Visual Abstract
In September 2023, the US Food and Drug Administration approved momelotinib for the treatment of myelofibrosis (MF) with anemia, marking the fourth US regulatory approval of a Janus kinase inhibitor for MF. A positive opinion from the European Medicines Agency followed in November 2023. Momelotinib’s ability to address splenomegaly, symptoms, and anemia, including in patients with thrombocytopenia (with platelet counts of ≥25 × 109/L), the ease of switching from ruxolitinib, and good tolerability uniquely position it to substantially impact the MF treatment landscape.
Introduction
Momelotinib (formerly CYT387) is an aminopyrimidine derivative that inhibits Janus kinase 1 (JAK1), JAK2, and tyrosine kinase 2 at low nanomolar concentrations.1 Mechanistic insights into the unexpected anemia benefit of momelotinib in patients with myelofibrosis (MF) were provided by the discovery, in a rodent model of anemia of chronic disease, that momelotinib directly inhibits bone morphogenic protein receptor kinase activin A receptor, type I, thus downregulating hepatocyte hepcidin production.2 This led to the hypothesis that momelotinib’s anemia benefits in patients with MF, a disease characterized by generalized inflammation, stem from mobilization of iron from the reticuloendothelial system and stimulation of erythropoiesis (Figure 1).3 This was validated in an open-label, phase 2, translational biology study in 41 red blood cell (RBC) transfusion–dependent (RBC-TD) patients with MF, of whom 41% achieved transfusion independence (TI) for ≥12 weeks.4 Of TI nonresponders, 78% achieved halving or better of their transfusion requirements for ≥8 weeks. This was associated with an acute and sustained decrease in blood hepcidin, associated with increased iron availability and markers of erythropoiesis.
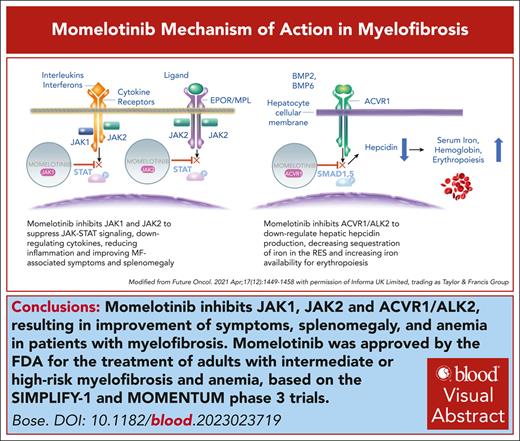
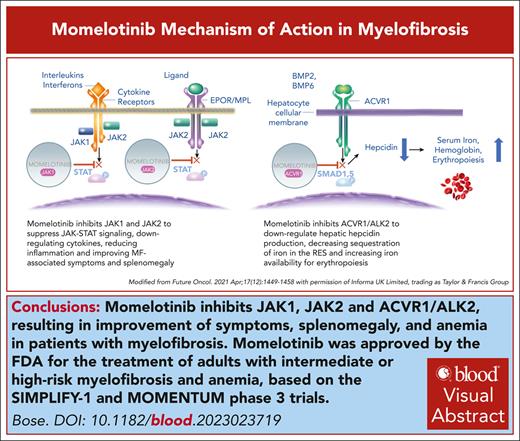
Systemic iron homeostasis and its regulation by the hepcidin-ferroportin axis. Momelotinib suppresses hepcidin expression in the liver via inhibition of the bone morphogenetic protein (BMP) 6/activin A receptor type 1 (ACVR1)/small mothers against decapentaplegic (SMAD) and interleukin 6 (IL-6)/JAK/STAT3 pathways, leading to reduced sequestration of iron in the reticuloendothelial system and enhanced iron availability for bone marrow erythropoiesis. Hepcidin is elevated in patients with MF due to aberrant hyperactivation of signaling via the BMP6-stimulated kinase, ACVR1/ALK2, and inflammatory cytokine signaling via IL-6, which is also elevated in patients with MF. Suppression of hepcidin expression in the liver increases circulating iron (transferrin [Tf]-Fe3+]2) and hemoglobin and stimulates erythropoiesis in the bone marrow. The lower panel lists the major causes of anemia in myelofibrosis. EPO, erythropoietin; ERFE, erythroferrone; H, hepcidin; MMB, momelotinib; RBC, red blood cell. Modified, with permission, from Chifotides HT, Bose P, Verstovsek S. J Hematol Oncol. 2022;15(1):7. Publication license: https://creativecommons.org/licenses/by/4.0/.
Systemic iron homeostasis and its regulation by the hepcidin-ferroportin axis. Momelotinib suppresses hepcidin expression in the liver via inhibition of the bone morphogenetic protein (BMP) 6/activin A receptor type 1 (ACVR1)/small mothers against decapentaplegic (SMAD) and interleukin 6 (IL-6)/JAK/STAT3 pathways, leading to reduced sequestration of iron in the reticuloendothelial system and enhanced iron availability for bone marrow erythropoiesis. Hepcidin is elevated in patients with MF due to aberrant hyperactivation of signaling via the BMP6-stimulated kinase, ACVR1/ALK2, and inflammatory cytokine signaling via IL-6, which is also elevated in patients with MF. Suppression of hepcidin expression in the liver increases circulating iron (transferrin [Tf]-Fe3+]2) and hemoglobin and stimulates erythropoiesis in the bone marrow. The lower panel lists the major causes of anemia in myelofibrosis. EPO, erythropoietin; ERFE, erythroferrone; H, hepcidin; MMB, momelotinib; RBC, red blood cell. Modified, with permission, from Chifotides HT, Bose P, Verstovsek S. J Hematol Oncol. 2022;15(1):7. Publication license: https://creativecommons.org/licenses/by/4.0/.
Early-phase clinical trials in MF
Momelotinib was tested in a 2-part (dose escalation and confirmation), phase 1/2 core study in 166 patients with International Prognostic Scoring System intermediate- or high-risk MF, followed by an open-label extension study for patients who completed 9 cycles on the core study.5 Three hundred milligrams daily was declared the maximum tolerated dose, and doses of 150 mg daily, 150 mg twice daily, and 300 mg daily were expanded in part 2 of the core study. Over two-thirds of RBC-TD patients achieved 12-week TI, and 28.2% of non-TD patients achieved a hemoglobin response lasting ≥12 weeks. Median increase in hemoglobin was 2.4 g/dL, and median duration of 12-week anemia response was 12.8 months. Forty percent of evaluable patients had a spleen response (clinical improvement per 2006 International Working Group for Myelofibrosis Research and Treatment criteria6). Spleen responses occurred rapidly and were durable. An overall improvement of constitutional symptoms was noted. Median overall survival (OS) had not been reached after a median follow-up of 19.5 months. Momelotinib did not reduce the variant allele frequency of JAK2V617F. Dizziness, nausea, hypotension, headache, and flushing after the first dose of momelotinib were seen. Peripheral neuropathy and thrombocytopenia were the most common adverse events leading to drug discontinuation.
One hundred of the aforementioned 166 patients enrolled and treated at the Mayo Clinic have formed the basis of several insightful reports. The mean elimination steady-state half-life of momelotinib was found to be 4 to 6 hours,7 which may explain the ease of switching from ruxolitinib to momelotinib without the need for tapering and/or overlapping to prevent ruxolitinib discontinuation syndrome.8 A median 3-kg increase in weight was observed.7 Momelotinib downregulated several cytokines, of which interleukin 1 receptor antagonist (IL-1RA) and IL-1β correlated with TI responses. Spleen responses were associated with decreases in IL-1RA, IL-1β, IL-2, fibroblast growth factor-basic, tumor necrosis factor α, and macrophage inflammatory protein-1β.7 Anemia responses to momelotinib were more likely in patients with postessential thrombocythemia MF, lower baseline serum ferritin, and shorter time from diagnosis to initiation of momelotinib, and they did not correlate with mutation status.9,10 Forty-four (44%) of the Mayo Clinic patients developed a treatment-emergent sensory peripheral neuropathy, nearly all grade 1, on momelotinib, with median time to onset of 32 weeks and median duration of 11 months.11 In general, the phenomenon did not appear reversible, but neither was it progressive. The only predictive factor was duration of momelotinib therapy. Interestingly, neither previous exposure to immunomodulatory drugs nor preexisting peripheral neuropathy increased the risk. Seven-year follow-up of the 100 patients showed the median duration of momelotinib therapy to be 1.4 years and median OS from momelotinib initiation of 3.2 years.12 In line with findings from SIMPLIFY-1 (discussed in “The SIMPLIFY studies”),13 anemia response to momelotinib had a favorable impact on survival in 72 JAK inhibitor-naïve Mayo Clinic patients.9
Phase 3 trials in MF
The SIMPLIFY studies
One of the largest trials conducted in MF, SIMPLIFY-1 randomly assigned JAK inhibitor-naïve patients with MF to receive momelotinib, 200 mg daily, or ruxolitinib, 20 mg twice daily or per label, for 24 weeks, after which patients receiving ruxolitinib could cross over to momelotinib (Table 1).14 SIMPLIFY-1 met its primary end point of noninferiority of momelotinib for spleen volume reduction of ≥35% (SVR35) at week 24. The key secondary end point of noninferiority of momelotinib for ≥50% reduction in total symptom score (TSS50) at week 24 was not met. The study used sequential testing to minimize type 1 error. As such, formal statistical testing was not performed, and only nominal significance was reported for the other secondary end points. All anemia-related end points favored momelotinib. Numerically, more momelotinib (38.6%) than ruxolitinib (34.6%) patients achieved ≥2 of the prespecified end points (SVR35, TSS50, or TI) at week 24, as well as all 3 end points (10.2% for momelotinib and 7.8% for ruxolitinib). Momelotinib dose intensity was better maintained than that of ruxolitinib.
Subsequent analyses of SIMPLIFY-1 have yielded several important insights. Momelotinib treatment led to a rapid increase in mean hemoglobin, reaching a significantly higher level at week 24 than at baseline, whereas the opposite was true for ruxolitinib.17 In >90% of ruxolitinib-randomized patients who crossed over to momelotinib (n = 197), a steep increase in mean hemoglobin occurred, with levels approaching those in patients on the momelotinib arm. Of 92 patients in the ruxolitinib group who were not transfusion independent at week 24 and crossed over to momelotinib, 42 (46%) became transfusion independent by week 12.8 Patients who were RBC transfusion independent at baseline were significantly more likely to remain so at week 24 on momelotinib than on ruxolitinib (81% vs 62%; P < .001).17 Conversion from RBC-transfusion dependence to TI for any 12-week period during the randomized phase was also more frequent on momelotinib (44% vs 25%; P = .034). Median duration of TI had not been reached for momelotinib-randomized patients after >3 years of follow-up. TI at week 24 in patients randomized to momelotinib was associated with improved OS,13 establishing TI as an important surrogate for long-term outcome, as documented for spleen response in the context of ruxolitinib.18,19 There were no meaningful differences in OS between the 2 arms.13 Mean platelet counts were generally stable on momelotinib, whereas they decreased by ∼100 × 109/L during the first 4 weeks on ruxolitinib and stayed low throughout the randomized phase, before recovering on crossover to momelotinib and converging with those of the momelotinib group by week 48.8 Evaluation of longitudinal change from baseline in TSS over the continuous 24-week period and individual symptom scores demonstrated similar overall symptom improvements for the 2 drugs, with a TSS difference of <1.5 points between them for each postbaseline visit.20 Similar proportions of patients were categorized as “improved” in the 2 groups. Using an absolute “meaningful change threshold (8 points),” symptom responses were achieved in similar proportions of patients (39% momelotinib, 41% ruxolitinib) at week 24.21 More weight gain was observed in the ruxolitinib group during the randomized phase; this did not worsen on crossover to momelotinib.8 Bone marrow fibrosis grade changes did not correlate with TI, hemoglobin levels, spleen/symptom outcomes, or OS,22 questioning whether this, as currently measured, is an indicator of “disease modification” in MF.23
SIMPLIFY-2 was a randomized, phase 3, open-label trial in ruxolitinib-intolerant (≥28 days of prior ruxolitinib with either need for RBC transfusions while on ruxolitinib or ruxolitinib dose reduction to <20 mg twice daily with grade ≥3 thrombocytopenia, anemia, and/or bleeding) patients with MF with a ≥5-cm palpable spleen.15 Patients were randomized to receive either 200 mg daily of momelotinib or best available therapy (BAT), which was ruxolitinib in 89% of patients. No washout from prior ruxolitinib was required. SIMPLIFY-2 did not meet its primary end point of SVR35 at week 24, plausibly because of the lack of a ruxolitinib washout in this study that essentially pitted momelotinib against continuation of ruxolitinib in a ruxolitinib-intolerant (rather than resistant) population. As such, statistical significance could not be claimed for the secondary end points. Twenty-six percent of evaluable momelotinib recipients vs 6% of evaluable BAT recipients achieved TSS50 at week 24 (nominal P = .0006). Again, all anemia-related end points favored momelotinib. A trend toward improved OS was noted in momelotinib-randomized patients who were transfusion independent at week 24 vs those who were not.13 Median OS from baseline was 2.9 years in patients randomized to momelotinib and 3.1 years in those who crossed over to momelotinib from BAT.
The MOMENTUM study
The most recent phase 3 trial, MOMENTUM, recruited JAK inhibitor-exposed (≥90 days or per the aforementioned SIMPLIFY-2 inclusion criteria for hematologic toxicity) patients with MF with splenomegaly who were symptomatic and anemic.24 Patients were randomly assigned to receive momelotinib, 200 mg daily, or danazol, an active control for anemia, 300 mg twice daily, in this placebo-controlled, blinded trial. A minimum 2-week washout from prior JAK inhibitor therapy and baseline platelets ≥25 × 109/L were required. All patients who remained on active therapy on the study beyond 24 weeks received open-label momelotinib. The primary end point of superiority of momelotinib for TSS50 at week 24 was met, as were the key secondary end points of superiority for SVR35 and noninferiority for TI.16 All other anemia-related end points also favored momelotinib. Spleen responders (both SVR35 and SVR25) to momelotinib had higher rates of week 24 TI than spleen nonresponders. The hazard ratio for OS over the entire study period was 0.73, favoring momelotinib. Superiority of momelotinib over danazol for spleen and symptom responses was maintained in the subgroups of patients with baseline platelets <100 × 109/L (n = 100) and <50 × 109/L (n = 31), and rates of TI were numerically higher. Ninety-three (72%) of the 130 originally momelotinib-randomized patients and 41 (63%) of the 65 patients originally randomized to danazol entered the momelotinib open-label extension period and were the subjects of a post hoc 48-week (median) analysis.16 In both groups, spleen, TSS50, and TI response rates improved from week 24 to week 48. After crossover, mean TSS and hemoglobin in the originally danazol-randomized patients approached and converged on those of the patients who continued momelotinib. Although new spleen responses were observed after crossover, the SVR35 rates at week 48 favored those who started with and continued on momelotinib (43% vs 13%).
Safety
Peripheral neuropathy with momelotinib was much less frequent in each of the phase 3 studies than in the aforementioned reports from the phase 1/2 study. Patients in all 3 studies could continue to receive momelotinib after completing study treatment on an extended access protocol. A total of 725 patients received momelotinib on the 3 studies, with 12% remaining on therapy for ≥5 years.25 The median duration of exposure to momelotinib was 11.3 months across these frontline and later-line studies. Diarrhea (3% grade ≥3), anemia, and thrombocytopenia occurred in 27%, 25%, and 23% of patients, respectively. Thrombocytopenia was the most common (4%) reason for discontinuation. The incidence of infections, blast transformation, hemorrhage, and peripheral neuropathy did not increase over time.
Which JAK inhibitor for cytopenic MF?
In recent years, the differences between the proliferative and cytopenic phenotypes of MF have increasingly been appreciated, and the availability of multiple JAK inhibitors allows for somewhat tailored therapy.26 Pacritinib, US Food and Drug Administration approved for the treatment of intermediate-/high-risk MF with severe thrombocytopenia (platelets, <50 × 109/L), was also recently recognized to be a potent inhibitor of activin A receptor, type I and to lead to a 24% rate of TI in non–transfusion-independent (RBC-TD or transfusion requiring without meeting formal criteria for RBC-TD) patients in the pivotal trial, PERSIST-2, using “SIMPLIFY” criteria, although these analyses27 used a “rolling” 12-week interval without transfusions or a hemoglobin level <8 g/dL, not a “terminal” one as used in the SIMPLIFY studies.28 Anemia and thrombocytopenia often coexist,29 raising the question of which JAK inhibitor may be optimal for frontline use in such patients, especially given momelotinib’s established anemia benefits and the absence of a platelet count restriction in its label. Momelotinib’s efficacy was preserved in thrombocytopenic patients (baseline platelets, <100 × 109/L; n = 126) across the 3 phase 3 trials, and dose intensity was better maintained than with ruxolitinib in the SIMPLIFY studies.30 Specifically, the rates of week 24 SVR35, TSS50, and TI with momelotinib in SIMPLIFY-1 were 27%, 28%, and 67%, respectively, in the overall population and 39%, 35%, and 61%, respectively, in the thrombocytopenic subgroup. However, relatively few patients with baseline platelets <50 × 109/L were randomized to receive momelotinib in the phase 3 trials, 18 in MOMENTUM and 9 in SIMPLIFY-2, and thrombocytopenia is one of the more common adverse effects of momelotinib, possibly favoring pacritinib in patients with platelets <50 × 109/L, consistent with its label.
Conclusion
In summary, momelotinib, given its ability to improve all 3 cardinal clinical features of MF, limited myelosuppressive potential, facilitating maintenance of dose intensity, good tolerability, and short half-life, represents an important and welcome new addition to the therapeutic armamentarium for MF, and is likely to increasingly be used as a JAK inhibitor backbone for future combination trials. Potential combination partners for momelotinib that make intuitive sense based on their success in combination with ruxolitinib include the bromodomain and extraterminal protein inhibitor pelabresib31 and luspatercept (to augment anemia responses).32 However, the first momelotinib-based combination to enter clinical testing may be that with the PIM kinase inhibitor, TP-3654 (NCT04176198), which has demonstrated encouraging clinical activity, especially with respect to symptom improvement, as monotherapy in the post-JAK inhibitor setting.33
Acknowledgments
The author acknowledges Helen T. Chifotides for her help with the figure and visual abstract.
This work was supported, in part, by MD Anderson Cancer Center support grant P30 CA016672 from the National Institutes of Health, National Cancer Institute.
Authorship
Contribution: P.B. wrote the manuscript.
Conflict-of-interest disclosure: P.B. discloses research support from Incyte, Bristol Myers Squibb (BMS), CTI BioPharma, Morphosys, Kartos, Telios, Sumitomo, Karyopharm, Disc Medicine, Ionis, Blueprint Medicines, Cogent, Geron, and Janssen; and honoraria/consulting fees from Incyte, BMS, GlaxoSmithKline, CTI BioPharma, AbbVie, Morphosys, Sumitomo, Karyopharm, Disc Medicine, Ionis, Pharma Essentia, Jubilant, Morphic, Novartis, Blueprint Medicines, Geron, and Cogent.
Correspondence: Prithviraj Bose, Department of Leukemia, University of Texas MD Anderson Cancer Center, 1400 Holcombe Blvd, FC4.3062, Houston, TX 77030; email: pbose@mdanderson.org.

![Systemic iron homeostasis and its regulation by the hepcidin-ferroportin axis. Momelotinib suppresses hepcidin expression in the liver via inhibition of the bone morphogenetic protein (BMP) 6/activin A receptor type 1 (ACVR1)/small mothers against decapentaplegic (SMAD) and interleukin 6 (IL-6)/JAK/STAT3 pathways, leading to reduced sequestration of iron in the reticuloendothelial system and enhanced iron availability for bone marrow erythropoiesis. Hepcidin is elevated in patients with MF due to aberrant hyperactivation of signaling via the BMP6-stimulated kinase, ACVR1/ALK2, and inflammatory cytokine signaling via IL-6, which is also elevated in patients with MF. Suppression of hepcidin expression in the liver increases circulating iron (transferrin [Tf]-Fe3+]2) and hemoglobin and stimulates erythropoiesis in the bone marrow. The lower panel lists the major causes of anemia in myelofibrosis. EPO, erythropoietin; ERFE, erythroferrone; H, hepcidin; MMB, momelotinib; RBC, red blood cell. Modified, with permission, from Chifotides HT, Bose P, Verstovsek S. J Hematol Oncol. 2022;15(1):7. Publication license: https://creativecommons.org/licenses/by/4.0/.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/7/10.1182_blood.2023023719/2/m_blood_bld-2023-023719-c-gr1.jpeg?Expires=1765964877&Signature=TqIue-HWODNdhtdWAXMUqa9bdiUssN2w9IbRsoMOOBA9HFXSrOu2pXCy9xd7F6ri2bfRBhk0R0S5hPi~4bf0N4tjqHN8qIN8cTN0EK9DB6tOX9KVGiDjzlB9ofSgB3Z5hBDM6bqQ9tb8wjXnSTGSIl0KYYNtWpNuq7yF~32hWBelnGIRmq51qOfv9~8uMl08WRwdiFBGzdnAfzEZHkGHkyStEyYi8lOeWddJW7vTDdLQkS0wJDxrB7d3f61wtieE7uH5mrko20MOu71mRxaIQOjT-Rt72Ag-4NtvUCGDAg1BjI-qF1sBTkq4OQWk4pq1vki4TrTeknJs6EpR-yos6w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Systemic iron homeostasis and its regulation by the hepcidin-ferroportin axis. Momelotinib suppresses hepcidin expression in the liver via inhibition of the bone morphogenetic protein (BMP) 6/activin A receptor type 1 (ACVR1)/small mothers against decapentaplegic (SMAD) and interleukin 6 (IL-6)/JAK/STAT3 pathways, leading to reduced sequestration of iron in the reticuloendothelial system and enhanced iron availability for bone marrow erythropoiesis. Hepcidin is elevated in patients with MF due to aberrant hyperactivation of signaling via the BMP6-stimulated kinase, ACVR1/ALK2, and inflammatory cytokine signaling via IL-6, which is also elevated in patients with MF. Suppression of hepcidin expression in the liver increases circulating iron (transferrin [Tf]-Fe3+]2) and hemoglobin and stimulates erythropoiesis in the bone marrow. The lower panel lists the major causes of anemia in myelofibrosis. EPO, erythropoietin; ERFE, erythroferrone; H, hepcidin; MMB, momelotinib; RBC, red blood cell. Modified, with permission, from Chifotides HT, Bose P, Verstovsek S. J Hematol Oncol. 2022;15(1):7. Publication license: https://creativecommons.org/licenses/by/4.0/.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/7/10.1182_blood.2023023719/2/m_blood_bld-2023-023719-c-gr1.jpeg?Expires=1765964878&Signature=pG1HWdRcnx5fm1NIev2Kd9R4D4mUIZL2RZnG9TkxfyfsCWud7FdIJMWpo4qZgCBkCYVkRV8Vu70d-NNNeqZEFg5gWxuaPHSX4XgV00aCvkM7w8oU5~jslzI3kNB-2fAl2Q-tnx2PgERmv1Stny9bBMuGnNWHmmghB6PFY5WN6Qox-Cp-QRrCf6N2js3~F8l4nJU0Heigi81i0Z~6pjpTSZ2SoQH0p6-KfzFSTjqmLdYFqM8bX1qh10SLhw9Nkz~nuT~AaCl1SKoguBQhPs9-sFBlNhGMieuyOW4davPjyMglWzj5R0bzpqKIqKiK17t-ktspTPQX8jpqujXXhzPxmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)