In this issue of Blood, Varricchio et al1 investigate how the expression of the megakaryocyte (MK) and platelet inhibitory receptor G6b on hematopoietic progenitor cells correlates to platelet counts in patients with myelofibrosis (MF) and identify a positive feedback loop linking an increased expression of the secreted glycoprotein YKL-40 in thrombocytopenic patients to impaired megakaryopoiesis.
MF is a clonal myeloid malignancy with an incidence rate of 0.33 per 100 000 people according to the US SEER (Surveillance, Epidemiology, and End Results) program.2 MF, together with essential thrombocythemia (ET) and polycythemia vera (PV), is a myeloproliferative neoplasm (MPN) caused by mutations in the thrombopoietin (TPO) receptor MPL, its downstream effector Janus kinase 2 (JAK2) or the CALR (calcium chaperone calreticulin). Although JAK2 inhibitors have significantly improved disease outcomes,3 patients may become resistant, and JAK2 inhibition has been associated with increased incidences of thrombocytopenia due to disrupted TPO signaling and subsequently, impaired megakaryopoiesis, reflecting the unmet need for new treatment options.
MKs are hematopoietic cells primarily residing in the bone marrow, whose complex differentiation process involves the upregulation of MK-specific markers such as the inhibitory MK and platelet marker G6b. Loss-of-function mutations within MPIG6B, the gene encoding for G6b, cause macrothrombocytopenia and progressive bone marrow fibrosis.4 Moreover, G6b was identified as a major regulator of megakaryopoiesis in murine studies, the loss of which resulted in impaired TPO signaling and downregulation of a master transcriptional regulator of MK differentiation, GATA-1.5 In a seminal study, Psaila et al6 uncovered that G6b was upregulated specifically on mutant CD34+ hematopoietic progenitor cells of patients with MF, suggesting therapeutic potential in targeting its expression. Advances in single-cell RNA sequencing have recently identified that MKs partake in both niche and immune-regulatory functions in addition to their capacity as platelet-producing cells, thus maintaining bone marrow homeostasis.7 Although MKs largely contribute to the secretion of profibrotic cytokines such as transforming growth factor β1 (TGFβ1),8 and defective MK differentiation accompanied by thrombocytopenia are commonly diagnosed in patients with MF, the underlying signaling defects conferring impaired MK maturation are incompletely understood.
Given the importance of G6b for MK maturation and building on the Psaila study,6 Varricchio et al sought to identify whether similar expression patterns of common MK-regulating genes in patients with MF with low platelet counts (MPN-MF-T) and a patient carrying a mutation within MPIG6B (MF-MPIG6B) would be causative for impaired MK maturation and subsequently, reduced platelet production. The authors reveal that, although MKs differentiated from cells derived from nonthrombocytopenic patients (MPN-MF-NT) exhibited higher G6b levels than healthy donor cells, patients with both MPN-MF-T and MF-MPIG6B can be distinguished due to low or absent G6b expression. This reduction correlated to lower expression of the late MK marker CD42b (glycoprotein Ibα), the transcription factor GATA-1, and a concomitant impairment in cytoplasmic maturation upon culture of mononuclear cells under MK differentiation conditions. Although plasma TGFβ1 levels were comparable between patients with MPN-MF-NT and MPN-MF-T, patients with thrombocytopenia presented with a distinct upregulation in tumor necrosis factor-α and TPO as well as a significant increase in the secreted glycoprotein YKL-40. YKL-40 is a proinflammatory cytokine, commonly upregulated in inflammatory diseases, and increased YKL-40 plasma levels were previously identified in patients with MF compared to patients suffering from ET or PV.9 To investigate whether an upregulation of YKL-40 may have functional consequences, the authors tested the effect of YKL-40 treatment on megakaryopoiesis and observed a reduced number of MKs after culture in MK-conditioning media. Conversely, treatment with recombinant TGFβ1 increased intracellular YKL-40 levels and treatment of patients with a TGFβ1 protein trap not only reduced plasma YKL-40 levels but concomitantly increased platelet counts, suggesting a positive feedback loop interconnecting TGFβ1 and YKL-40 to MK differentiation.
Varricchio et al address an important question that has puzzled researchers for decades: why do MKs in MF display maturation defects and fail to differentiate toward mature, platelet-producing cells? Previous studies have identified reduced GATA-1 expression, low ploidy, and cytoplasmic maturation defects in MKs derived from patients with MF.8 Building on these findings, the present study reveals that MK maturation defects observed in a patient with MF-MPIG6B were recapitulated in MPN-MF-T patients, highlighting the importance of G6b during megakaryopoiesis (see figure). However, while the findings strongly support the notion that G6b regulates MK differentiation, it remains unknown whether defective G6b expression is the first cue instigating impaired MK maturation in MF or whether other intracellular signals reduce MK maturation and consequently G6b expression. It would be interesting to test whether the observed upregulation of YKL-40 in MKs derived from patients with MF-MPIG6B and MPN-MF-T is G6b-dependent, that is, by investigating what causes an upregulation of YKL-40 in G6b-deficient cells and whether it can be reversed by re-introducing G6b. Psaila et al6 previously suggested using a bispecific antibody targeting CD34 and G6b to specifically clear mutant clones from the bone marrow of patients with MF, however, given the findings presented here, platelet counts and MK maturation defects should be put into account when selecting patients for immunotherapy.
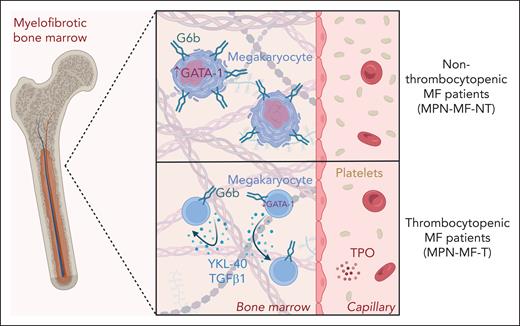
G6b expression and YKL-40 secretion correlate with platelet counts in patients with MF. Thrombocytopenia is a common symptom of MF and can be exacerbated upon treatment with JAK2 inhibitors. In nonthrombocytopenic patients with MF, MKs express high levels of the MK and platelet inhibitory receptor G6b, which correlates to high GATA-1 levels and sufficient cytoplasmic maturation. Thrombocytopenia in MF, however, is associated with impaired G6b expression, low GATA-1 levels and increased inflammatory cytokine secretion. Autocrine and paracrine signaling of both the secreted glycoprotein YKL-40 and the profibrotic TGFβ1 further attenuate MK maturation. The figure was created with BioRender.com. Roweth H. (2025) https://BioRender.com/up5g4as.
G6b expression and YKL-40 secretion correlate with platelet counts in patients with MF. Thrombocytopenia is a common symptom of MF and can be exacerbated upon treatment with JAK2 inhibitors. In nonthrombocytopenic patients with MF, MKs express high levels of the MK and platelet inhibitory receptor G6b, which correlates to high GATA-1 levels and sufficient cytoplasmic maturation. Thrombocytopenia in MF, however, is associated with impaired G6b expression, low GATA-1 levels and increased inflammatory cytokine secretion. Autocrine and paracrine signaling of both the secreted glycoprotein YKL-40 and the profibrotic TGFβ1 further attenuate MK maturation. The figure was created with BioRender.com. Roweth H. (2025) https://BioRender.com/up5g4as.
The study also strikingly confirms the relevance of considering how changes to the microenvironment may enhance defective MK maturation. Although studies have demonstrated the beneficial effect of targeting the inflammatory microenvironment on MF progression,10 the present results shed important light on the role of MKs therein. MKs do not only respond to YKL-40 or TGFβ1 treatment, but are also a major source for both cytokines, thus verifying the important role of MKs in regulating the hematopoietic microenvironment in disease. However, more research on the effect of YKL-40 on other cells is needed to decide whether it might be a novel therapeutic target for the treatment of MF. Moreover, although the study refers to an inflammatory profile in MKs derived from patients with MPN-MF-T, the exact nature of this and whether it correlates to previously described immune-regulatory signatures discovered in MKs7 remains to be determined.
Overall, the study by Varricchio et al reveals an interesting autocrine signaling pathway in MKs that promotes MK dysfunction and adds a little piece to the puzzle of understanding the causes of MK dysplasia in MF.
Conflict-of-interest disclosure: The author declares no competing financial interests.