Older at HSCT | |||
| Sex/age at HSCT . | Parameter . | Outcome . | Busulfan–based conditioning regimen . | TBI–based conditioning regimen . |
|---|---|---|---|---|
| Adult women who underwent transplantation before puberty | Ovarian hormone function28 | Immediate POI∗ (pubertal induction required) | 60% | |
| Spontaneous menarche with secondary POI during the 10 y after puberty | 20% | |||
| Spontaneous menarche without POI at 10 y after puberty | 20% | |||
| Favorable risk factor | Younger age at HSCT | |||
| Uterus volume29 | Uterine volume† (vs general population) | 45% volume reduction | 75% volume reduction | |
| Favorable risk factor | Hormone level‡ and younger age at HSCT | None | ||
| Adult men | Leydig cell function11 | Total testosterone deficiency§ | 25% | 45% |
| Partial testosterone deficiency | 15% | 15% | ||
| No testosterone deficiency | 60% | 35% | ||
| Favorable risk factor | Older at HSCT | |||
The data presented are from the LEA program.
Premature ovarian insufficiency (POI) is defined as the association of an oligo/amenorrhea for at least 4 months and an elevated follicle-stimulating hormone (FSH) level >25 IU/L on 2 occasions at 4 weeks apart before age 40 years of age.
Using MRI evaluation.
Either normal ovarian function or hormone replacement therapy.
Total testosterone deficiency was defined as a testosterone level <12 nmol/L or testosterone replacement therapy. Partial testosterone deficiency was defined as normal testosterone levels with elevated luteinizing hormone (LH) levels >10 IU/L. Leydig-cell function was considered normal when testosterone and LH levels were normal without hormone substitution.
HSCT
The range of complications after busulfan-based myeloablative conditioning regimen largely overlaps with those observed after TBI, particularly for the risk of gonadal toxicity. However, many late effects such as metabolic syndrome, secondary cancer, height growth impairment, and cataracts are less frequent, leading to an overall lower burden of health conditions instead of an increased risk of permanent partial alopecia.26,30
Chronic graft-versus-host disease (GVHD) is a graft immune response against host organs primarily mediated by T lymphocytes. The severity of chronic GVHD can vary from mild defects to severe organ injury and can affect several organs or systems. Pediatric chronic GVHD tends to be less frequent and severe than in adults.31,32 However, extensive data on chronic GVHD in children are lacking, resulting in limited understanding of disease occurrence and the impact on childhood survivors. Based on data from the LEA cohort, chronic GVHD affects ∼25% of patients who received transplantation and persists long-term in ∼20% of patients.33 The most frequently affected organs include the eyes, skin, mouth, and lungs. Severe forms of long-term chronic GVHD represent ∼20% of cases. These severe forms include a lung defect characterized by airflow obstruction called bronchiolitis obliterans. Alloimmune reaction results in bronchioles inflammation, fibrosis, and irreversible constriction of the terminal air passages. Transplantation during adolescence and second transplantation constitute risk factors of chronic GVHD. Chronic GVHD significantly alters the long-term physical and psychological aspects of quality of life.
CNS irradiation
Historically, CNS irradiation has resulted in a major improvement in cure rates for childhood ALL by efficiently preventing CNS relapse. Cranial or craniospinal fields were considered with doses commonly ranging from 12 to 24 Gy. To minimize long-term sequelae, CNS irradiation has been nearly eliminated from most modern ALL protocols, although patients with CNS relapse still require this therapy. The main long-term complications after CNS radiation include metabolic syndrome, precocious puberty, height growth retardation, neurocognitive impairments, and secondary brain, skin, and thyroid tumors.34 Metabolic syndrome observed after CNS irradiation is usually associated with obesity, thus suggesting a unique pathophysiology.35 The neuropsychological effects of CNS radiation have been extensively studied. The most severe impairments are observed in survivors treated with higher radiation doses (ie, 24-28 Gy).36-38 CNS irradiation impairs height growth, depending on the radiation dose and field.39,40
Steroids
Osteonecrosis is the main long-term complication caused by the administration of high-dose corticosteroids.41,42 This complication particularly affects adolescents. Female sex is also a risk factor for osteonecrosis. The diagnosis is confirmed using magnetic resonance imaging (MRI) centered on the affected structure. Osteonecrosis frequently affects weight-bearing joints and multiple sites. Specific orthopedic management is difficult and can range from simple monitoring to joint replacement.43 This complication generally occurs during or within several months after treatment. It is frequently responsible for long-term abnormal joint mobility, pain, activity limitations, and altered quality of life.44,45 Genetic studies have shown an association between osteonecrosis and constitutional variants near genes involved in bone metabolism, glutamate metabolism, endothelial cell function, angiogenesis, and cellular migration such as glutamate receptor genes and NWD2 gene.46,47 Screening for osteonecrosis using serial MRI during leukemia therapy may be investigated to detect actionable early signs of this complication.
Anthracycline-related cardiomyopathy
Anthracyclines and anthraquinones are antileukemia drugs used to treat almost all children with acute leukemia. Survivors treated with these drugs are at risk of developing cardiomyopathy.48-50 A strong dose-dependent relationship has been observed between anthracycline chemotherapy exposure and risk of cardiomyopathy. Anthracycline and anthraquinone doses are usually calculated as the doxorubicin-equivalent dose, applying cardiotoxicity conversion ratios (Table 6).51 The risk is markedly increased when the cumulative anthracycline dose is >250 mg/m2.58 Younger children are at higher risk of anthracycline-induced cardiomyopathy.52,53 The probable additive cardiotoxic effect of combined anthracycline and TBI exposure is still not well documented.54,55 Variants in genes involved in anthracycline transportation and metabolism, generation of reactive oxygen species, and cardiac diseases have been associated with cardiotoxicity, thus suggesting a genetic predisposition to this condition. Anthracycline-related cardiomyopathy is a progressive disorder with a natural history that frequently involves an asymptomatic phase. During this phase, the asymptomatic cardiomyopathy can be diagnosed using echocardiography, which is recommended every 2 to 5 years after anthracycline exposure.59 Echocardiographic monitoring should include global longitudinal strain, which is a more sensitive technique to diagnose cardiac dysfunction.60 Discontinuation of follow-up after a given monitoring time is not recommended because cardiopathy can occur many years after anthracycline exposure.61 Cardio-protectants such as dexrazoxane may minimize the long-term cardiac toxicity56 without affecting relapse or secondary cancer risk.57 Patients who plan to engage in high-intensity exercise or who have symptomatic or asymptomatic cardiomyopathy should consult a cardiologist.
Anthracycline-related cardiomyopathy
| Risk factors, pathophysiology, prevention, management, and current research efforts . | |
|---|---|
| Main risk factor: anthracycline and anthraquinone exposure51 | The risk for cardiac dysfunction increases with anthracycline dose Cardiotoxicity conversion ratios: doxorubicin: 1 (reference), daunorubicin: 0.6, epirubicin: 0.8, idarubicin: 5, mitoxantrone: 10.5 |
| Other risk factors52-55 | Younger age at anthracycline and anthraquinone exposure Female sex Chest radiation exposure (TBI?) |
| Pathophysiology48-50 | Single cell myocytolysis → patchy myocardial necrosis → focal myocardial fibrosis → multifocal myocardial fibrosis (clinically overt congestive heart failure) Genetic predisposition |
| Clinical classification48-50 | Acute cardiotoxicity (withing weeks of anthracycline and anthraquinone exposure; impaired myocardial contractility) Early-onset chronic cardiotoxicity (<1 y after leukemia therapy completion; dilated/hypokinetic cardiomyopathy) Late-onset chronic cardiotoxicity (>1 y after leukemia therapy completion; dilated/hypokinetic cardiomyopathy) |
| Cardioprotection56,57 | Limiting anthracycline/anthraquinone cumulated dose Use of cardioprotectant drug: dexrazoxane Use of liposome-encapsulated versions of anthracyclines |
| Interventions for cardiomyopathy48-50 | Early use of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, or beta-blockers (may prevent severe contractile dysfunction and improve cardiac function) |
| Current research efforts49 | Early detection of anthracycline-related cardiomyopathy Polygenic risk scores Patient-specific hiPSC-CMs |
| Risk factors, pathophysiology, prevention, management, and current research efforts . | |
|---|---|
| Main risk factor: anthracycline and anthraquinone exposure51 | The risk for cardiac dysfunction increases with anthracycline dose Cardiotoxicity conversion ratios: doxorubicin: 1 (reference), daunorubicin: 0.6, epirubicin: 0.8, idarubicin: 5, mitoxantrone: 10.5 |
| Other risk factors52-55 | Younger age at anthracycline and anthraquinone exposure Female sex Chest radiation exposure (TBI?) |
| Pathophysiology48-50 | Single cell myocytolysis → patchy myocardial necrosis → focal myocardial fibrosis → multifocal myocardial fibrosis (clinically overt congestive heart failure) Genetic predisposition |
| Clinical classification48-50 | Acute cardiotoxicity (withing weeks of anthracycline and anthraquinone exposure; impaired myocardial contractility) Early-onset chronic cardiotoxicity (<1 y after leukemia therapy completion; dilated/hypokinetic cardiomyopathy) Late-onset chronic cardiotoxicity (>1 y after leukemia therapy completion; dilated/hypokinetic cardiomyopathy) |
| Cardioprotection56,57 | Limiting anthracycline/anthraquinone cumulated dose Use of cardioprotectant drug: dexrazoxane Use of liposome-encapsulated versions of anthracyclines |
| Interventions for cardiomyopathy48-50 | Early use of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, or beta-blockers (may prevent severe contractile dysfunction and improve cardiac function) |
| Current research efforts49 | Early detection of anthracycline-related cardiomyopathy Polygenic risk scores Patient-specific hiPSC-CMs |
hiPSC-CMs, human induced pluripotent stem cell–derived cardiomyocytes.
Transfusions
Patients who underwent blood product transfusion before 1970 to 1995 (depending on the virus and the country) are susceptible to potential HIV, hepatitis C virus, or hepatitis B virus infection.62 Advancements in the safety measures implemented throughout the blood transfusion process have now nearly eliminated the risk of viral contamination.
Iron overload is a common complication of red blood cell transfusions in survivors of childhood leukemia. Prevalence rates are higher for those who undergo HSCT and those who receive TBI.63,64 Other factors that contribute to the risk include older age at the time of HSCT and intensity of chemotherapy (eg, AML or high-risk ALL treatment). The long-term impact of iron overload in survivors of childhood leukemia is unknown, but it may exacerbate cardiac, hepatic, and metabolic toxicities. When iron overload is suspected (>20 red blood cell transfusions, ferritin levels >1000 ng/mL, and HSCT), iron liver content should be evaluated using T2∗ MRI. Confirmed iron overload can be efficiently treated using phlebotomy after hematological recovery (eg, 10 mL/kg with a maximum of 500 mL every 3 weeks for 1 year). Oral iron chelators such as deferasirox constitute an alternative for patients with phlebotomy contraindication (eg, anemia, cardiovascular instability, and pregnancy) or poor peripheral venous access.
How do patient clinical characteristics and environmental factors modulate the risk of long-term complications?
Clinical case
A 10-and-a-half-year-old girl was diagnosed with KMT2A-rearranged ALL. She had a relapse on therapy at the age of 11 treated with second-line chemotherapy and HSCT. Transplantation conditioning involved TBI. Ovarian cryopreservation was performed before HSCT. Long-term follow-up included monitoring by a pediatric endocrinologist. She developed a premature ovarian insufficiency, necessitating hormonal treatment for puberty induction. By the age of 20, despite 12 months of regular and unprotected sexual intercourse with her partner, she had not achieved pregnancy. She faced various social challenges, including unemployment, which delayed access to medically assisted reproduction. This case demonstrates the role of patient clinical characteristics and environmental factors in the occurrence and management of long-term complications.
Age and sex
Age at HSCT and sex are major determinants of the risk of decreased growth velocity after HSCT, as described in the previous section of the manuscript. For young children who have undergone transplantation, growth velocity is often near-normal until pubertal age, although the peripubertal growth spurt is very frequently impaired.65,66
Age at HSCT and sex have a major impact on gonadal toxicity after HSCT; 65% of girls aged <5 years at the time of HSCT had spontaneous menarche, whereas 85% of prepubertal girls aged >10 years at the time of HSCT suffered premature ovarian insufficiency requiring hormone treatment to induce puberty.28 In patients who received HSCT after puberty, gonadal toxicity was even higher.67 Careful evaluation by a pediatric endocrinologist is crucial to manage the complex interaction between abnormalities of puberty, gonadal dysfunction, growth hormone deficiency, and peripubertal growth spurt.
Infants with leukemia face heightened vulnerability primarily due to the aggressive nature of the disease, with leukemia relapse being the predominant cause of mortality in this population.68 Those who achieve long-term survival are often expected to be at higher risk of severe long-term complications. However, patients who received chemotherapy or HSCT during the first years of life during infancy did not show a significantly increased risk of most late sequelae compared with older children.68,69 Children aged <6 months at the time of diagnosis probably require special consideration because of an increased risk of growth failure, underweight, and thyroid dysfunction.
Osteonecrosis particularly affects adolescents. This may be attributed to a specific bone vulnerability due to epiphyseal closure, the procoagulant effects of sex hormones, and increased bone metabolic activity secondary to the peak of growth hormone and insulin-like growth factor 1 during puberty.44
Underlying diseases
When compared with other survivors of leukemia, patients with Down syndrome have a higher risk of cataracts, hypothyroidism, obesity, and bone mineral density abnormalities.70 Most observed complications are at least partly related to the constitutional disease itself rather than leukemia therapy.
Patients with constitutional telomere disease receiving HSCT for leukemia are at high risk of late life-threatening complications, including pulmonary complications, liver failure, and microangiopathy.71
Patients with ataxia telangiectasia and Li-Fraumeni syndrome are predisposed to secondary malignant neoplasms.72,73 Constitutional (including synonymous) and somatic TP53 variants are enriched in survivors with secondary cancers who do not necessarily fulfill the classic Li-Fraumeni syndrome criteria.74 Constitutional TP53 variants may identify patients at high risk of secondary malignant cancers that would benefit from adapted surveillance program. There is currently insufficient data to support reduction of treatment intensity in these patients, notably regarding HSCT conditioning. However, unnecessary radiation should be avoided, and alternative approaches (such as immunotherapy) should be further evaluated.
Children with Fanconi anemia who survive leukemia therapy and/or allogeneic HSCT are at high risk of a wide range of secondary malignant neoplasms, including vulvar, vaginal, and head and neck squamous cell carcinoma.75 Frequent gynecologic examinations, as well as head and neck examinations (eg, every 6 months), including careful oral cavity evaluations and flexible laryngoscopy should be performed in these patients. To minimize the long-term toxicity of HSCT, these patients receive specific low-intensity fludarabine-based conditioning regimen.
Modifiable risk factors
Leukemia survivorship shares several medical risks with smoking, alcohol consumption, unhealthy diet, and physical inactivity. Shared risks include cardiovascular complications, pulmonary disorders, and cancer development, which the survivors treated with HSCT are particularly susceptible to. Survivors include a substantial proportion of consistent smokers.76 The survivors of childhood cancers with healthy lifestyle and no cardiovascular risk factors are at reduced risk of late mortality,6 although it remains unclear whether these modifiable risk factors carry more than additive risks. Preventing smoking and alcohol use, as well as implementing physical activity and nutritional intervention are important aspects of health promotion within this population.
Socioeconomic determinants
Socioeconomic and racial parameters have been increasingly recognized as determinants of long-term health and loss to follow-up of survivors of childhood leukemia. Among survivors of childhood cancer, loss to follow-up rates were higher among patients from ethnic minority groups and those who resided in areas associated with lower socioeconomic status at the time of diagnosis.77 This may jeopardize their long-term survival, treatment-related health conditions, and quality of life. Neighborhood deprivation was also independently associated with overweight and obesity in survivors of childhood leukemia, particularly those in the highest quartile of the area deprivation index.78 Collecting comprehensive information on social determinants of health is important to target interventions to improve long-term follow-up and health among disadvantaged survivors of childhood leukemia.
Challenges and unmet needs
Surveillance of patients at lower risk of long-term complications
Patients at lower risk of long-term complications are those treated with chemotherapy alone, without intensive therapy for relapse, CNS irradiation, or HSCT. The cumulative dose of anthracyclines should also be taken into account, with lowest risk among those treated with a cumulative anthracycline dose of <100 mg/m2.58 The first 10 years after diagnosis is the period of greatest risk for complications.79 After 10 years postdiagnosis, cardiovascular risk factors and cardiotoxicity are the most frequent and severe long-term complications.79 However, the optimal modalities of long-term follow-up in these patients remain undetermined.
Organization of long-term follow-up and transition
Organization of long-term follow-up and transition practices are widely heterogeneous and largely depend on the community setting or country-wide health care systems.80 American and European guidelines have been developed (eg, Children Oncology Group and International Guideline Harmonization Group/PanCare guidelines) and inform risk stratification and follow-up. The French leukemia survivor follow-up program LEA consists of dedicated follow-up visits organized in pediatric centers. The visits start 1 year after leukemia therapy and repeat every 2 to 4 years thereafter. During the initial visit, the risk of long-term complications is stratified based on factors such as leukemia type, therapeutic exposure, and age at the time of evaluation. Every visit includes a clinical evaluation with appropriate laboratory analysis and imaging. Specific questionnaires are used to evaluate the patient’s quality of life as well as the socioeconomic factors of the patient and their family. Patients may be referred to pediatric or adult subspecialists for diagnosis and treatment of complications (eg, cataracts and cardiomyopathy). Patients at risk of multiple late complications (eg, patients who received irradiation or transplantation) greatly benefit from specialized follow-up centers that can provide the multiple expertise needed for their management. Transition to an adult-focused provider and environment is a standard in most settings.81 However, transition is challenging and several barriers have been identified,82 including social issues (eg, health insurance that does not cover long-term follow-up), the lack of adult providers familiar with survivorship care, and insufficient knowledge and empowerment among survivors.
Surveillance of investigational or recently approved therapies
Long-term follow-up of children and adolescents treated with investigational or recently approved therapies is crucial. However, the lack of long-term safety data may result in inconsistent screening practices. It is essential to establish a comprehensive follow-up program for these patients. Tyrosine kinase inhibitors, primarily imatinib, are administered to children and adolescents with chronic myeloid leukemia and Philadelphia-positive ALL. Several safety concerns regarding these drugs have been identified, including growth alterations and bone metabolism abnormalities.83 The impact on growth is most notable in children who initiate imatinib therapy before puberty.84 Recent reports have indicated that survivors with prolonged exposure to tyrosine kinase inhibitors may be at risk of specific pulmonary outcomes and pericardial effusion, which are typically reversible. There is currently no available long-term safety data for chimeric antigen receptor (CAR) T cells and T-cell engagers (eg, blinatumomab), which are increasingly used to treat children with ALL. It is crucial to monitor patients treated with immunotherapies, especially those who experience neurotoxicity. CD19-directed CAR T cells may be associated with prolonged B-cell aplasia with hypogammaglobulinemia requiring immunoglobulin replacement therapy. Recently, T-cell malignancies, including CAR-positive lymphoma, in patients treated with CD19-directed CAR T cells were reported to be under investigation.85 Treosulfan is an alkylating agent that is increasingly used in conditioning regimens for HSCT. Regarding long-term effects, data suggest that treosulfan-based conditioning may have a limited gonadotoxicity compared with busulfan.86 The follow-up program of patients treated with treosulfan should be similar to that proposed for patients who received other transplantations.
Conclusion
Because therapy and cure rates of childhood leukemia have made significant headway, health care objectives have expanded to improve long-term health and quality of life. The implementation of comprehensive follow-up programs plays a crucial role in childhood leukemia survivor care, enabling early detection and treatment of long-term complications. Risk-adapted therapies have reduced the use of HSCT, radiotherapy, and high-intensity chemotherapy, which are associated with increased long-term toxicity. However, these approaches remain essential to cure patients with aggressive disease. Innovative therapies (eg, antibody-drug conjugates and immunotherapy) are expected to have a better long-term safety profile, although diligent long-term toxicity monitoring is necessary. Advances in understanding genetic susceptibility to long-term complications hold promise regarding tailored therapeutic strategies for leukemia treatment and optimizing follow-up programs.
Acknowledgments
The authors thank Pascal Auquier, the LEA investigators, and the patients and families involved in the LEA survivorship program, Sophie Beliard and Blandine Courbière for insightful discussions on metabolic syndrome and gonadal toxicity, and Sandra Moore for revising the paper.
Authorship
Contribution: P.S. and G.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul Saultier, Department of Pediatric Hematology, Immunology and Oncology, La Timone Children’s Hospital, APHM and Aix Marseille Université, 264 rue Saint-Pierre, 13385 Marseille, France; email: paul.saultier@ap-hm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal