Eosinophils promote arterial thrombosis in hematopoietic LNK deficiency.
Eosinophil LNK is essential in suppression of arterial thrombosis.
Visual Abstract
Increased eosinophil counts are associated with cardiovascular disease and may be an independent predictor of major cardiovascular events. However, the causality and underlying mechanisms are poorly understood. Genome-wide association studies have shown an association of a common LNK variant (R262W, T allele) with eosinophilia and atherothrombotic disorders. LNK(TT) reduces LNK function, and Lnk-deficient mice display accelerated atherosclerosis and thrombosis. This study was undertaken to assess the role of eosinophils in arterial thrombosis in mice with hematopoietic Lnk deficiency. Hematopoietic Lnk deficiency increased circulating and activated eosinophils, JAK/STAT signaling in eosinophils, and carotid arterial thrombosis with increased eosinophil abundance and extracellular trap formation (EETosis) in thrombi. Depletion of eosinophils by anti–Siglec-F antibody or by the ΔdbIGata1 mutation eliminated eosinophils in thrombi and markedly reduced thrombosis in mice with hematopoietic Lnk deficiency but not in control mice. Eosinophil depletion reduced neutrophil abundance and NETosis in thrombi without altering circulating neutrophil counts. To assess the role of Lnk specifically in eosinophils, we crossed Lnkf/f mice with eoCre mice. LnkΔeos mice displayed isolated eosinophilia, increased eosinophil activation, and accelerated arterial thrombosis associated with increased EETosis and NETosis in thrombi. DNase I infusion abolished EETs and neutrophil extracellular traps (NETs) in thrombi and reversed the accelerated thrombosis. Human induced pluripotent stem cell–derived LNK(TT) eosinophils showed increased activation and EETosis relative to isogenic LNK(CC) eosinophils, demonstrating human relevance. These studies show a direct link between eosinophilia, EETosis, and atherothrombosis in hematopoietic Lnk deficiency and an essential role of eosinophil LNK in suppression of arterial thrombosis.
Introduction
Atherothrombotic cardiovascular disease (ACD) is the major cause of morbidity and mortality in the United States.1 Residual cardiovascular risk remains substantial even in clinical trials with marked low-density lipoprotein cholesterol reduction,2-4 pointing to the need to understand the remaining risk factors and underlying mechanisms and to develop new treatments. Innate immunity and inflammation have long been implicated in ACD, and recent clinical trials have validated the critical role of innate immunity and inflammation in ACD.5,6 Leukocytosis is associated with increased risk of ACD,7-10 and monocytes and neutrophils are key effector cells in atherosclerosis and atherothrombosis. Although representing only a small fraction of leukocytes, eosinophils are important in innate immunity, and dysregulated eosinophil production and activity are common in eosinophilic inflammatory disorders.11 Observational evidence indicates association of eosinophilia with an increased risk of ACD in general populations.12,13 Eosinophil cationic protein is a marker of eosinophil activation and degranulation, and blood eosinophil cationic protein levels have been linked to ACD, including coronary heart disease and ischemic stroke.9,14 Patients with hypereosinophilia also suffer from an increased incidence of atypical thrombotic events.15,16 However, the causality has been controversial.17-19
Genome-wide association studies have identified an association of a single nucleotide polymorphism (SNP) in SH2B3/LNK, rs3184504 (T allele) with increased risk of cardiovascular disease, including myocardial infarction10 and venous thrombosis.20 The risk SNP in LNK causes a nonconservative amino acid change at position 262 (R262W); the same SNP is associated with thrombocytosis and leukocytosis, suggesting that the risk allele of LNK might worsen ACD via effects on hematopoiesis and platelet and neutrophil activation. LNK functions as a negative regulator of cytokine signaling and cell proliferation. Targeted deletion of Lnk in mice causes expansion of hematopoietic stem cells and increased myelopoiesis, thrombocytosis, and leukocytosis,21-23 due to a lack of negative-feedback regulation of thrombopoietin on its receptor myeloproliferative leukemia (MPL).21 LNK(R262W) is a loss of function LNK variant.24,25 Our studies in hematopoietic LNK-deficient mice linked excess myelopoiesis, platelet overproduction and activation, and platelet interaction with leukocytes to accelerated atherogenesis and thrombosis.24 More recently, we showed the essential role of enhanced NETosis in accelerated arterial thrombosis in Lnk−/− mice, which involved priming of neutrophils for NETosis by oxidized phospholipids (OxPLs) from activated platelets.25
Human genome wide association studies have shown that for white blood cells (WBCs) increased in LNK(R262W) carriers, the effect on the size of eosinophils is most pronounced.10 However, the potential role of eosinophils in the LNK(R262W)-associated risk of ACD has not been evaluated. In this study, we sought to assess the role of eosinophils in arterial thrombosis in mice with hematopoietic Lnk deficiency. Using both genetic and pharmacological approach to deplete eosinophils, we show that eosinophils promote arterial thrombosis by enhancing extracellular trap formation (EETosis) and NETosis in LNK deficiency. Moreover, using a newly developed Lnkf/f mouse model, we show that deletion of Lnk specifically in eosinophils accelerates arterial thrombosis in association with increased EETs and NETs in thrombi. Treatment with DNase I eliminates EETs and NETs in thrombi and reverses the accelerated thrombosis in eosinophil specific Lnk deficiency.
Materials and methods
On reasonable request to the corresponding author, the data, analytic methods, and study materials will be made available to other researchers. All supporting data are available within the article and in the supplemental Expanded methods, available on the Blood website. Institutional review board approval was obtained for this study where applicable.
Patient samples were obtained with informed consent under protocols approved by a local institutional review board at Memorial Sloan Kettering Cancer Center, the Icahn School of Medicine, Mount Sinai, New York, NY, and the University of Patras Medical School.
Animals
Wild-type (WT; C57BL/6J), Ldlr−/− (B6.129S7-Ldlrtm1Her) and ΔdblGATA (C.129S1(B6)-Gata1tm6Sho/J) mice were purchased from The Jackson Laboratory. eoCre has been generated by knockin of Cre recombinase in the Epx locus and widely used to target floxed genes specifically in eosinophils26-29 (provided by Jacobsen, Mayo Clinic).
Generation of eosinophils from human iPSCs
Induced pluripotent stem cells (iPSCs) were cultured on mitotically inactivated mouse embryonic fibroblasts with human embryonic stem cell medium supplemented with 6 ng/mL fibroblast growth factor-2, as previously described.30 Hematopoietic lineage specification was performed following a previously described spin-embryoid body–based protocol to generate hematopoietic progenitor cells through a hemogenic endothelium intermediate. Following an established protocol31 with minor modifications, cells were collected every other day and analyzed by flow cytometry to detect CD45, CCR3, and Siglec8. The immature myeloid cells were collected, with continued exposure for an additional 10 days to human recombinant interleukin-5 (IL-5; 10 ng/mL, Peprotech) in Iscove modified Dulbecco medium culture media (Gibco) with fetal bovine serum and 2-mercaptoethanol to generate terminally differentiated eosinophils. After differentiation, p-STAT5 and CD11b mean fluorescence intensity were analyzed in LNK(TT) or isogenic LNK(CC) eosinophils by flow cytometry. CC and TT eosinophils were incubated with platelet-activating factor (PAF; 5 μM) for 4 hours, then analyzed for EET formation. Eosinophils were identified using eosinophil major basic protein (EMBP) (1:50; Santa Cruz; sc-365701). EETs were defined as EMBP+-citrullinated histone–positive (H3Cit+). To assess tissue factor intensity, eosinophils were permeabilized and stained with anti–major basic protein (MBP) antibody (Santa Cruz, sc-365701) and anti-human tissue factor antibody (LSBio, c393246). Total tissue factor intensity was quantified from MBP+TF+ cells.
Results
Characterization of eosinophils in LNK deficiency
Consistent with increased eosinophil counts associated with LNK(R262W) in humans, chow-fed normocholesterolemic (NC) Lnk−/− bone marrow transplantation (BMT) mice displayed eosinophilia when compared with controls, and this was markedly exacerbated by hypercholesterolemia (HC) in western-type diet (WTD) fed Ldlr−/− recipient mice reconstituted with Lnk−/− BM (Figure 1A). Eosinophil activation increases eosinophil degranulation.32,Lnk−/− eosinophil had increased surface CD11b and CD69 (Figure 1B), markers of eosinophil activation.33,34 pSTAT5 was increased in Lnk−/− eosinophils in response to IL-5 or granulocyte-macrophage colony-stimulating factor (GM-CSF; Figure 1C; supplemental Figure 1), suggesting that LNK negatively regulates IL-5 and GM-CSF signaling in eosinophils. Eosinophil activation could lead to EETosis, and EETosis has been implicated in thrombus stabilization.17 Therefore, we evaluated EETosis in LNK deficiency in vitro, using BM cell–derived eosinophils.35 Siglec-F was used as an eosinophil marker, and CCR3 allowed for identification of mature vs immature eosinophils.35 Almost all of the BM derived cells coexpressed Siglec-F and CCR3 (supplemental Figure 3A). The identity of eosinophils was also confirmed by positive staining of MBP, an eosinophil granule protein (supplemental Figure 3B). EETosis was assessed after incubation of WT or Lnk−/− eosinophils with thrombin-activated WT or Lnk−/− platelets, and staining with H3Cit. H3Cit+ eosinophils were increased in the Lnk−/− eosinophil population under basal conditions and further increased when Lnk−/− eosinophils were incubated with activated Lnk−/− platelets or PAF (Figure 1D). Together, the data indicate increased eosinophil counts and activation as well as EETosis in hematopoietic Lnk deficiency.
Characteristics of eosinophils and increased EETs in LNK deficiency. (A) Eosinophil counts or percentage in CD45+ cells in chow-fed and WTD mice. (B) Eosinophil activation marker (CD11b and CD69) expression in eosinophils from peripheral blood as assessed by flow cytometry. (C) Phospho-stat5 expression at basal and IL-5–stimulated conditions in WT and Lnk−/− BM-derived eosinophils assessed by flow cytometry. (D) Representative images and quantification of EETs (H3Cit+, red and MBP, green) at basal and stimulated conditions in WT and Lnk−/− BM-derived eosinophils. Scale bar, 100 μm. IgG, immunoglobulin G.
Characteristics of eosinophils and increased EETs in LNK deficiency. (A) Eosinophil counts or percentage in CD45+ cells in chow-fed and WTD mice. (B) Eosinophil activation marker (CD11b and CD69) expression in eosinophils from peripheral blood as assessed by flow cytometry. (C) Phospho-stat5 expression at basal and IL-5–stimulated conditions in WT and Lnk−/− BM-derived eosinophils assessed by flow cytometry. (D) Representative images and quantification of EETs (H3Cit+, red and MBP, green) at basal and stimulated conditions in WT and Lnk−/− BM-derived eosinophils. Scale bar, 100 μm. IgG, immunoglobulin G.
Eosinophils contribute to arterial thrombosis in hematopoietic LNK deficiency
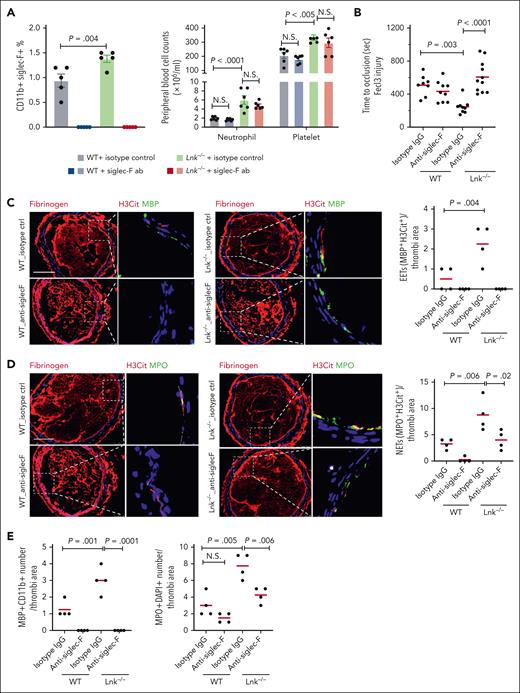
To assess the role of eosinophils in thrombosis, we treated chow-fed WT and Lnk−/− BMT mice with eosinophil-depleting antibody (anti–Siglec-F) or isotype-matched control immunoglobulin G, as described.36 Anti–Siglec-F antibody depleted eosinophils but had no significant effect on counts or activation of neutrophils, monocytes, or platelets, as reported36 (Figure 2A; supplemental Figure 2). As we reported,25 carotid arterial thrombosis was accelerated in Lnk−/− mice (Figure 2B). Once formed, the thrombus was stable, and we did not detect spontaneous thrombolysis (not shown). Strikingly, eosinophil depletion by Siglec-F antibody completely reversed the accelerated thrombosis in Lnk−/− mice while having no effect on thrombosis in the WT control (Figure 2B). These results indicate a major role of eosinophils in the accelerated arterial thrombosis in hematopoietic LNK deficiency. Eosinophil depletion had no significant impact on time to occlusion in carotid thrombosis in the chow-fed WT control, consistent with previous reports.37 Although eosinophils constitute a small fraction of WBCs, EETs represent up to ∼27% of leukocyte extracellular traps in carotid thrombi and may contribute to thrombus stability in Apoe−/− mice.17 To explore a potential role of EETs in arterial thrombosis in LNK deficiency, we assessed carotid thrombotic EETs in WT or Lnk−/− BMT mice by staining for MBP, H3Cit and 4′,6-diamidino-2-phenylindole (Figure 2C). Indeed, eosinophil abundance and EETs were increased in thrombi in Lnk−/− mice (Figure 2C,E). As expected, Siglec-F antibody eliminated eosinophils and EETs in thrombi of both WT and Lnk−/− mice. As reported,25 hematopoietic Lnk deficiency increased neutrophil abundance and NETs in thrombi (Figure 2D-E; supplemental Figure 4). Interestingly, eosinophil depletion also reduced neutrophil abundance and NETs in Lnk−/− mice (Figure 2D-E; supplemental Figure 4). Neutrophil abundance and NETs in thrombi also trended down or were significantly reduced by eosinophil depletion in the control mice when assessed using myeloperoxidase or Ly6G in combination with H3Cit as the NET marker (Figure 2E; supplemental Figure 4). Since enhanced NETosis is critical in accelerated arterial thrombosis in Lnk deficiency,25 the data suggest that eosinophils promote arterial thrombosis in part by facilitating NETosis.
Eosinophil depletion by anti–Siglec-F reverses arterial thrombosis and reduces EETs and NETs in Lnk−/− mice. (A) Eosinophil, neutrophil, and platelet counts in WT and Lnk−/− mice treated with isotype control antibody or anti–Siglec-F antibody (20 μg per mice, 24 hours, IV injection). (B) FeCl3-induced carotid artery occlusion in WT and Lnk−/− mice treated with isotype control antibody or anti–Siglec-F antibody. (C) Representative images of Fibrinogen (red) staining and EETs staining in carotid artery thrombi of WT and Lnk−/− treated with isotype control antibody or anti–Siglec-F antibody. EETs were quantified using MBP (green) with H3Cit (red); n = 4 per group. (D) Representative images and quantifications of NETs using myeloperoxidase (MPO) (green) with H3Cit (red) in thrombi of WT and Lnk−/− treated with isotype control antibody or anti–Siglec-F antibody (n = 4 per group). (E) Quantification of eosinophil number (MBP+CD11b+) and neutrophil number (MPO+DAPI+) per thrombi. Scale bar, 100 μm. DAPI, 4′,6-diamidino-2-phenylindole.
Eosinophil depletion by anti–Siglec-F reverses arterial thrombosis and reduces EETs and NETs in Lnk−/− mice. (A) Eosinophil, neutrophil, and platelet counts in WT and Lnk−/− mice treated with isotype control antibody or anti–Siglec-F antibody (20 μg per mice, 24 hours, IV injection). (B) FeCl3-induced carotid artery occlusion in WT and Lnk−/− mice treated with isotype control antibody or anti–Siglec-F antibody. (C) Representative images of Fibrinogen (red) staining and EETs staining in carotid artery thrombi of WT and Lnk−/− treated with isotype control antibody or anti–Siglec-F antibody. EETs were quantified using MBP (green) with H3Cit (red); n = 4 per group. (D) Representative images and quantifications of NETs using myeloperoxidase (MPO) (green) with H3Cit (red) in thrombi of WT and Lnk−/− treated with isotype control antibody or anti–Siglec-F antibody (n = 4 per group). (E) Quantification of eosinophil number (MBP+CD11b+) and neutrophil number (MPO+DAPI+) per thrombi. Scale bar, 100 μm. DAPI, 4′,6-diamidino-2-phenylindole.
Accelerated thrombosis in HC-LNK–deficient mice is partially reversed by Siglec-F antibody–mediated eosinophil depletion
In addition to increasing eosinophilia, HC exacerbates neutrophilia and platelet activation in hematopoietic Lnk deficiency.25 Priming and activation of neutrophils for NETosis by OxPL released from activated platelets are important mechanisms responsible for the accelerated atherothrombosis in HC Lnk−/− mice.25 To assess the role of eosinophils in atherothrombosis in HC Lnk−/− mice, Ldlr−/− mice received transplantation with WT and Lnk−/− BM and were fed with the WTD for 10 weeks. Then, WT and Lnk−/−Ldlr−/− recipient mice received anti–Siglec-F or isotype-matched control immunoglobulin G. As expected, HC Lnk–/– mice displayed heightened eosinophilia (Figure 3A). As in chow-fed mice, anti–Siglec-F antibody depleted eosinophils but had no significant effect on neutrophil and platelet counts (Figure 3A). Carotid artery atherothrombosis was substantially accelerated in HC Lnk–/– mice, and this was largely reversed by Siglec-F antibody, whereas Siglec-F antibody had no effect on thrombosis in the WT controls (Figure 3B). The incomplete reversal of the accelerated atherothrombosis when compared with the same treatment in chow-fed mice (Figure 2B) may be explained by increased platelet activation and platelet-neutrophil interaction in HC-Lnk−/− mice.24 EETs and eosinophil abundance in thrombi were further increased in HC-Lnk−/− mice (Figure 3C,E), with the reversal by Siglec-F antibody treatment. Eosinophil depletion also reduced NETs and neutrophils in thrombi in HC Lnk−/− mice but not in HC WT mice (Figure 3D-E; supplemental Figure 5).
Eosinophil depletion by anti–Siglec-F reverses arterial thrombosis and reduces EETs and NETs in 10 weeks WTD-fed Lnk−/−Ldlr−/− recipient mice. (A) Eosinophil, neutrophil, and platelet counts in Ldlr−/− and Lnk−/−Ldlr−/− recipient mice treated with isotype control antibody or anti–Siglec-F antibody (20 μg per mice, 24 hours, IV injection). (B) FeCl3-induced carotid artery occlusion in Ldlr−/− and Lnk−/−Ldlr−/− recipient mice treated with isotype control antibody or anti–Siglec-F antibody. (C) Representative images of Fibrinogen (red) staining and EETs staining in carotid artery thrombi of WT and Lnk−/− treated with isotype control antibody or anti–Siglec-F antibody. EETs were quantified using MBP (green) with H3Cit (red); n = 5 per group. (D) Representative images and quantifications of NETs using MPO (green) with H3Cit (red) in thrombi of Ldlr−/− and Lnk−/−Ldlr−/− recipient mice treated with isotype control antibody or anti–Siglec-F antibody (n = 5-6 per group). (E) Quantification of eosinophil number (MBP+CD11b+) and neutrophil number (MPO+DAPI+) per thrombi. Scale bar, 100 μm.
Eosinophil depletion by anti–Siglec-F reverses arterial thrombosis and reduces EETs and NETs in 10 weeks WTD-fed Lnk−/−Ldlr−/− recipient mice. (A) Eosinophil, neutrophil, and platelet counts in Ldlr−/− and Lnk−/−Ldlr−/− recipient mice treated with isotype control antibody or anti–Siglec-F antibody (20 μg per mice, 24 hours, IV injection). (B) FeCl3-induced carotid artery occlusion in Ldlr−/− and Lnk−/−Ldlr−/− recipient mice treated with isotype control antibody or anti–Siglec-F antibody. (C) Representative images of Fibrinogen (red) staining and EETs staining in carotid artery thrombi of WT and Lnk−/− treated with isotype control antibody or anti–Siglec-F antibody. EETs were quantified using MBP (green) with H3Cit (red); n = 5 per group. (D) Representative images and quantifications of NETs using MPO (green) with H3Cit (red) in thrombi of Ldlr−/− and Lnk−/−Ldlr−/− recipient mice treated with isotype control antibody or anti–Siglec-F antibody (n = 5-6 per group). (E) Quantification of eosinophil number (MBP+CD11b+) and neutrophil number (MPO+DAPI+) per thrombi. Scale bar, 100 μm.
ΔdbIGata1 mutation–mediated eosinophil depletion reverses atherothrombosis in HC-LNK deficiency
To confirm the results obtained using antibody-mediated eosinophil depletion, we performed studies using ΔdbIGata1 mice with genetic deficiency in eosinophils. ΔdbIGata1 mice were generated by deletion of a high-affinity GATA-binding site in the Gata1 promoter, leading to selective loss of the eosinophil lineage without affecting the development of other GATA-1 dependent blood cells in erythroid, megakaryocytic, or mast cell lineages.38 ΔdbIGata1 mice in the B6 background were crossbred with Lnk−/− mice. Female Ldlr−/− mice reconstituted with WT, ΔdbIGata1, Lnk−/−, and ΔdbIGata1/Lnk−/− BM cells were fed with the WTD for 10 weeks. The ΔdbIGata1 mutation substantially reduced eosinophils but had no significant effect on neutrophil, platelet or monocyte counts or activation in HC Lnk−/− and HC WT mice (Figure 4A; supplemental Figure 6), as reported.38 ΔdbIGata1 partially reversed the accelerated carotid artery atherothrombosis in HC Lnk–/– mice while having no effect on thrombosis in the HC WT controls (Figure 4B). EETs in thrombi were increased in HC Lnk−/− mice, and ΔdbIGata1 abolished EETs in thrombi of both WT and Lnk−/− mice (Figure 4C). Eosinophil depletion by ΔdbIGata1 also significantly reduced NETs and neutrophils in thrombi of HC Lnk−/− mice but not of WT mice (Figure 4D-E; supplemental Figure 7). Both pharmacological and genetic eosinophil depletion confirm a major role of eosinophils in accelerated atherothrombosis in hematopoietic Lnk deficiency.
Eosinophil depletion by GATA1 deficiency reverses arterial thrombosis and reduces EETs and NETs in 10 weeks WTD-fed Lnk−/−Ldlr−/− recipient mice. (A) Eosinophil, neutrophil, and platelet counts in WT, △dblGATA, Lnk−/−, Lnk−/−△dblGATA BM recipient mice. (B) FeCl3-induced carotid artery occlusion in WT, △dblGATA, Lnk−/−, and Lnk−/−△dblGATA BM recipient mice. (C) Representative images of Fibrinogen (red) staining and EETs staining in carotid artery thrombi of WT, △dblGATA, Lnk−/−, and Lnk−/−△dblGATA BM recipient mice. EETs were quantified using MBP (green) with H3Cit (red). (D) Representative images and quantifications of NETs using MPO (green) with H3Cit (red) in thrombi of WT, △dblGATA, Lnk−/−, Lnk−/−△dblGATA BM recipient mice (n = 13 per group). (E) Quantification of eosinophil number (MBP+CD11b+) and neutrophil number (MPO+DAPI+) per thrombi. Scale bar, 100 μm. BM, bone marrow.
Eosinophil depletion by GATA1 deficiency reverses arterial thrombosis and reduces EETs and NETs in 10 weeks WTD-fed Lnk−/−Ldlr−/− recipient mice. (A) Eosinophil, neutrophil, and platelet counts in WT, △dblGATA, Lnk−/−, Lnk−/−△dblGATA BM recipient mice. (B) FeCl3-induced carotid artery occlusion in WT, △dblGATA, Lnk−/−, and Lnk−/−△dblGATA BM recipient mice. (C) Representative images of Fibrinogen (red) staining and EETs staining in carotid artery thrombi of WT, △dblGATA, Lnk−/−, and Lnk−/−△dblGATA BM recipient mice. EETs were quantified using MBP (green) with H3Cit (red). (D) Representative images and quantifications of NETs using MPO (green) with H3Cit (red) in thrombi of WT, △dblGATA, Lnk−/−, Lnk−/−△dblGATA BM recipient mice (n = 13 per group). (E) Quantification of eosinophil number (MBP+CD11b+) and neutrophil number (MPO+DAPI+) per thrombi. Scale bar, 100 μm. BM, bone marrow.
Eosinophil selective Lnk deficiency increases eosinophil production and activation, promoting atherothrombosis
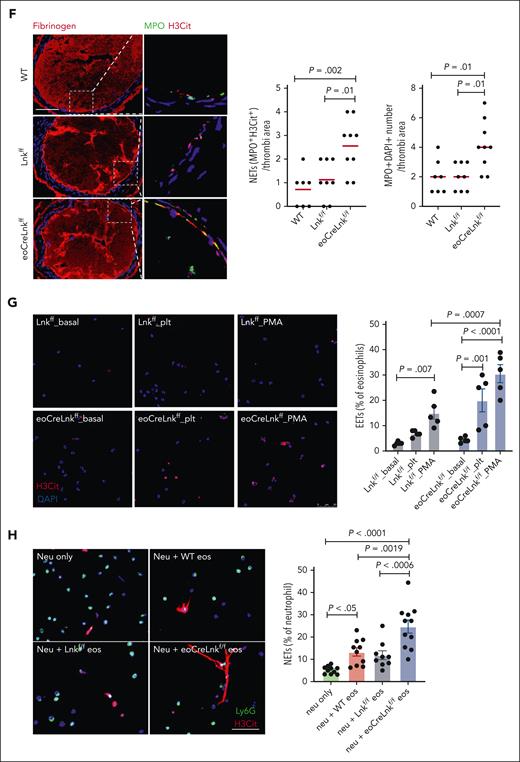
Although the data indicate the critical role of eosinophils in accelerated atherothrombosis in hematopoietic Lnk deficiency, LNK is expressed in monocytes, neutrophils and platelets; thus, the specific role of eosinophil Lnk in regulation of eosinophil function and thrombosis is unknown. We, therefore, generated Lnkf/f mice by floxing a region containing Lnk exons 4 to 8. Lnk floxed mice in B6 background were crossed with eoCre mice26 to produce eosinophil lineage specific Lnk deficiency. eoCre has been generated by knockin of Cre recombinase in the Epx locus and widely used to target floxed genes specifically in eosinophils.26-29,eoCre directs Cre expression in eosinophil progenitors and eosinophils but not in granulocyte-macrophage progenitors.29 eoCre+/− mice, with 1 functional Epx allele, do not show any signs of altered eosinophil or other blood cell development and function or any other detectable defects.26 LNK protein and Lnk messenger RNA (mRNA) levels were markedly reduced in BM derived eosinophils from LnkΔeos mice (supplemental Figure 8A-B). Consistently, Lnk mRNA levels were substantially reduced in circulating eosinophils but not circulating neutrophils or monocytes from LnkΔeos mice relative to Lnkf/f or WT mice (supplemental Figure 8C), indicating robust lineage-specific Lnk deletion.
LnkΔeos mice showed increased eosinophil counts but no significant change of platelet, WBC, neutrophil, or monocyte counts or activation when compared with Lnkf/f or WT mice (Figure 5A; supplemental Figure 9A-C); the increase in eosinophils was comparable with that in chow-fed Lnk−/− mice (Figure 1A). NET formation in LnkΔeos and control Lnkf/f neutrophils showed no difference under basal or platelet stimulated conditions (supplemental Figure 9D). Plasma sVCAM1 levels, a marker of endothelial activation, were similar between LnkΔeos and Lnkf/f groups or between hematopoietic Lnk−/− and WT mice (supplemental Figure 9E). As in Lnk−/− mice, pSTAT5 and surface CD69 in eosinophils were increased in LnkΔeos mice (Figure 5B-C), indicating eosinophil activation. LnkΔeos eosinophils also showed increased EETosis in vitro relative to Lnkf/f eosinophils in response to platelet or phorbol 12-myristate 13-acetate stimulations (Figure 5G). Importantly, carotid artery thrombosis was markedly accelerated in the chow-fed LnkΔeos mice relative to Lnkf/f or WT mice (Figure 5D). These data recapitulate the findings in hematopoietic Lnk−/− mice and indicate for the first time, to our knowledge, the essential role of eosinophil LNK in suppression of arterial thrombosis. They also indicate that the phenotypic changes of Lnk−/− eosinophils are likely cell-autonomous as a result of eosinophil Lnk deficiency. EET, NET, eosinophil, and neutrophil counts in thrombi were all increased in LnkΔeos compared those in Lnkf/f or WT mice (Figure 5E-F; supplemental Figure 10).
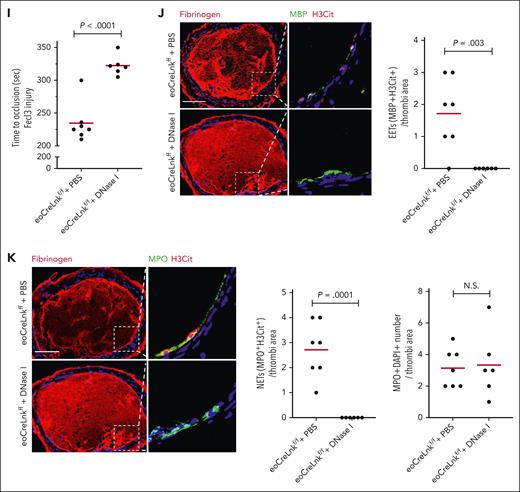
Eosinophil LNK deficiency promotes EETs and thrombosis. (A) Eosinophil, neutrophil, monocyte, and platelet counts in WT, Lnkf/f, and eoCreLnkf/f mice. (B-C) P-stat5 (B) and CD69 (C) levels in eosinophils. (D) FeCl3-induced carotid artery occlusion in WT, Lnkf/f, and eoCreLnkf/f mice. (E) Representative images and quantifications of EETs using MBP (green) with H3Cit (red) in thrombi of WT, Lnkf/f and eoCreLnkf/f mice. (F) Quantification of NETs using MPO with H3Cit (MPO+H3Cit+) per thrombi. (G) Representative images and quantification of EETs (H3Cit+, red) at basal and stimulated conditions in WT, Lnkf/f, and eoCreLnkf/f BM-derived eosinophils. (H) Representative images and quantification of NETs (Ly6G+H3Cit+/Ly6G+Dapi+) in neutrophils incubated with WT, Lnkf/f or eoCreLnkf/f BM derived eosinophils for 4 hours. (I) FeCl3-induced carotid artery occlusion and quantification of NETs in eoCreLnkf/f mice treated with and without 400 U DNase I. (J-K) Quantification of EETs (J) and NETs (K) per thrombi. Unpaired t test. (L) FeCl3-induced carotid artery occlusion in Lnkf/f and eoCreLnkf/f mice with isotype control antibody or anti-Ly6G antibody. IV injection (250 μg per mice) 3 times per week. (M) Representative images and quantifications of EETs using MBP (green) with H3Cit (red) in thrombi of Lnkf/f and eoCreLnkf/f mice with isotype control or anti-Ly6G antibody. (N) Representative images and quantifications of NETs using MPO (green) with H3Cit (red) in thrombi of Lnkf/f and eoCreLnkf/f mice with isotype control or anti-Ly6G antibody. Data are expressed as mean (red bar) ± standard error of the mean (SEM). Scale bar, 100 μm.
Eosinophil LNK deficiency promotes EETs and thrombosis. (A) Eosinophil, neutrophil, monocyte, and platelet counts in WT, Lnkf/f, and eoCreLnkf/f mice. (B-C) P-stat5 (B) and CD69 (C) levels in eosinophils. (D) FeCl3-induced carotid artery occlusion in WT, Lnkf/f, and eoCreLnkf/f mice. (E) Representative images and quantifications of EETs using MBP (green) with H3Cit (red) in thrombi of WT, Lnkf/f and eoCreLnkf/f mice. (F) Quantification of NETs using MPO with H3Cit (MPO+H3Cit+) per thrombi. (G) Representative images and quantification of EETs (H3Cit+, red) at basal and stimulated conditions in WT, Lnkf/f, and eoCreLnkf/f BM-derived eosinophils. (H) Representative images and quantification of NETs (Ly6G+H3Cit+/Ly6G+Dapi+) in neutrophils incubated with WT, Lnkf/f or eoCreLnkf/f BM derived eosinophils for 4 hours. (I) FeCl3-induced carotid artery occlusion and quantification of NETs in eoCreLnkf/f mice treated with and without 400 U DNase I. (J-K) Quantification of EETs (J) and NETs (K) per thrombi. Unpaired t test. (L) FeCl3-induced carotid artery occlusion in Lnkf/f and eoCreLnkf/f mice with isotype control antibody or anti-Ly6G antibody. IV injection (250 μg per mice) 3 times per week. (M) Representative images and quantifications of EETs using MBP (green) with H3Cit (red) in thrombi of Lnkf/f and eoCreLnkf/f mice with isotype control or anti-Ly6G antibody. (N) Representative images and quantifications of NETs using MPO (green) with H3Cit (red) in thrombi of Lnkf/f and eoCreLnkf/f mice with isotype control or anti-Ly6G antibody. Data are expressed as mean (red bar) ± standard error of the mean (SEM). Scale bar, 100 μm.
Assessing the mechanism of the prothrombotic effect, we showed that tissue factor activity was significantly increased in LnkΔeos compared with that in Lnkf/f eosinophils (supplemental Figure 11A). A similar increase was found in Lnk−/− eosinophils (supplemental Figure 11B-D). Increased expression and activity of tissue factor in LNK-deficient eosinophils may contribute to the accelerated arterial thrombosis and also promote coagulation. However, a clotting assay using whole blood from Lnkf/f vs LnkΔeos mice showed no change of prothrombin time or activated partial thromboplastin time (supplemental Figure 11E). In contrast to the impaired hemostasis caused by eosinophil depletion,37 LnkΔeos did not affect tail-vein bleeding time (supplemental Figure 11F). These findings indicate that eosinophil-specific LNK deficiency does not affect whole blood coagulation or hemostasis, whereas it does accelerate arterial thrombosis.
Contribution of NETs to accelerated thrombosis in eosinophil LNK deficiency
To investigate whether eosinophil specific Lnk deficiency increases NETosis, we incubated WT neutrophils with C5α pre-activated WT, Lnkf/f or LnkΔeos eosinophils. Compared ith the neutrophil only group, NETs were significantly increased when neutrophils were coincubated with activated eosinophils. The largest induction of NETosis was found in neutrophils coincubated with LnkΔeos eosinophils (Figure 5H). To study the potential role of EETs and NETs in thrombosis in LnkΔeos mice, we treated LnkΔeos with phosphate-buffered saline or DNase I, which can effectively degrade DNA in EETs and NETs and reduce EET-dependent thrombosis in vivo.39,40 We showed previously that DNase I treatment did not alter arterial thrombosis in the WT control mice but specifically reversed the accelerated arterial thrombosis in hematopoietic Lnk−/− mice.25 Treatment with DNase I substantially reduced carotid artery thrombosis in LnkΔeos mice (Figure 5I), similar to the findings in hematopoietic Lnk−/− mice.25 DNase I abolished EETs and NETs in thrombi but had no effect on neutrophil abundance (Figure 5J-K; supplemental Figure 12). These data indicate that eosinophil specific Lnk deficiency promotes arterial thrombosis via facilitating EETosis and NETosis.
In order to separate the effects of EETs and NETs, we depleted neutrophils in Lnkf/f and LnkΔeos mice by injection of anti-Ly6G antibody. The depletion efficiency was confirmed by complete blood count. Depletion of neutrophils partly reversed the accelerated arterial thrombosis in LnkΔeos mice but did not change occlusion time in the Lnkf/f group (Figure 5L). This was associated with a complete reversal of the increased NETs but no significant impact on the increased EETs in thrombotic lesions in LnkΔeos mice (Figure 5M-N). Together with the reversal of the accelerated arterial thrombosis by DNase I, these data indicate that both EETs and NETs contribute to the accelerated arterial thrombosis in Lnk deficiency.
We carried out further studies to elucidate potential mechanisms responsible for the recruitment of neutrophils by LNK deficient-eosinophils. First, we used a transwell neutrophil migration assay in vitro, which showed that neutrophil migration and infiltration were markedly increased when Lnk−/− neutrophils were paired with Lnk−/− eosinophils relative to the WT neutrophil/eosinophil pair (supplemental Figure 13A). Neutrophil migration also trended higher when Lnk−/− eosinophils or neutrophils were paired with their WT counterpart relative to the WT pairs. Together, these findings suggest that both Lnk−/− eosinophils and neutrophils contribute to the enhanced neutrophil migration. Next, we endeavored to understand how LNK-deficient eosinophils modulate neutrophil migration. We showed previously that OxPLs from activated Lnk−/− platelets activate neutrophils and promote NETosis via ligation of platelet-activating factor receptor (PAFR) in neutrophils. PAFR activation has been shown to be a potent chemotaxis signaling in neutrophils.41,42 We hypothesized that OxPL generation in LNK-deficient eosinophils is increased, facilitating neutrophil recruitment. We assessed OxPL exposure on WT and Lnk−/− eosinophil surface. Flow cytometry showed increased binding of E06-scFv to Lnk−/− monocyte and eosinophils in the circulation or in bone marrow–derived Lnk−/− eosinophils after adenosine diphosphate (ADP stimulation (supplemental Figure 13B-C), indicating increased OxPL exposure. To assess the potential role of OxPL released by eosinophils, we prepared Lnk−/− neutrophils and added WT eosinophils or Lnk−/− eosinophils with or without ADP stimulation in the transwell neutrophil migration assay. ADP-stimulated Lnk−/− eosinophils significantly increased Lnk−/− neutrophil migration (supplemental Figure 13D). Finally, we showed that WEB2086, a potent PAFR antagonist, largely reversed the increased neutrophil migration in Lnk deficiency while having no significant effect when the WT neutrophil/eosinophil pair was used in the assay (supplemental Figure 13E). These findings suggest that Lnk−/− eosinophils may facilitate recruitment of neutrophils via OxPL/PAFR pathway.
Increased EETosis in iPSC-derived LNK(TT) eosinophils
To test the human relevance of these findings, we used isogenic LNK(TT) and LNK(CC) iPSCs, as reported previously,25 to generate isogenic LNK(TT) and LNK(CC) eosinophils. Following an established protocol,31 we were able to produce eosinophils from iPSCs, as evidenced by co-expression of CD45, Siglec-8, CCR3, CD11b and MBP (Figure 6; supplemental Figure 15A). LNK(TT) eosinophils showed increased pSTAT5 and surface CD11b levels relative to the isogenic LNK(CC) eosinophils (Figure 6A-B), suggesting increased eosinophil activation. Importantly, LNK(TT) eosinophils also showed increased EETosis relative to LNK(TT) eosinophils when stimulated with PAF (Figure 6C). We showed that tissue factor content, tissue factor mRNA and tissue factor activity were all significantly increased in LNK(TT) compared with LNK(CC) eosinophils (supplemental Figure 16). These findings with LNK(TT) and LNK(CC) eosinophils recapitulate the results from studies with Lnk−/− or LnkΔeos eosinophils, demonstrating translational significance.
Increased activation and EETosis in LNK(TT) cells. (A) p-STAT5 level was analyzed on day 18 in CD45+CD11b+Siglec8+ LNK(TT) and LNK(CC) cells (n = 3). Unpaired t test. (B) Surface CD11b level on day 18 in LNK(TT) and LNK(CC) cells (n = 3). (C) LNK(TT) and LNK(CC) eosinophils were stimulated with PAF for 4 hours. Representative images of EETosis. Green, MBP; red, citrullinated histone (H3Cit); blue, DAPI. Eosinophil extracellular trap (EET) numbers were normalized to MBP positive cell numbers; 2-way analysis of variance. Scale bar, 50 μm. Data are expressed as mean ± SEM.
Increased activation and EETosis in LNK(TT) cells. (A) p-STAT5 level was analyzed on day 18 in CD45+CD11b+Siglec8+ LNK(TT) and LNK(CC) cells (n = 3). Unpaired t test. (B) Surface CD11b level on day 18 in LNK(TT) and LNK(CC) cells (n = 3). (C) LNK(TT) and LNK(CC) eosinophils were stimulated with PAF for 4 hours. Representative images of EETosis. Green, MBP; red, citrullinated histone (H3Cit); blue, DAPI. Eosinophil extracellular trap (EET) numbers were normalized to MBP positive cell numbers; 2-way analysis of variance. Scale bar, 50 μm. Data are expressed as mean ± SEM.
Discussion
Our study indicates a major role of eosinophils in accelerated atherothrombosis in hematopoietic Lnk deficiency and demonstrates the essential role of eosinophil Lnk in suppression of arterial thrombosis. To our knowledge, this is the first study showing that knocking out a gene specifically in eosinophils promotes arterial thrombosis. Mechanistically, LNK deficiency in eosinophils increases signaling of cytokines like GM-CSF and IL-5, leading to increased eosinophil priming and activation and enhanced EETosis. As a result of eosinophilia, increased eosinophil activation, and EETosis, LNK-deficient eosinophils promote atherothrombosis. This may be partly mediated by facilitating NETosis, indicating functional interactions between eosinophils and neutrophils during atherothrombosis in Lnk deficiency.
LNK is a negative regulator of various hematopoietic cytokine signaling pathways, many involving JAK2/STATs as the mediator.21 IL-5 and GM-CSF are important cytokines promoting eosinopoiesis, eosinophil activation, and EETosis,11,43,44 and both IL-5 and GM-CSF signaling are mediated by JAK2/STATs.45,46 LNK negatively regulates GM-CSF signaling in bone marrow hematopoietic cells.24 Increased pSTAT5 levels in response to IL-5 or GM-CSF in Lnk-deficient eosinophils indicate that LNK also negatively regulates IL-5 and GM-CSF signaling in eosinophils. Eosinophilia in Lnk−/− and LnkΔeos mice are likely at least partly explained by increased IL-5 and GM-CSF signaling. Increased IL-5 and GM-CSF signaling also could explain increased eosinophil activation and EETosis in vivo, promoting arterial thrombosis in Lnk−/− or LnkΔeos mice.
Depletion of eosinophils has differential impact on arterial thrombosis in Lnk deficiency under NC vs HC state. The complete reversal of the accelerated arterial thrombosis by eosinophil depletion in NC in hematopoietic Lnk deficiency indicates the indispensable role of eosinophils in this setting. In contrast, the accelerated arterial thrombosis in the setting of HC was only partially reversed by pharmacological or genetic depletion of eosinophils, indicating other factors that also contribute to atherothrombosis in hematopoietic Lnk deficiency. In hematopoietic Lnk deficiency, HC exacerbates neutrophilia24 and eosinophilia. Importantly, priming and activation of platelets were substantially increased by HC in Lnk deficiency,24 reflecting enhanced thrombopoietin/MPL signaling and HC mediated suppression of SHIP-1 activity acting convergently to increase AKT and platelet activation.24 Increased platelet activation, platelet-neutrophil complex formation, and induced neutrophil activation and NETosis by OxPLs released from the activated platelets in HC Lnk−/− mice contribute to atherothrombosis.
Emerging evidence suggests that eosinophils act to stabilize thrombus during thrombosis, which may involve EETosis, although the causal role of EETosis has not been directly assessed.17,37 Consistently, our data also support a critical role of EETosis to promote arterial thrombosis in hematopoietic Lnk−/− or LnkΔeos mice. Moreover, this effect of eosinophils appears to be mediated, at least partly, by facilitating NETosis, indicating functional interactions between eosinophils and neutrophils in accelerated atherothrombosis in Lnk deficiency. This functional interaction may involve increased OxPL production from eosinophils and enhanced neutrophil PAFR signaling in Lnk deficiency.
Targeting eosinophils as a therapeutic approach has proved to be successful in certain types of eosinophilic disorders. Antibodies against IL-5 or IL-5Ra and reducing eosinophils have been marketed for the treatment of eosinophilic asthma.11 Lirentelimab, an antibody against Siglec-8, a human paralog of Siglec-F, reduces eosinophils and improves symptoms in eosinophilic gastroenteritis or duodenitis.47 Our study suggests that targeting eosinophils in a genetically defined population, such as those carrying LNK(TT), may benefit to reduce the risk of atherothrombosis. The full benefit may be achieved in combination with active management of blood cholesterol levels.
In summary, our findings show that eosinophils play a critical role for accelerated atherothrombosis in LNK deficiency, involving a mechanism promoting EETosis and NETosis. Eosinophil LNK is essential in suppression of arterial thrombosis. Therapeutic targeting of eosinophils may prove to be beneficial to reduce the risk of atherothrombosis in genetically defined populations.
Acknowledgments
The authors thank Elizabeth Jacobsen from Mayo Clinic for providing the eoCre line of mice.
This work was supported by National Institutes of Health (NIH), National Heart, Lung, and Blood Institute grants R01HL137663 (A.R.T.), R01HL155431 (A.R.T.), R01 HL118567 (N.W.), and R01 HL148071 (N.W.) and Fondation Leducq grant 18CVD04. H.D. is supported by American Heart Association Career Development Award 927064. E.P.P. is supported by NIH, National Cancer Institute grants R01CA225231, R01CA271418, R01CA260711 and R01CA271331, a Leukemia and Lymphoma Society Scholar Award, an Edward P. Evans Foundation Discovery Research Grant, an Leukemia and Lymphoma Society Blood Cancer Discoveries Grant, and by a 2021 AACR-MPM Oncology Charitable Foundation Transformative Cancer Research Grant (21-20-45-PAPA).
Authorship
Contribution: H.D., R.W., M.T., T.X., and M.O. conducted the research and analyzed data. E.P.P. provided human LNK(CC) and LNK(TT) iPSCs; H.D., N.W., and A.R.T. wrote the manuscript; and all authors read and commented on the manuscript.
Conflict-of-interest disclosure: A.R.T. is a consultant for Amgen, CSL Behring, AstraZeneca, and Foresite Laboratories and is on the science advisory boards of Staten Biotech, Fortico Biotech, and Beren Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Nan Wang, Division of Molecular Medicine, Columbia University Medical Center, PS 8-401, 630 W 168th St, New York, NY 10032; email: nw30@cumc.columbia.edu; and Huijuan Dou, Division of Molecular Medicine, Columbia University Medical Center, PS 8-401, 630 W 168th St, New York, NY 10032; email: hd2400@cumc.columbia.edu.
References
Author notes
Data, analytic methods, and study materials will be made available to other researchers on reasonable request to the corresponding authors, Nan Wang (nw30@cumc.columbia.edu) and Huijuan Dou (hd2400@cumc.columbia.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal