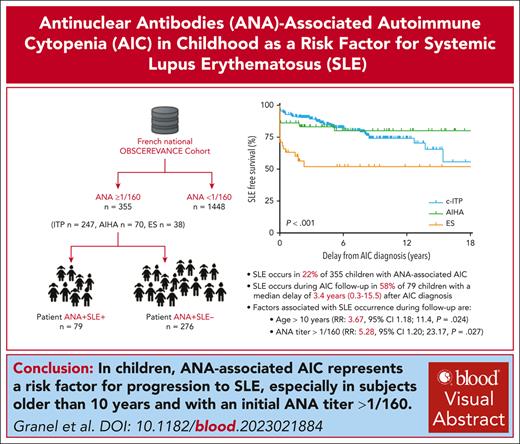
SLE occurs in 22% of children with ANA-associated AIC.
Children of age >10 years and ANA titer >1/160 must be monitored long-term, for progression to SLE.
Visual Abstract
Autoimmune cytopenia (AIC) in children may be associated with positive antinuclear antibodies (ANA) and may progress to systemic lupus erythematosus (SLE). We evaluated the risk of progression to SLE of childhood-onset ANA-associated AIC. In the French national prospective OBS’CEREVANCE cohort, the long-term outcome of children with ANA-associated AIC (ANA titer ≥1/160) and a subgroup of children who developed SLE were described. ANA were positive in 355 of 1803 (20%) children with AIC. With a median follow-up of 5.8 (range, 0.1-29.6) years, 79 of 355 (22%) patients developed SLE at a median age of 14.5 (1.1-21.4) years; 20% of chronic immune thrombocytopenic purpura, 19% of autoimmune hemolytic anemia, and 45% of Evans syndrome. None of the patients with ANA-negative test developed SLE. Severe manifestations of SLE were observed in 21 patients, and 2 patients died. In multivariate analysis including patients with positive ANA within the first 3 months after AIC diagnosis, age >10 years at AIC diagnosis (relative risk [RR], 3.67; 95% confidence interval [CI], 1.18-11.4; P = .024) and ANA titer >1/160 (RR, 5.28; 95% CI, 1.20-23.17; P = .027) were associated with the occurrence of SLE after AIC diagnosis. ANA-associated AIC is a risk factor for progression to SLE, especially in children with an initial ANA titer >1/160 and an age >10 years at AIC diagnosis. ANA screening should be recommended in children with AIC, and patients with ANA should be monitored long-term for SLE, with special attention to the transition period. This trial was registered at www.ClinicalTrials.gov as #NCT05937828.
Introduction
Childhood-onset systemic lupus erythematosus (cSLE) is a rare autoimmune disease characterized by heterogeneous clinical and biological manifestations associated with the presence of antinuclear antibodies (ANA).1 The prevalence of cSLE is 3.3 to 8.8 cases per 100 000 children, representing 10% to 20% of all cases of systemic lupus erythematosus (SLE).1 The course of cSLE is more severe than that in adults, with high prevalence rates of renal and neurological involvement and an estimated 10-year mortality rate of 8% to 19%.1,2
Hematological manifestations are present in 75% of patients at the initial presentation of cSLE.3 Among them, autoimmune cytopenia (AIC), manifesting as immune thrombocytopenic purpura (ITP), autoimmune hemolytic anemia (AIHA), autoimmune neutropenia, or Evans syndrome (ES), are found in one-third of the patients with cSLE.4 Moreover, their presence is associated with a more severe disease course.4,5
In adults, isolated ANA are found in the sera of most patients up to 3 years before the diagnosis of SLE.6 Similarly, AIC, either isolated or associated with positive ANA, may precede the diagnosis of SLE.4 Lambers et al defined incomplete lupus erythematosus (ILE) as ANA positivity associated with clinical or laboratory manifestations suggestive of SLE but did not satisfy the classification criteria for SLE.7 The ANA-associated AIC satisfies this definition. Patients with ILE are at an increased risk of progression to classifiable SLE.8 In small retrospective or single-center studies, childhood ITP was associated with ANA positivity in 7% to 29% of cases, and 0% to 42% of this subgroup later developed SLE9-13 (supplemental Table 1). Further comprehensive and long-term studies of the predictive factors for progression to SLE in childhood AIC are required.
Early identification of subgroups of children with AIC at risk of developing SLE is important to prevent severe disease manifestations. Hydroxychloroquine (HCQ) is effective in adults and children with SLE, and with ANA-associated ITP14,15 but its ability to prevent SLE for patients with ILE is still discussed.8
Here, we present a comprehensive description of the long-term outcomes of children with ANA-associated AIC from the French nationwide prospective OBS’CEREVANCE cohort, including a subgroup of children who later developed SLE. Specifically, we investigated the predictive factors associated with progression to SLE.
Patients and Methods
OBS’CEREVANCE cohort
Since 2004, French patients with chronic ITP (cITP) and newly diagnosed AIHA and ES diagnosed before the age of 18 years were consecutively enrolled in the prospective OBS’CEREVANCE cohort.16,17 The inclusion criteria, exclusion criteria, and definitions are presented in supplemental Table 2.18 Written informed consent for data collection was obtained from the parents/guardians of all patients. This cohort study was approved by our institutional ethics committee and the database was registered with the national data protection authority (CNIL, 1396823V1) and ClinicalTrial.gov (NCT05937828). On 1 March 2022, 1803 patients were registered: 1089 (60%) cITP, 605 (34%) AIHA, and 109 (6%) simultaneous ES. The median follow-up was 5.1 (0-38.6) years, and 53% of patients are currently adults.
Patient selection
Patients from the OBS’CEREVANCE cohort were included in this study if ANA-positivity was detected at least once, at AIC diagnosis, or during follow-up. In this real-life data collection, only positive results were reported. ANA positivity was defined as a titer ≥1/160. Patients with SLE diagnosed before the development of AIC or with known secondary etiologies of AIC at initial diagnosis were excluded. Data were extracted from the OBS’CEREVANCE cohort database, and the medical and follow-up records of each participating center were checked if found to be incomplete. Data collection was stopped on 1 March 2022.
Definitions
Clinical and biological manifestations of SLE were defined according to the International Working Group SLICC 2012 and EULAR/ACR 2019 criteria.19,20 The date of SLE diagnosis was when the SLICC 2012 and/or EULAR/ACR 2019 diagnostic criteria were met. AIC and SLE were considered concomitant if SLE occurred within 3 months after AIC diagnosis. Severe SLE was defined as renal, neurological, thoracic, or digestive involvement or a thrombotic event.
Patients classified as having simultaneous ES were those with bi- or tri-cytopenia within the first 3 months after the initial diagnosis. Patients with ES presenting with sequential cytopenia were classified as the first one: ITP or AIHA. For ITP, clinical severity was defined as a Buchanan score ≥3 and hematological severity as a platelet count <10 G/L.21 For AIHA, clinical severity was defined by the presence of coma, collapse, or acute renal insufficiency, and hematological severity by a hemoglobin level <7 g/dL.22
Immunopathological manifestations (IM) were defined as predefined clinical or biological manifestations associated with AIC. IM diagnoses were based on CEREVANCE criteria or international classification (if available; eg, for antiphospholipid syndrome).23 IM were assessed at clinical and biological follow-ups (at least every 6-12 months) according to CEREVANCE guidelines.22,24 Second-line treatments were those other than steroids and intravenous immunoglobulins, which were managed according to French guidelines (http://www.cerevance.org).
Statistical analysis
We compared continuous and categorical variables using the nonparametric Wilcoxon–Mann-Whitney test and Fisher exact test, respectively. To determine the risk factors for progression to SLE, we included patients with available data on ANA titers within the first 3 months after AIC diagnosis, who developed SLE >3 months after AIC diagnosis. Univariate and multivariate Cox proportional hazard analyses were used to assess the effects of several parameters present at the initial diagnosis of AIC on SLE occurrence: that is, first-degree or second-degree familial IM, sex, age at AIC diagnosis, clinical and hematological severity of AIC, type of AIC, and ANA titer. The Kaplan-Meier method was used to analyze SLE-free survival, and the groups were compared using the log-rank test. Statistical analyses were performed using RStudio (version 2022.07.1 Build 554; RStudio PBC, Boston, MA) and Prism (version 5.01; GraphPad Software, Inc, San Diego, CA) software. All tests were 2-sided, and P < .05 was taken to indicate statistical significance.
Results
Patients with ANA (n = 355)
On 1 March 2022, 355 of 1803 (20%) patients were followed up in the 30 centers of the OBS’CEREVANCE cohort, with at least 1 ANA-positive test (titer ≥1/160), and were classified as patients with ANA: 23% of patients with cITP (247/1089), 12% of patients with AIHA (70/605), and 35% of patients with simultaneous ES (38/109). The details of patient selection are presented in supplemental Figure 1. The median age a first ANA titer ≥1/160 was 12.0 (0.2-28.9). Among patients with AIHA, isolated or in the context of an ES, 97 of 108 (90%) had an IgG or IgG + complement direct antiglobulin test.
Among patients with ANA, ANA positivity was first detected in 146 of 355 (41%) patients within the first 3 months after AIC diagnosis and in 220 of 355 (62%) patients within the first year after AIC diagnosis. AIC was diagnosed at a median age of 10.5 (0.2-17.6) years and before the age of 8 years for 120 of 355 (34%) patients (80 patients with cITP, 28 patients with AIHA, and 12 patients with simultaneous ES), and 252 of 355 (71%) were girls. The median follow-up from AIC diagnosis was 5.8 (0.1-29.6) years. The characteristics of the patients with ANA are presented in Table 1.
Characteristics of 355 patients with ANA-associated AIC
| . | Patients with ANA, n = 355 . | Patients with ANA and SLE, n = 79 . | Patients with ANA but negative for SLE, n = 276 . | P value . |
|---|---|---|---|---|
| Sex ratio (female/male) | 252/103 | 72/7 | 180/96 | 2.3e-6 |
| Median age at AIC diagnosis, y (min-max) | 10.5 (0.2-17.6) | 12.4 (0.8-17.0) | 9.7 (0.2-17.6) | 4.7e-5 |
| AIC type at initial diagnosis, n (%) cITP | 247 (70%) | 49 (62%) | 198 (72%) | .13 |
| isolated AIHA | 70 (20%) | 13 (16%) | 57 (21%) | .52 |
| simultaneous ES | 38 (10%) | 17 (22%) | 21 (8%) | .001 |
| Severity of AIC at initial diagnosis, n (%) | ||||
| Clinical severity, n (%) | 69/214 (32%) | 24/52(46%) | 45/162 (28%) | .02 |
| Hematological severity, n (%) | 138/340 (41%) | 38/77 (49%) | 100/263 (38%) | .08 |
| First-degree or second-degree familial IM, n (%) | 131/355 (37%) | 35/79 (44%)∗ | 96/276 (35%) | .14 |
| cIM other than SLE during the follow-up, n (%) | 62/355 (17%) | 15/79 (19%)† | 51/276 (18%) | 1 |
| ANA titer during follow-up ≥1/320, n (%) | 225/355 (64%) | 65/79 (82%) | 160/276 (58%) | 6e-5 |
| Patients with second-line treatments, n (%) | 238/355 (67%) | 72/79 (91%) | 166/276 (60%) | 5.9e-8 |
| Hydroxychloroquine, n (%) | 143 (40%) | 65 (82%) | 78 (28%) | 4.7e-18 |
| IS (AZA, MMF, CYC, CSA, VCR), n (%) | 104 (29%) | 31 (39%) | 73 (26%) | .04 |
| Rituximab, n (%) | 93 (26%) | 23 (29%) | 70 (25%) | .56 |
| Splenectomy, n (%) | 38 (11%) | 11 (14%) | 27 (10%) | .30 |
| Patients died, n (%) | 7 (2%) | 2 (3%) | 5 (1%) | .65 |
| Patients in CR at 5 years from initial AIC diagnosis, n (%) | 107/207 (51%) | 32/51 (63%) | 75/156 (46%) | .07 |
| Median follow-up from AIC diagnosis, y (min-max) | 5.8 (0.1-29.6) | 5.4 (0.2-20.1) | 5.8 (0.1-29.6) | .99 |
| . | Patients with ANA, n = 355 . | Patients with ANA and SLE, n = 79 . | Patients with ANA but negative for SLE, n = 276 . | P value . |
|---|---|---|---|---|
| Sex ratio (female/male) | 252/103 | 72/7 | 180/96 | 2.3e-6 |
| Median age at AIC diagnosis, y (min-max) | 10.5 (0.2-17.6) | 12.4 (0.8-17.0) | 9.7 (0.2-17.6) | 4.7e-5 |
| AIC type at initial diagnosis, n (%) cITP | 247 (70%) | 49 (62%) | 198 (72%) | .13 |
| isolated AIHA | 70 (20%) | 13 (16%) | 57 (21%) | .52 |
| simultaneous ES | 38 (10%) | 17 (22%) | 21 (8%) | .001 |
| Severity of AIC at initial diagnosis, n (%) | ||||
| Clinical severity, n (%) | 69/214 (32%) | 24/52(46%) | 45/162 (28%) | .02 |
| Hematological severity, n (%) | 138/340 (41%) | 38/77 (49%) | 100/263 (38%) | .08 |
| First-degree or second-degree familial IM, n (%) | 131/355 (37%) | 35/79 (44%)∗ | 96/276 (35%) | .14 |
| cIM other than SLE during the follow-up, n (%) | 62/355 (17%) | 15/79 (19%)† | 51/276 (18%) | 1 |
| ANA titer during follow-up ≥1/320, n (%) | 225/355 (64%) | 65/79 (82%) | 160/276 (58%) | 6e-5 |
| Patients with second-line treatments, n (%) | 238/355 (67%) | 72/79 (91%) | 166/276 (60%) | 5.9e-8 |
| Hydroxychloroquine, n (%) | 143 (40%) | 65 (82%) | 78 (28%) | 4.7e-18 |
| IS (AZA, MMF, CYC, CSA, VCR), n (%) | 104 (29%) | 31 (39%) | 73 (26%) | .04 |
| Rituximab, n (%) | 93 (26%) | 23 (29%) | 70 (25%) | .56 |
| Splenectomy, n (%) | 38 (11%) | 11 (14%) | 27 (10%) | .30 |
| Patients died, n (%) | 7 (2%) | 2 (3%) | 5 (1%) | .65 |
| Patients in CR at 5 years from initial AIC diagnosis, n (%) | 107/207 (51%) | 32/51 (63%) | 75/156 (46%) | .07 |
| Median follow-up from AIC diagnosis, y (min-max) | 5.8 (0.1-29.6) | 5.4 (0.2-20.1) | 5.8 (0.1-29.6) | .99 |
AZA, azathioprine; cIM, clinical immune manifestation; CR, complete remission; CSA, cyclosporin A; CYC, cyclophosphamide; IM, immunopathological manifestations; IS, immunosuppressants; MMF, mycophenolate mofetil; VCR, vincristine. Boldfaced P values indicate P value > .05.
Main significant familial history, that is, first- or second-degree relatives with IM for patients with ANA who had SLE: cancer (n = 10), SLE (n = 8), thyroid disease (n = 7), arthritis (n = 5), inflammatory bowel disease (n = 4), myasthenia (n = 2), celiac disease (n = 2), and type 1 diabetes (n = 2).
Clinical IM observed for patients with ANA who had SLE: autoimmune thyroiditis (n = 8), acrosyndrome (n = 2), cryoglobulinemia type IIa (n = 1), inflammatory bowel disease (n = 1), neuromyelitis optica associated with antiaquaporin 4 antibodies (n = 1), pernicious anemia (n = 1), and rheumatoid arthritis (n = 1).
On 1 March 2022, 79 of 355 (22%) patients with ANA developed SLE according to the SLICC 2012 and/or EULAR/ACR 2019 criteria, representing 20% of cITP−ANA+ (49/247), 19% of AIHA−ANA+ (13/70), and 45% of simultaneous ES−ANA+ (17/38) patients. No patient with an ANA-negative test from the OBS’CEREVANCE cohort developed SLE and among those with ANA along with AIHA, none of the patients with a complement direct antiglobulin test developed SLE. Of note, 51 of 276 (18.5%) patients with ANA who were negative for SLE, had clinical IM, and various primary immune deficiencies (PID) were confirmed in 15 of 276 (5%), of which 9 were previously published25,26: autoimmune lymphoproliferative syndrome (n = 5), KRAS variant (n = 2), IKZF1 variant (n = 2), ADAR1 deficiency (n = 1), CBL variant (n = 1), CTLA4 variant (n = 1), SOCS1 haploinsufficiency (n = 1), gain-of-function STAT3 mutation (n = 1), and TLR6-ligand deficiency (n = 1). Among the 15, 11 (73%) were under 8 years of age at AIC diagnosis, representing 9% (11/120) of the patients with ANA who had AIC diagnosed before the age of 8 years.
Patients with SLE (n = 79)
Patients with ANA who developed SLE were older at AIC diagnosis (median age of 12.4 (0.8-17) years) than patients with ANA who were negative for SLE (median age of 9.7 [0.2-17.6] years; P = 4.7e-5) and were more often girls (72/79 [91%] vs 180/276 [65%]; P = 2.3e-6). The characteristics of the patients with ANA who developed SLE compared with those who were negative for SLE are presented in Table 1. Among the patients with ANA who developed SLE, those with cITP and AIHA were younger at AIC diagnosis (median age, 11.4 [1.1-17.3] years and 12.5 [3.8-16.7] years, respectively) than those with ES (median age, 14.3 [0.8-17] years; P = .02). The median age at SLE diagnosis was 14.5 (1.1-21.4) years and was not significantly different according to initial AIC. Among patients with ANA along with SLE, 13 of 79 (16%) were under 8 years old at AIC diagnosis, representing 11% (13/120) of patients with ANA who had AIC diagnosed before the age of 8 years. Among the 13, 10 had cITP with a diagnosis of SLE >1 year after ITP diagnosis. Regarding ANA positivity, it was first detected in 56 of 79 (71%) patients within the first 3 months after AIC diagnosis.
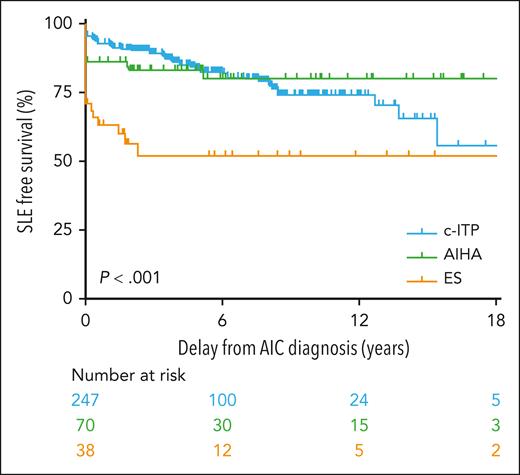
In 33 of 79 (42%) patients, the SLE criteria were met concomitantly with AIC diagnosis, whereas they were met during follow-up in the remaining 46 (58%) patients, with a median delay of 3.4 years (0.3-15.5) after AIC diagnosis (supplemental Table 3). In most patients with cITP (37/49; 75%), SLE occurred during the follow-up of AIC, whereas most of patients with AIHA (10/13, 77%) and ES (11/17, 65%) had concomitant SLE and AIC diagnoses (P < .001). SLE-free survival rates according to the initial AIC are presented in Figure 1. For each patient, the age at AIC diagnosis and SLE diagnosis are represented in Figure 2.
SLE-free survival in 355 patients with at least one positive antinuclear antibody test during follow-up according to the initial AIC: AIHA, cITP, and ES.
SLE-free survival in 355 patients with at least one positive antinuclear antibody test during follow-up according to the initial AIC: AIHA, cITP, and ES.
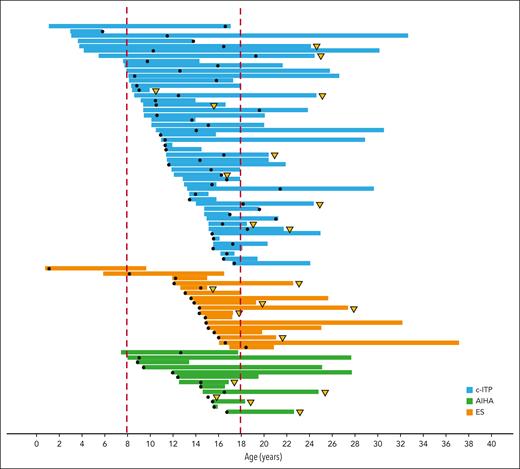
Representation of follow-up of 79 of 355 patients with AIC associated with SLE, regardless of the time of SLE onset (concomitant or sequential). Each horizontal band represents a single patient, from the age at diagnosis of AIC to the age at the last follow-up. The black dot represents the age at diagnosis of SLE. Patients with severe manifestations of SLE are marked with a yellow triangle.
Representation of follow-up of 79 of 355 patients with AIC associated with SLE, regardless of the time of SLE onset (concomitant or sequential). Each horizontal band represents a single patient, from the age at diagnosis of AIC to the age at the last follow-up. The black dot represents the age at diagnosis of SLE. Patients with severe manifestations of SLE are marked with a yellow triangle.
Nonhematological clinical manifestations of SLE were observed in 51 of 79 (65%) patients and are described in Table 2 according to the type of AIC at the last follow-up. Joint involvement, including arthralgia and arthritis, and cutaneous manifestations, mainly acute cutaneous lupus, were observed in 34 of 79 (43%) patients. Severe manifestations were observed in 21 of 79 (27%) patients during follow-up. Among them, lupus glomerulonephritis was more frequently observed in the ES group, whereas neurological manifestations (mainly ischemic complications) and thoracic involvement were more frequently observed in the AIHA group.
Clinical and biological manifestations of SLE according to AIC at the last follow-up
| Manifestations . | Total n = 79 n (%) . | cITP n = 43 n (%) . | AIHA n = 12 n (%) . | ES n = 24 n (%) . | Median delay from AIC diagnosis, y (min-max) . |
|---|---|---|---|---|---|
| Articular | 34 (43%) | 17 (40%) | 3 (25%) | 14 (58%) | 2.3 (0-13.8) |
| Cutaneous | 34 (43%) | 18 (42%) | 5 (42%) | 11 (46%) | 1.7 (0-15.5) |
| Renal | 10 (13%) | 4 (9%) | 1 (8%) | 5 (21%) | 4.9 (0-10.2) |
| Neurological | 8 (10%) | 3 (7%) | 3 (25%) | 2 (8%) | 0.4 (0-12.9) |
| Thoracic | 6 (8%) | 2 (5%) | 2 (17%) | 2 (8%) | 5.4 (0-10.3) |
| Digestive | 1 (1%) | 0 | 0 | 1 (4%) | NA |
| APS | 2 (3%) | 1 (2%) | 1 (8%) | 0 | NA |
| TTP | 2 (3%) | 0 | 0 | 2 (8%) | NA |
| Anti-dsDNA or anti-Smith Abs | 65 (82%) | 38 (88%) | 9 (75%) | 18 (75%) | 1.1 (0-11.5) |
| Low C3 and/or C4 | 43 (54%) | 18 (42%) | 8 (67%) | 17 (71%) | 0.3 (0-15) |
| Antiphospholipid | 40 (51%) | 22 (51%) | 8 (67%) | 10 (42%) | 0.9 (0-14.5) |
| Leukopenia | 23 (29%) | 11 (26%) | 6 (50%) | 6 (25%) | 0.1 (0-20) |
| Manifestations . | Total n = 79 n (%) . | cITP n = 43 n (%) . | AIHA n = 12 n (%) . | ES n = 24 n (%) . | Median delay from AIC diagnosis, y (min-max) . |
|---|---|---|---|---|---|
| Articular | 34 (43%) | 17 (40%) | 3 (25%) | 14 (58%) | 2.3 (0-13.8) |
| Cutaneous | 34 (43%) | 18 (42%) | 5 (42%) | 11 (46%) | 1.7 (0-15.5) |
| Renal | 10 (13%) | 4 (9%) | 1 (8%) | 5 (21%) | 4.9 (0-10.2) |
| Neurological | 8 (10%) | 3 (7%) | 3 (25%) | 2 (8%) | 0.4 (0-12.9) |
| Thoracic | 6 (8%) | 2 (5%) | 2 (17%) | 2 (8%) | 5.4 (0-10.3) |
| Digestive | 1 (1%) | 0 | 0 | 1 (4%) | NA |
| APS | 2 (3%) | 1 (2%) | 1 (8%) | 0 | NA |
| TTP | 2 (3%) | 0 | 0 | 2 (8%) | NA |
| Anti-dsDNA or anti-Smith Abs | 65 (82%) | 38 (88%) | 9 (75%) | 18 (75%) | 1.1 (0-11.5) |
| Low C3 and/or C4 | 43 (54%) | 18 (42%) | 8 (67%) | 17 (71%) | 0.3 (0-15) |
| Antiphospholipid | 40 (51%) | 22 (51%) | 8 (67%) | 10 (42%) | 0.9 (0-14.5) |
| Leukopenia | 23 (29%) | 11 (26%) | 6 (50%) | 6 (25%) | 0.1 (0-20) |
Abs, antibodies; APS, antiphospholipid syndrome; NA, not available; TTP, thrombotic thrombocytopenic purpura.
Two deaths related to SLE were observed (2.6%), both of which were young women (aged 21 and 28 years) with ES and antiphospholipid antibodies. Of note, 5 patients died in the ANA with SLE group (1.8%): 4 had ES and 1 had AIHA. The characteristics of the 7 deceased patients are detailed in supplemental Table 4. Among the surviving patients, 41 of 77 (53%) are currently followed by adult teams.
Factors associated with the development of SLE
Among 146 patients with isolated ANA in the first 3 months after AIC diagnosis (99 with cITP, 33 with AIHA, and 14 with simultaneous ES), 19 developed nonconcomitant SLE. In univariate analysis, age >10 years at AIC diagnosis (relative risk [RR], 3.14; 95% confidence interval [CI], 1.02-9.60; P = .04) and ANA titer >1/160 within the first 3 months after AIC diagnosis (RR, 4.54; 95% CI, 1.04-19.74; P = .043) were associated with SLE occurrence during follow-up. Female sex (RR, 4.15; 95% CI, 0.96-18.24; P = .056) and clinically severe AIC at the initial diagnosis (RR, 2.52; 95% CI, 0.97-6.15; P = .056) tended to be associated with SLE occurrence. Significant familial history and type of AIC at diagnosis were not associated with the occurrence of SLE. SLE-free survival curves are presented in Figure 3. In multivariate analysis, age >10 years at AIC diagnosis (RR, 3.67; 95% CI, 1.18-11.4; P = .024) and ANA titer >1/160 within the first 3 months after AIC diagnosis (RR, 5.28; 95% CI, 1.20-23.17; P = .027) were associated with SLE occurrence during follow-up.
SLE-free survival analysis. SLE-free survival in the subgroup of 146 of 355 of patients with SLE with positive ANA within the first 3 months of AIC according to age (A), ANA titer (B), sex (C), and clinical initial severity of AIC (D) at AIC diagnosis.
SLE-free survival analysis. SLE-free survival in the subgroup of 146 of 355 of patients with SLE with positive ANA within the first 3 months of AIC according to age (A), ANA titer (B), sex (C), and clinical initial severity of AIC (D) at AIC diagnosis.
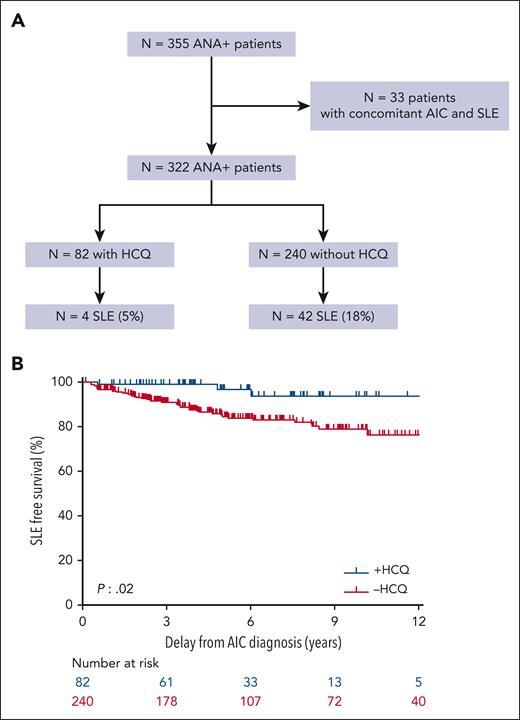
The role of HCQ in the subsequent development of SLE has also been studied retrospectively. Among the 322 patients diagnosed as ILE, that is, patients with ANA but without AIC and without a concomitant diagnosis of SLE, 82 received HCQ for their isolated AIC. The dosage, pharmacological follow-up, treatment duration, and patient adherence were heterogeneous. However, among these 82 patients, 4 (5%) receiving HCQ, developed SLE later compared with 42 of 240 (18%) not treated with HCQ for AIC (P = .003; OR, 4.12; 95% CI, 1.42-16.37) (Figure 4A-B).
Impact of HCQ on SLE occurrence. Patients with SLE (A) and SLE free survival (B) for patients with positive antinuclear antibody (patients with ANA) without a concomitant diagnosis of SLE at AIC diagnosis (n = 322) according to the previous use of HCQ for AIC.
Impact of HCQ on SLE occurrence. Patients with SLE (A) and SLE free survival (B) for patients with positive antinuclear antibody (patients with ANA) without a concomitant diagnosis of SLE at AIC diagnosis (n = 322) according to the previous use of HCQ for AIC.
Discussion
This unbiased French National Prospective OBS’CEREVANCE cohort with long-term follow-up provided comprehensive data on the risk of progression to SLE in children with ANA-associated AIC. Patients with ANA represented 20% of patients with AIC, and 22% of them developed SLE, representing 4.4% of all patients with AIC. No SLE occurred for patients with ANA-negative test. Significant predictive factors for progression to SLE were age >10 years and ANA titer >1/160 at the AIC diagnosis. For most patients (58%), mainly cITP, progression to SLE occurred in a median of 3.4 years after the diagnosis of AIC, similar to the delay of 2.9 years reported by Song et al in their cohort of 50 children who developed SLE after diagnosis of ITP.27 Of note, this delay was up to 15.5 years in our study, highlighting the importance of long-term follow-up of patients with ANA and avoiding loss to follow-up during the transition period. We, therefore, recommend that all children with chronic AIC should at least be monitored annually, for a long term, for connective tissue disease with ANA, by complement level and proteinuria screening.
ANA screening is not strictly recommended for the initial ITP diagnosis in children or adults.28 In most French centers, ANA screening is performed in older children (>8 years old) or in cases of chronic ITP or an autoimmune familial history.29 In this study, only children with chronic ITP were included; children aged <8 years at initial diagnosis represented 34% of patients with ANA, and 20% of them were diagnosed with SLE (11%) or PID (9%). All children with SLE and ITP diagnosed before the age of 8 years evolved to SLE >1 year after ITP diagnosis. We were not able to provide the rate of ANA screening, as only positive results at any moment of follow-up were reported through this real-life data collection. Nevertheless, in this population of cITP, ANA positivity was first detected within the first 3 months after AIC diagnosis in most patients with SLE (71%), and 42% of SLE occurred within the first 3 months after AIC diagnosis. Therefore, an early ANA screening at AIC diagnosis appears relevant, because its positivity would lead to an earlier detection of SLE and thus, to a reduction in the significant morbidity and mortality related to the disease, particularly for patients with AIHA and ES, who develop earlier and more severe SLE. These data are consistent with the French recommendations for ANA screening in newly diagnosed ITP after the age of 8 years, in chronic ITP, and in all cases of AIHA and ES, whatever the age at initial diagnosis. Indeed, ANA positivity, mainly in ES, can reveal PID or be associated with PID. In a recent study on 299 PID, AIC was the most commonly associated autoimmune disease (10.4%) and ANA was the most commonly observed autoantibody (10%).30
AIC accounted for 33% of cases of cSLE in a previous retrospective cohort study of 130 children with SLE, but the types of AIC were not reported.4 According to previous studies, the prevalence of AIC in cSLE was newly diagnosed, persistent, or chronic ITP (range 17%-25%), AIHA (range 14%-16%), and ES (1.3%).31-34 In this study, the initial AIC associated with SLE were as follows (in order of frequency): cITP (62%), ES (22%), and AIHA (16%). The 79 patients diagnosed with SLE and with childhood-onset AIC as described in this study, seem to have some particularities compared with “classic” cSLE. Indeed, cutaneous and joint involvement were observed in less than half of our patients (43%), whereas they were observed in ∼70% of patients in a French National cSLE cohort of 155 patients.3 This reduced occurrence of cutaneous and joint involvement in AIC-associated SLE was also observed in previous studies by Ogbu et al and Lube et al.4,34 The absence of these classic SLE manifestations could mislead clinicians in the diagnosis of SLE during follow-up of AIC. Of note, renal and neurological involvement were observed in 13% and 10% of cases in our AIC–associated SLE cohort, respectively, less than in previous cSLE studies.35,36 Ogbu et al also reported a lower incidence of renal involvement in AIC-associated SLE.4 As they suggested, we hypothesized that the treatments used for AIC before the occurrence of SLE may have an impact on the occurrence of these severe manifestations. Nevertheless, severe manifestations were observed in 27% of our patients with SLE, mainly when the initial AIC was AIHA or ES. Furthermore, 2 patients died of SLE at a young age (<30 years); both had ES and antiphospholipid antibodies. The high burden of pediatric-onset ES was recently demonstrated by our group in a long-term series of 151 patients with a 15-year mortality rate of 16%, including those SLE cases.24 Therefore, early diagnosis of SLE in a child with ANA-associated AIC is a challenge to prevent potentially severe and fatal complications of the disease at an adult age.
To our knowledge, the risk of progression from childhood AIC to SLE has only been studied retrospectively in children with ITP, and there are no conclusive data on AIHA and ES. Most previous pediatric studies, presented in supplemental Table 1, were heterogeneous, monocentric, and retrospective, including small samples of both acute and chronic ITP with various definitions.9-13 Among children with ITP, ANA, older age at ITP diagnosis, and cITP tended to be risk factors for progression to SLE; no other major predictive factors have been demonstrated.9,10,13 The risk of progression to SLE from ITP−ANA+ also remains unpredictable in adults.37,38 The large-scale prospective OBS’CEREVANCE cohort study with long-term follow-up allowed us to provide a robust method to determine risk factors that could be assessed at an early stage by clinicians in children with ANA-associated AIC. The homogeneous inclusion of only chronic ITP is an advantage of our cohort, as chronic ITP has already been identified as a risk factor for progression to SLE.9,10,13 However, children with ITP-ANA at initial diagnosis who did not develop chronic ITP, either spontaneously or under treatment, were not accounted. Although it reduced the sample size, to identify early predictive factors to enhance the early identification of at-risk patients, we analyzed only patients with information regarding ANA titer within the first 3 months after AIC diagnosis who developed SLE >3 months after AIC diagnosis. In multivariate analysis, age >10 years at AIC diagnosis and ANA titer >1/160 within the first 3 months after AIC diagnosis were associated with SLE occurrence during follow-up, with RR of 3.7 and 5.3, respectively. To our knowledge, this is the first prospective report to describe an ANA titer cutoff (>1/160) and age cutoff (>10 years) as risk factors for progression to SLE in children with ANA-associated AIC. The heterogeneity of ANA assays performed in different laboratories could limit the power of our analysis. However, our ANA titer cutoff >1/160 is consistent with a retrospective study by McGhee et al reporting that an ANA titer ≤1/360 had a negative predictive value for SLE.39 Female sex tended to be associated with SLE, although the association was not significant, probably due to the small sample size, whereas it was associated with progression to SLE from ITP in 2 previous retrospective single-center studies.10,13 A low complement level was also associated with the occurrence of SLE in childhood-onset ITP in a previous retrospective case–control study27 but we were not able to include the complement level in our analysis, as complement tests were heterogeneously documented by our data collection in our cohort of patients with ANA.
Once identified, children with ANA at risk of progression to SLE could benefit from targeted interventions. Among the available therapies, HCQ has been shown to be effective in adult and pediatric ANA-associated ITP and remains the gold standard for the treatment of SLE.14,15,40 Khellaf et al reported a 60% overall response rate to HCQ in 40 adults with ITP (12 patients with SLE-ITP and 28 with isolated ANA), with a higher response rate seen in the SLE group.14 Our pediatric group reported a 57% overall response rate to HCQ in 46 children with ANA-associated ITP.15 In recent international guidelines, the place of HCQ among second-line treatments for ITP in children and adults is not clearly defined: “hydroxychloroquine may be an effective treatment if ANAs are present especially in young women.”28 In our national pediatric experience, the indications and modalities were historically heterogeneous as well, but it has improved since 2004 with the diffusion of national guidelines from the CEREVANCE reference center (PNDS 2009, 2017) and by real-life case-by-case discussions. In this study, among ANA-associated patients with AIC without a concomitant diagnosis of SLE, treatment with HCQ for AIC before SLE onset, tended to be associated with a lower rate of progression to SLE; for those, the treatment is presently maintained in the long-term. To demonstrate this difference conclusively, a prospective pediatric randomized trial with homogeneous dosages and pharmacological monitoring is required. James et al also reported that patients treated with HCQ before SLE diagnosis had a longer delay between the onset of the first clinical symptoms and SLE classification.41 Olsen and Karp hypothesized that the use of HCQ early in the disease process, that is in ILE, would delay the onset of complete SLE, which may then be less severe or even completely absent.8 However, further prospective and controlled robust studies of the long-term favorable benefit-risk balance of HCQ in ILE are required, including detailed data on dosage, duration, and patient adherence.
Conclusions
In children with cITP, AIHA, or ES, the presence of a significantly positive ANA is a risk factor for progression to SLE, especially in girls aged >10 years at AIC diagnosis and if the initial ANA titer is >1/160. We suggest that AIC associated with positive ANA should no longer be called “primary,” for both clinical settings and adequate follow-up, and patient stratification for clinical trials, as, according to the definitions of Rodeghiero et al, “other causes or disorders might be associated in the follow-up.”18 Therefore, ANA screening, at a relatively low cost, should be included in the basic evaluation of all children with AIHA, ES, and chronic ITP, regardless of age, and in children aged >8 years with newly diagnosed ITP. Even if initially normal, ANA, complement level, and proteinuria screening should be added to the annual long-term workup of patients with chronic AIC. Patients with ANA-associated AIC require long-term monitoring for progression to SLE and organizing transition to an adult specialist even if AIC is in remission. The absence of cutaneous and joint involvement should not exclude the diagnosis of SLE, and the renal and neurological symptoms should be carefully investigated in this population. The ILE period preceding SLE, including ANA-associated AIC, is a critical period in which the issue should be early identification of at-risk patients and early initiation of targeted treatment with HCQ.
Lastly, several biomarkers are promising for identifying adult patients with ILE at risk of progression to complete SLE, including the expression of type I and II interferon gene products and serum B-cell activating factor of the tumor necrosis factor family.42 Type I interferon level begins to increase ∼2 years before the diagnosis of SLE.43 Further studies are ongoing to determine their value in predicting and limiting severe complications and mortality related to SLE in children with AIC.
Acknowledgments
The authors thank all the patients and their families, and the medical and paramedical teams involved in the CEREVANCE prospective cohort study from 2004 onwards. The authors thank the pediatricians and their teams in charge of pediatric patients at Bordeaux (n = 47), APHP-Trousseau (n = 44), APHP-Robert Debré (n = 27), Lyon (n = 23), Toulouse (n = 23), Marseille (n = 18), APHP-Bicêtre (n = 17), Montpellier (n = 14), Nantes (n = 13), Rennes (n = 13), Besancon (n = 11), Strasbourg (n = 11), Lille (n = 10), Nancy (n = 10), APHP-Necker (n = 10), Poitiers (n = 10), Tours (n = 10), Amiens (n = 7), Grenoble (n = 6), Dijon (n = 5), Brest (n = 4), Caen (n = 4), Limoges (n = 3), Nice (n = 3), Rouen (n = 3), Saint-Etienne (n = 3), Angers (= 2), Reims (n = 2), Clermont-Ferrand (n = 1), and Martinique (n = 1). The authors also thank the adult teams, who regularly gave information on patients who had become adults.
This work was financially supported since 2004 by the French Ministry of Health (Programme Hospitalier de Recherche Clinique 2005; Rare Disease Plan 2007, 2017, 2023), the Association Bordelaise pour l’Avancement des Sciences Pédiatriques research charity, the Association pour la Recherche et les Maladies Hématologiques de l’Enfant research charity, the Association Française du Syndrome d’Evans, the O-CYTO patients’ association.
Authorship
Contribution: B.B.-M. first initiated this study; J.G., H.F., N.A., S.H., and T.L. designed the study; J.G., H.F., and N.A. performed the research, analyzed and interpreted data, and drafted the manuscript; J.G. and H.F. performed statistical analysis; and N.A., S.H., T.L., B.B-M., G.L., S.D., M.F., M.P., N.G., V.B., C.G., E.J., C.T., S.B., N.C., C. Paillard, W.A.C., P.C., B.N., F.M., J.L., V.L.-T.T., C.A.-A., C.B., L.C., M.D., C. Piguet, J.B., A.M.-C., J.-L.S., I.P., C. Pluchart, E.D., K.M., A.G., O.R., and P.P., participated in patient recruitment, prospective data collection, and data interpretation and revised the manuscript for critical content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nathalie Aladjidi, Unité d’hématologie pédiatrique, Centre de Référence National des Cytopénies Auto-immunes de l’enfant CEREVANCE, Hôpital des Enfants, Hôpital Pellegrin, Place Amélie Raba Léon, 33000 Bordeaux, France; email: nathalie.aladjidi@chu-bordeaux.fr.
References
Author notes
J.G. and H.F. contributed equally to this study as first authors.
All authors are members of the French Reference Center for Pediatric Autoimmune Cytopenia (CEREVANCE).
Original data are available upon request from the corresponding author, Nathalie Aladjidi (nathalie.aladjidi@chu-bordeaux.fr).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal