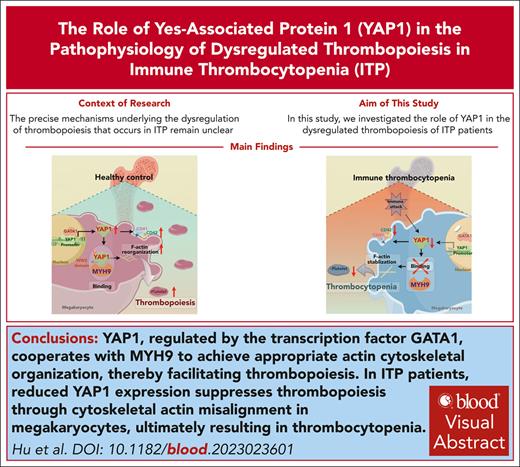
Key Points
Reduced YAP1 expression suppresses thrombopoiesis through cytoskeletal actin misalignment in MKs, causing thrombocytopenia during ITP.
YAP1, regulated by GATA-binding protein 1, cooperates with myosin heavy chain 9 to reach actin reorganization, facilitating thrombopoiesis.
Visual Abstract
Immune thrombocytopenia (ITP) is a complicated bleeding disease characterized by a sharp platelet reduction. As a dominating element involved in ITP, megakaryocytes (MKs) are responsible for thrombopoiesis. However, the mechanism underlying the dysregulation of thrombopoiesis that occurs in ITP remains unidentified. In this study, we examined the role of Yes-associated protein 1 (YAP1) in thrombopoiesis during ITP. We observed reduced YAP1 expression with cytoskeletal actin misalignment in MKs from patients with ITP. Using an experimental ITP mouse model, we showed that reduced YAP1 expression induced aberrant MK distribution, reduced the percentage of late MKs among the total MKs, and caused submaximal platelet recovery. Mechanistically, YAP1 upregulation by binding of GATA-binding protein 1 to its promoter promoted MK maturation. Phosphorylated YAP1 promoted cytoskeletal activation by binding its WW2 domain to myosin heavy chain 9, thereby facilitating thrombopoiesis. Targeting YAP1 with its activator XMU-MP-1 was sufficient to rescue cytoskeletal defects and thrombopoiesis dysregulation in YAP1+/− mice with ITP and patients. Taken together, these results demonstrate the crucial role of YAP1 in thrombopoiesis, providing potential for the development of diagnostic markers and therapeutic options for ITP.
Introduction
Immune thrombocytopenia (ITP) is an acquired hemorrhagic autoimmune disease characterized by severe thrombocytopenia.1 Despite advances in knowledge, its intricate mechanisms remain inadequately elucidated. The pathogenesis revolving around immune dysregulation, particularly among T cells, B cells, dendritic cells, and myeloid-derived suppressor cells,1,2 manifested as accelerated platelet destruction and decreased production.3 Autoantibodies trigger abnormal apoptosis of platelets and inhibit megakaryocyte (MK) maturation, hindering platelet release.4 Although cytotoxic T lymphocytes (CTLs) mediate direct platelet destruction5 and indirectly suppress platelet production by inhibiting MK apoptosis.6 A synergistic interplay between humoral and cellular immunity fuels thrombocytopenia, leading to subsequent bleeding manifestations in various tissues such as skin, mucous membranes, and internal organs.7
Megakaryopoiesis involves the intricate differentiation of MK progenitors to functional platelets from hematopoietic stem cells (HSCs).8 A critical step in proplatelet formation (also known as thrombopoiesis) is the migration of MKs from the bone marrow (BM) periosteal niche toward the BM sinusoidal blood vessels.9 Once localized in the proximity of marrow sinusoids, MKs extend to form proplatelets through the endothelial barrier.10 Studies indicate significant reductions in MK maturation and distribution within the BM periosteal niche in ITP, suggesting impaired megakaryopoiesis is critical in the pathogenesis of ITP.11 However, the intricate mechanisms underlying this process during ITP remain poorly understood.
Yes-associated protein 1 (YAP1), identified as a consequence of its interaction with the Src-homology 3 domain of the tyrosine kinase yes,12 serves as a core regulator in the Hippo signaling pathway.13 Once entering the nucleus, YAP1 forms complexes with TEA domain transcription factors to activate the transcription of genes involved in cell proliferation, invasion, and metastasis.14 Functionally, YAP1 is essential for regulating cytoskeletal tension15 and GATA-binding protein 1 (GATA1)–mediated tumor suppressor Ras association domain family member 1a, which acts upstream of YAP1, during the transition from pluripotency to differentiation.16 Although YAP1 has been extensively studied in tumors and hematologic malignancies,17-19 its role in megakaryopoiesis is inconclusive. Royet al showed YAP1 depletion hindered platelet generation due to mitochondrial defect in MKs,20 whereas Lorthongpanich et al revealed YAP1 upregulation promotes expansion of CD41+ immature MKs.21
Our study disclosed a reduced YAP1 expression in ITP MKs, linked to a defective cytoskeleton. In ITP mice, decreased YAP1 levels correlated with aberrant MK distribution and insufficient thrombopoiesis. Mechanistically, YAP1 was regulated by GATA1, promoting MK maturation. Furthermore, phosphorylated YAP1 (P-YAP1) partnering with myosin heavy chain 9 (MYH9) facilitated cytoskeletal rearrangement for proplatelet formation. Using a YAP1+/− ITP mouse model and MKs from patients with ITP, we showed that the administration of XMU-MP-1, a YAP1 agonist, restored thrombopoietic function in YAP1+/− mice, implicating YAP1 as a promising therapeutic target for ITP.
Methods
More details on the materials and methods are provided in the supplemental Material, available on the Blood website.
Patients and controls
Healthy donors and patients with ITP were recruited at the First Affiliated Hospital of Soochow University. This study was approved by the Medical Ethical Committees of the First Affiliated Hospital of Soochow University. Written informed consent was obtained from each patient and healthy donor in accordance with the Declaration of Helsinki.
Animal ITP model
All animal protocols were approved by the Soochow University Animal Care and Use Committee. YAP1+/− mice were generated via clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated 9 (CRISPR/Cas9) technology and IV injected with an anti-platelet monoclonal CD41 antibody at an initial dose of 0.2 mg/kg body weight for 7 days.
HSC generation and primary MK cultures
CD34+ HSCs were isolated from peripheral blood using an immunomagnetic bead cell-sorting system and cultured with the human cytokines thrombopoietin, stem cell factor, and interleukin-3 over a period of 12 days.
Chromatin immunoprecipitation
The cells were crosslinked with 1% formaldehyde and quenched with glycine for 5 minutes. Pellets were lysed and sonicated after centrifugation. Sheared chromatin was then incubated with the primary antibody overnight, followed by elution and reverse crosslinking at 65°C overnight. Tris-EDTA buffer was added, followed by RNase treatment at 37°C for 30 minutes and proteinase K treatment at 51°C for 1 hour, after which the DNA was subsequently isolated and purified.
Luciferase reporter assay
HEK293T cells were cultured and transfected with pcDNA3.1-GATA1 and pGL3-YAP1-wild type/mutant type using Lipofectamine 2000. Five hours after transfection, the medium was replenished with a medium containing curcumin. Forty-eight hours after transfection, luciferase activity was measured using the Dual-Luciferase Reporter Assay System.
Statistical analysis
All data are shown as the mean ± standard error of the mean and were analyzed using PRISM software (GraphPad software version 8.0). Statistical significance was established using the Student t test, as described in the legends, or the 1-way analysis of variance, followed by the pairwise multiple comparison procedures. A P value <.05 indicated statistically significant results.
Results
YAP1 expression is reduced in MKs from patients with ITP
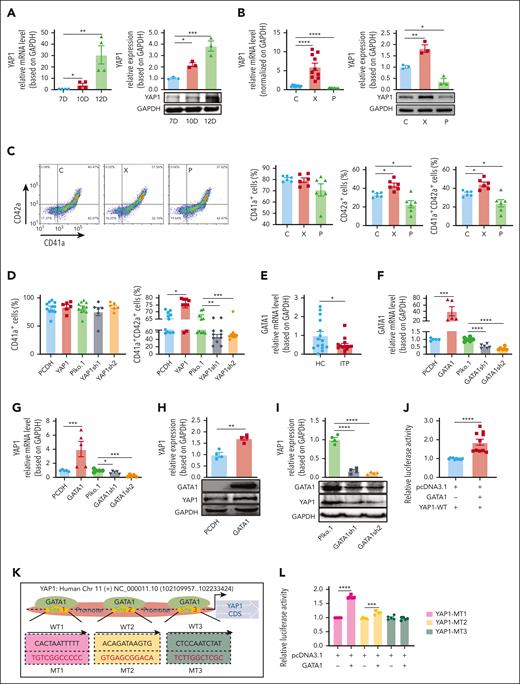
YAP1, a vital component of the Hippo signaling pathway, governs cell proliferation and differentiation to maintain homeostasis,22 and patients with ITP exhibit decreased platelet production due to MK maturation disorders. To investigate the potential role of YAP1 in ITP, we performed a flow cytometry analysis of MKs from the BM of patients with ITP. Patients with ITP displayed a lower percentage of YAP1+ MKs, both overall MKs (ITP 45.29% vs healthy 95.25%; P < .05; Figure 1A) and among mature MKs (ITP 38.77% vs healthy 82.34%; P < .01; Figure 1B). Messenger RNA (mRNA) analysis of ITP (n = 9) and healthy (n = 7) donor MKs showed a 54.87% reduction in YAP1 mRNA expression (P < .0001), consistent with other MK markers (CD41, CD42a, CD42b, and CD61; Figure 1C). Intriguingly, compared with the even distribution in the nucleus and cytoplasm in healthy MKs, YAP1 exhibited cytoplasmic dominance in MKs from patients with ITP (Figure 1D).
Reduced YAP1 expression is involved in megakaryopoiesis during ITP development. (A-B) Flow cytometry analysis of the percentage of YAP1+CD41+ MKs among CD41+ MKs (A) and YAP1+CD41+CD42+ MKs among CD41+CD42+ MKs (B) (n = 5). (C) Verification of YAP1 and CD41/CD42/CD61 mRNA levels in MKs from the BM of 9 patients with ITP relative to MKs from the BM of 7 healthy controls (HCs) by real-time quantitative polymerase chain reaction (qPCR). (D) Confocal images of immature and mature MKs from patients with ITP and healthy controls after immunostaining for YAP1 (green, YAP1) and MKs (red, CD41). Scale bar, 10 μm. (E) mRNA levels of YAP1 in WT mice before and after ITP establishment (n ≥ 5). (F) Changes in peripheral platelet levels in mice ar antibody administration. (G) Confocal images of BM sections from WT and YAP1+/− mice after immunostaining for YAP1 (green, YAP1), vascular sinusoids (yellow, laminin A), and MKs (red, CD41). Scale bar, 50 μm. Analysis of MK numbers (left) and number of MKs located around the sinusoids (right; n ≥ 10). (H) Cell clusters in the BM were identified, shown in a t-distributed stochastic neighbor embedding (tSNE) plot. The colors indicate the cell types. (I) Representative gene set enrichment analysis of early and late MKs. (J) Representative Gene Ontology (GO) terms showing differentially expressed genes in the late MK cluster between WT and YAP1+/− mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. DAPI, 4ʹ,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HC-imMK, healthy control–immature MK; HC-mMK, healthy control–mature MK; ITP-imMK, ITP-immature MK; ITP-mMK, ITP-mature MK; MEP, MK-erythroid progenitor; NES, normalized enrichment score; NK, natural killer cell; PDC, plasma dendritic cell.
Reduced YAP1 expression is involved in megakaryopoiesis during ITP development. (A-B) Flow cytometry analysis of the percentage of YAP1+CD41+ MKs among CD41+ MKs (A) and YAP1+CD41+CD42+ MKs among CD41+CD42+ MKs (B) (n = 5). (C) Verification of YAP1 and CD41/CD42/CD61 mRNA levels in MKs from the BM of 9 patients with ITP relative to MKs from the BM of 7 healthy controls (HCs) by real-time quantitative polymerase chain reaction (qPCR). (D) Confocal images of immature and mature MKs from patients with ITP and healthy controls after immunostaining for YAP1 (green, YAP1) and MKs (red, CD41). Scale bar, 10 μm. (E) mRNA levels of YAP1 in WT mice before and after ITP establishment (n ≥ 5). (F) Changes in peripheral platelet levels in mice ar antibody administration. (G) Confocal images of BM sections from WT and YAP1+/− mice after immunostaining for YAP1 (green, YAP1), vascular sinusoids (yellow, laminin A), and MKs (red, CD41). Scale bar, 50 μm. Analysis of MK numbers (left) and number of MKs located around the sinusoids (right; n ≥ 10). (H) Cell clusters in the BM were identified, shown in a t-distributed stochastic neighbor embedding (tSNE) plot. The colors indicate the cell types. (I) Representative gene set enrichment analysis of early and late MKs. (J) Representative Gene Ontology (GO) terms showing differentially expressed genes in the late MK cluster between WT and YAP1+/− mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. DAPI, 4ʹ,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HC-imMK, healthy control–immature MK; HC-mMK, healthy control–mature MK; ITP-imMK, ITP-immature MK; ITP-mMK, ITP-mature MK; MEP, MK-erythroid progenitor; NES, normalized enrichment score; NK, natural killer cell; PDC, plasma dendritic cell.
To improve our understanding of the reduced expression and abnormal distribution of YAP1 in patients with ITP, we conducted an RNA sequencing analysis of total BM RNA from patients with ITP and healthy controls (supplemental Table 1). Gene ontology enrichment analysis (supplemental Figure 1A) revealed that the differentially expressed genes between the 2 groups were mainly associated with immune response and membrane vesicle formation. Notably, among 7 randomly selected samples, at least 42.86% of patients with ITP showed a reduction in YAP1 expression compared with healthy donors (supplemental Figure 1B). Taken together, these results indicate an indispensable role of YAP1 in MKs from patients with ITP.
Reduced YAP1 expression induces aberrant MK distribution and submaximal platelet recovery in an experimental ITP mouse model
To investigate the role of YAP1 in ITP development, we first generated an ITP mouse model (supplemental Figure 2A), as previously described.23 Consistent with expectations, the expression of YAP1, along with CD41 and CD42a, was reduced in WT MKs after ITP induction (Figure 1E; supplemental Figure 2B-C). Considering that YAP1 knockout is embryonically lethal in mice, next, we used CRISPR/Cas9 technology to generate a heterozygous YAP1 mouse model. After ITP induction, submaximal platelet recovery was observed in YAP1+/− mice compared with that in WT mice with ITP (Figure 1F). Furthermore, compared with WT mice with ITP, YAP1+/− mice exhibited a decreased number of total MKs (Figure 1G, bottom left; P < .0001) with reduced localization around the sinusoidal endothelium (Figure 1G, bottom right; P < .0001), suggesting a role for YAP1 in MK migration toward the BM sinusoids.
After single-cell RNA sequencing of BM cells from WT and YAP1+/− mice after ITP induction (supplemental Figure 2D), 12 cell subpopulations (HSCs, MK-erythroid progenitors, MKs, basophils, plasma dendritic cells, natural killer cells, monomacrophages, macrophages, T cells, monocytes, B cells, and neutrophils) were identified using a t-distributed stochastic neighbor embedding plot based on characteristic markers (Figure 1H; fraction of cells and characteristic marker genes of each subset are presented in supplemental Figures 2E and 3). Notably, the total proportion of MKs was reduced in YAP1+/− mice after ITP induction, whereas the proportion of MK-erythroid progenitors remained unchanged compared with that in WT mice (supplemental Figure 2G-H). Based on the expression of immature MK markers (GATA2, Stat3, and FOXP1) and mature MK markers (PF4, Mef2c, Cited2, and CD61), we further subdivided the MKs into early MKs and late MKs (supplemental Figure 2F). The abundance of total MKs (CD41+), especially late MKs, was reduced in YAP1+/− mice (in total MKs, YAP1 128 vs WT 239; in early MKs, YAP1 56 vs WT 87; in late MKs, YAP1 72 vs WT 152; supplemental Figure 2I-J). To investigate the distinct functions of these 2 subsets, we analyzed the differential gene expression profiles (supplemental Figure 2K-L). Gene set enrichment analysis revealed that early MKs were involved in immune regulation and antigen presentation, whereas late MKs focused on genes linked to the cytoplasmic membrane, pivotal for thrombopoiesis (Figure 1I; supplemental Table 6). Furthermore, gene ontology analysis of the differential genes between the late MKs from WT and YAP1+/− mice highlighted disruptions in proplatelet formation in YAP1+/− MKs (Figure 1J). Taken together, these results indicate that a reduction in YAP1 expression alters the proportion of late MKs and the regulation of thrombopoiesis during ITP development.
YAP1 expression is regulated by GATA1 and contributes to MK maturation
Next, we sought to examine whether YAP1 expression facilitates MK maturation using a CD34+ HSC–based in vitro induction system (supplemental Figure 4A) and showed that YAP1 expression gradually increased with MK maturation (Figure 2A). Notably, the diameter of MKs (supplemental Figure 4B), CD41/CD42 expression (supplemental Figure 4C), and the proportion of CD42a+ MKs and CD41a+CD42a+ mature MKs (Figure 2C) were remarkably increased by XMU-MP-1 (YAP1 activator) treatment. Conversely, inhibiting YAP1 with peptide 17 (YAP1 inhibitor) showed a decrease in these maturation indicators. To consolidate these findings, we manipulated YAP1 levels through overexpression (by PCDH-YAP1) or knockdown (using YAP1-short hairpin RNA) in CD34+ HSCs for MK induction. As expected, YAP1 overexpression in the PCDH-YAP1 group increased CD41a+ and CD42a+ mature MKs compared with the vehicle control (PCDH group; Figure 2D). In contrast, YAP1 knockdown (YAP1-short hairpin RNA) led to reduced maturation of MKs compared with vehicle controls (Figure 2D). These results collectively indicate that YAP1 contributes to MK maturation.
YAP1 expression is regulated by GATA1 and contributes to MK maturation. (A) Fold changes in YAP1 mRNA (left) and protein expression (right) were assessed by real-time qPCR and western blotting on D10 and D12 compared with D7 during MK differentiation (n ≥ 3). (B) Fold changes in YAP1 mRNA (left) and protein expression (right) were assessed by real-time qPCR and western blotting after XMU-MP-1 (X, 300 nM) or peptide 17 (P, 2 μM) treatment (n ≥ 3). (C) Flow cytometry analysis of the percentages of CD41a+ MKs, CD42a+ MKs, and CD42a+ in CD41a+ MKs (n = 6). (D) Percentages of CD41a+ MKs (left) and CD41a+CD42a+ MKs (right) after YAP1 overexpression (PCDH-YAP1–labeled YAP1) or YAP1 knockdown (Plko.1-YAP1sh1/2–labeled YAP1sh1/2; n ≥ 6). (E) Fold changes in GATA1 mRNA levels in 13 patients with ITP relative to those in 14 healthy controls by real-time qPCR. (F-G) Assessment of GATA1/YAP1 mRNA expression in HEK293T cells after GATA1 overexpression (PCDH-GATA1–labeled GATA1) or knockdown (Plko.1-GATA1sh1/2–labeled GATA1sh1/2; n ≥ 5). (H-I) Representative immunoblots showing total GATA1 and YAP1 levels in HEK293T cells and the measurement of GATA1 and YAP1 levels based on GAPDH after GATA1 overexpression and knockdown (n = 4). (J) Measurement of luciferase activity to assess the binding activity of YAP1-WT (n ≥ 3). (K) Diagram depicting the domains of the YAP1 promoter with potential binding sequences (site 1, site 2, and site 3) for GATA1. Three mutants for the proposed binding sites (MT1, MT2, and MT3) were constructed. Binding site mutations (MT1, MT2, and MT3) are highlighted in red. (L) Measurement of luciferase activity to assess the binding activity of YAP1-MT1, YAP1-MT2, and YAP1-MT3 (n = 3). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. C, control; CDS, coding sequence; MT, mutant type; P, peptide 17; WT, wild type; X, XMU-MP-1.
YAP1 expression is regulated by GATA1 and contributes to MK maturation. (A) Fold changes in YAP1 mRNA (left) and protein expression (right) were assessed by real-time qPCR and western blotting on D10 and D12 compared with D7 during MK differentiation (n ≥ 3). (B) Fold changes in YAP1 mRNA (left) and protein expression (right) were assessed by real-time qPCR and western blotting after XMU-MP-1 (X, 300 nM) or peptide 17 (P, 2 μM) treatment (n ≥ 3). (C) Flow cytometry analysis of the percentages of CD41a+ MKs, CD42a+ MKs, and CD42a+ in CD41a+ MKs (n = 6). (D) Percentages of CD41a+ MKs (left) and CD41a+CD42a+ MKs (right) after YAP1 overexpression (PCDH-YAP1–labeled YAP1) or YAP1 knockdown (Plko.1-YAP1sh1/2–labeled YAP1sh1/2; n ≥ 6). (E) Fold changes in GATA1 mRNA levels in 13 patients with ITP relative to those in 14 healthy controls by real-time qPCR. (F-G) Assessment of GATA1/YAP1 mRNA expression in HEK293T cells after GATA1 overexpression (PCDH-GATA1–labeled GATA1) or knockdown (Plko.1-GATA1sh1/2–labeled GATA1sh1/2; n ≥ 5). (H-I) Representative immunoblots showing total GATA1 and YAP1 levels in HEK293T cells and the measurement of GATA1 and YAP1 levels based on GAPDH after GATA1 overexpression and knockdown (n = 4). (J) Measurement of luciferase activity to assess the binding activity of YAP1-WT (n ≥ 3). (K) Diagram depicting the domains of the YAP1 promoter with potential binding sequences (site 1, site 2, and site 3) for GATA1. Three mutants for the proposed binding sites (MT1, MT2, and MT3) were constructed. Binding site mutations (MT1, MT2, and MT3) are highlighted in red. (L) Measurement of luciferase activity to assess the binding activity of YAP1-MT1, YAP1-MT2, and YAP1-MT3 (n = 3). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. C, control; CDS, coding sequence; MT, mutant type; P, peptide 17; WT, wild type; X, XMU-MP-1.
GATA1, a crucial transcription factor in hematopoietic development,24 plays a pivotal role in MK maturation. GATA1 mutations within the N-terminal zinc finger domain are associated with myeloproliferative disorders25 and acute megakaryoblastic leukemia.26 Embryonic stem cells with decreased GATA1 expression exhibit insufficient expression of megakaryocytic markers,27 underscoring its essential role in the maturation of MKs. Upon investigation, we observed lower GATA1 mRNA levels in the BM of 14 patients with ITP than in those of 15 healthy individuals (Figure 2E; P < .05). To explore the interplay between GATA1 and YAP1, GATA1-overexpression and GATA1-knockdown HEK293T cells (supplemental Figure 4D-E) were generated. Overexpression of GATA1 resulted in the upregulation of YAP1, whereas GATA1 knockdown suppressed YAP1 expression (Figure 2F-I). A luciferase assay demonstrated that transfection with the GATA1 plasmid enhanced YAP1 transcriptional activity (Figure 2J), suggesting that GATA1 directly regulates YAP1 by binding to its promoter. Subsequently, binding sites for GATA1 in the YAP1 promoter were screened using the JASPAR and PROMO databases, and 3 potential binding sites (named sites 1, 2, and 3) were proposed (Figure 2K). Luciferase experiments revealed that site 3 (CTCCAATCTAT) was a specific site for GATA1 binding (Figure 2L). Moreover, the chromatin immunoprecipitation–quantitative polymerase chain reaction assay validated the interaction of binding between GATA1 and YAP1 promoter (supplemental Figure 4F). Collectively, these results suggest GATA1 as a regulator of YAP1 expression via its binding to the YAP1 promoter.
YAP1 promotes actin cytoskeletal reorganization
Cytoskeletal regulation in MKs, especially the activation of microtubules and F-actin assembly, is crucial for proplatelet formation. Thus, we examined the potential role of YAP1 in the MK cytoskeletal activation. Pretreatment with XMU-MP-1 promoted actin polymerization and abundant stress fiber formation (Figure 3A, left and middle). However, F-actin presented as an entwined pattern at the periphery of the MKs, with a disrupted assembly of F-actin (Figure 3A, left, yellow) after YAP1 inhibition. As a consequence of defective actin cytoskeletal dynamics, the spreading area was diminished in YAP1-inhibited MKs compared with normal controls (252.9 μm2 of peptide 17 vs 470.2 μm2 of normal control; P < .01; Figure 3A, right). Moreover, there were no differences in tubulin levels or distribution between the YAP1 upregulation and knockdown groups (Figure 3A, left, green), suggesting that YAP1 is a regulator of actin rather than tubulin dynamics in MKs. Consistently, MKs from healthy donors exhibited well-developed actin stress fibers with larger spreading areas than those from patients with ITP (healthy 644.7 μm2 vs ITP 133.2 μm2; P < .0001; Figure 3B). In line with these observations, YAP1-deficient MKs derived from YAP1+/− mice displayed defective cytoskeletal activation with reduced spreading area (159.3 μm2 for YAP1 vs 528.7 μm2 for WT; P < .0001; Figure 3C). Taken together, these findings emphasize the critical role of YAP1 in MK maturation, where it functions as a key regulator of actin dynamics during platelet biogenesis.
P-YAP1 facilitates cytoskeletal activation by binding its WW2 domain to MYH9. (A) Confocal images of MKs treated with a YAP1 activator (XMU-MP-1, X) or YAP1 inhibitor (Peptide 17, P) after immunostaining for tubulin (green, tubulin), F-actin (yellow, phalloidin), and MKs (red, CD41). Scale bar, 10 μm. Proplatelet formation is indicated by the red arrows. Assessment of percentage of stress fiber formation (middle) and spreading area (right) in the 3 groups (n ≥ 5). (B) Confocal images of MKs from patients with ITP and healthy controls after immunostaining for YAP1 (green, YAP1), F-actin (yellow, phalloidin), and MKs (red, CD41). Scale bar, 10 μm. Right: analysis of the MK spreading area in the ITP group vs the control group (n = 10). (C) MKs were isolated from WT and YAP1+/− mice to observe the cytoskeleton (left) and the spreading area (right; n = 10). (D) Coimmunoprecipitation assay to verify the interaction between YAP1 and MYH9 (n = 3). (E) Confocal images of PCDH-YAP1-flag-DAMI (YAP1-flag) after immunostaining for MYH9 (yellow, MYH9) and YAP1 (red, CD41). Scale bar, 10 μm. (F) P-YAP1 binding to MYH9 was confirmed by coimmunoprecipitation using flag antibodies (n ≥ 3). (G) P-YAP1 was responsible for MYH9 binding in MKs. After P-YAP1 coimmunoprecipitation, the total binding protein complex and the protein supernatant that did not bind to P-YAP1 were collected. Flag coimmunoprecipitation was performed to verify the interaction between unphosphorylated YAP1 and MYH9 in the MKs (n ≥ 3). (H) Compared with full-length YAP1, WW1 deletion (WW1-del) led to P-YAP1 reduction, whereas WW2 deletion (WW2-del) and WW1-WW2 double deletion (WW1-WW2-del) showed no decrease or even an increase in P-YAP1. The cooperation between MYH9 and the 4 isoforms of YAP1 was demonstrated by coimmunoprecipitation. (I) The WW2 domain fragment plays a critical role in MYH9 binding, and WW2 deletion leads to the upregulation of dysfunctional P-YAP1, leading to severe defects in MYH9 binding (n ≥ 3). ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Flag, PCDH-flag-DAMI; IP, immunoprecipitation.
P-YAP1 facilitates cytoskeletal activation by binding its WW2 domain to MYH9. (A) Confocal images of MKs treated with a YAP1 activator (XMU-MP-1, X) or YAP1 inhibitor (Peptide 17, P) after immunostaining for tubulin (green, tubulin), F-actin (yellow, phalloidin), and MKs (red, CD41). Scale bar, 10 μm. Proplatelet formation is indicated by the red arrows. Assessment of percentage of stress fiber formation (middle) and spreading area (right) in the 3 groups (n ≥ 5). (B) Confocal images of MKs from patients with ITP and healthy controls after immunostaining for YAP1 (green, YAP1), F-actin (yellow, phalloidin), and MKs (red, CD41). Scale bar, 10 μm. Right: analysis of the MK spreading area in the ITP group vs the control group (n = 10). (C) MKs were isolated from WT and YAP1+/− mice to observe the cytoskeleton (left) and the spreading area (right; n = 10). (D) Coimmunoprecipitation assay to verify the interaction between YAP1 and MYH9 (n = 3). (E) Confocal images of PCDH-YAP1-flag-DAMI (YAP1-flag) after immunostaining for MYH9 (yellow, MYH9) and YAP1 (red, CD41). Scale bar, 10 μm. (F) P-YAP1 binding to MYH9 was confirmed by coimmunoprecipitation using flag antibodies (n ≥ 3). (G) P-YAP1 was responsible for MYH9 binding in MKs. After P-YAP1 coimmunoprecipitation, the total binding protein complex and the protein supernatant that did not bind to P-YAP1 were collected. Flag coimmunoprecipitation was performed to verify the interaction between unphosphorylated YAP1 and MYH9 in the MKs (n ≥ 3). (H) Compared with full-length YAP1, WW1 deletion (WW1-del) led to P-YAP1 reduction, whereas WW2 deletion (WW2-del) and WW1-WW2 double deletion (WW1-WW2-del) showed no decrease or even an increase in P-YAP1. The cooperation between MYH9 and the 4 isoforms of YAP1 was demonstrated by coimmunoprecipitation. (I) The WW2 domain fragment plays a critical role in MYH9 binding, and WW2 deletion leads to the upregulation of dysfunctional P-YAP1, leading to severe defects in MYH9 binding (n ≥ 3). ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. Flag, PCDH-flag-DAMI; IP, immunoprecipitation.
P-YAP1 facilitates cytoskeletal activation through its WW2 domain binding to MYH9
To explore the target cytoskeletal proteins downstream of YAP1 in MKs, proteins that potentially interacted with YAP1 were identified by mass spectrometry (supplemental Table 3). Notably, MYH9 was validated as a binding protein, and the interactions were confirmed by coimmunoprecipitation (Figure 3D). Confocal microscopy images further demonstrated the colocalization of YAP1 with MYH9 (Figure 3E). Upon YAP1 overexpression, P-YAP1 and MYH9 levels were upregulated (supplemental Figure 5A). Coimmunoprecipitation experiments substantiated the interaction between P-YAP1 and MYH9, verifying that P-YAP1 interacts with MYH9 to activate the downstream cytoskeleton (Figure 3F; supplemental Figure 5B). However, no interaction binding was detected between unphosphorylated YAP1 and MYH9 after P-YAP1 coimmunoprecipitation in MKs (Figure 3G), indicating that the phosphorylated conformation of YAP1 plays a fundamental role in YAP1-MYH9 binding in MKs.
Using ZDOCK combined with Discovery Studio, several potential binding sites were identified between MYH9 and the WW domains of YAP1 (which are universally acknowledged binding sites for interacting proteins28). Subsequently, mutant constructs were generated to determine specific binding sites (supplemental Figure 5C). The absence of WW1 led to a decrease in P-YAP1 levels, whereas WW2 deletion and double WW1-WW2 deletion did not decrease or even increase P-YAP1 levels (Figure 3H). Coimmunoprecipitation pinpointed the WW2 domain as the functional binding site (Figure 3H-I), as WW2 deletion led to an increased level of dysfunctional P-YAP1 with crucial defects in MYH9 binding (Figure 3H-I). Immunofluorescence staining of full-length YAP1 and its mutants for colocalization of MYH9 and YAP1 further confirmed the defective interaction after WW2 deletion (supplemental Figure 5F). In summary, these data suggested that P-YAP1 interacts with MYH9 through its WW2 domain, supporting the potential role of YAP1 in orchestrating actin filament dynamics during thrombopoiesis.
YAP1 contributes to MK thrombopoiesis by orchestrating actin cytoskeletal reorganization through MYH9
Because F-actin in ITP MKs displayed peripheral localization without filamentous networks, further proplatelet formation was decreased in the ITP group compared with that in healthy individuals (ITP 1.83 vs healthy 14.83; P < .0001; Figure 4A). Meanwhile, YAP1+/− mice showed reduced proplatelet formation compared with WT (6.8 for YAP1+/− vs 15.3 for WT; P < .0001; Figure 4B). Moreover, XMU-MP-1 treatment enhanced proplatelet production (Figure 4C, red arrows), with a 52.70% increase in thrombopoiesis (Figure 4D, bottom; P < .01) but a 40.42% decrease upon peptide 17 inhibition (Figure 4D, bottom; P < .05). Collectively, these observations suggested that YAP1 contributes to thrombopoiesis.
YAP1 promotes thrombopoiesis through F-actin reorganization. (A) Proplatelet formation in MKs from patients with ITP and healthy controls after immunostaining for F-actin (yellow, phalloidin) and MKs (red, CD41). The arrows indicate proplatelet formation. Scale bar, 10 μm. Right, assessment of proplatelet formation in MKs from patients with ITP and healthy controls (n = 6). (B) MKs were isolated from WT and YAP1+/− mice to observe the cytoskeleton and proplatelet formation. Right: proplatelet numbers were calculated for WT and YAP1+/− mice (n = 10). (C) Confocal images of MKs from the YAP1 upregulation or reduction group after immunostaining for actin/stress fibers (yellow, phalloidin) and MKs (red, CD41). Scale bar, 10 μm. (D) The number of proplatelet formation (top) was analyzed and percentage of proplatelet formation (bottom) were assessed by flow cytometry (n ≥ 10). (E) Flow cytometry analysis of the percentage of CD41+ MKs and CD41+CD42+ MKs after XMU-MP-1 and blebbistatin treatment in D7, D10, and D12 (n = 3). (F-G) Measurement for adhered (F) and migrated (G) MKs in the untreated group and YAP1 activation (XMU-MP-1) or inhibition (Peptide 17) groups after blebbistatin (5 μM), cytochalasin D (500 nM), and narciclasine (50 nM) treatment (n ≥ 3). (H-J) Confocal images of MK thrombopoiesis in the untreated group (H) and YAP1 activation (XMU-MP-1; I) or inhibition (Peptide 17; J) groups after blebbistatin, cytochalasin D, and narciclasine treatment, after immunostaining for F-actin/stress fibers (yellow, phalloidin) and MKs (red, CD41). Scale bar, 10 μm. (K-L) Measurement of proplatelet formation in YAP1 activation (XMU-MP-1; K) or inhibition (Peptide 17; L) groups after blebbistatin, cytochalasin D, and narciclasine treatment (n ≥ 5). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. B, blebbistatin; XB, XMU-MP-1 + blebbistatin; Cy, cytochalasin D; N, narciclasine.
YAP1 promotes thrombopoiesis through F-actin reorganization. (A) Proplatelet formation in MKs from patients with ITP and healthy controls after immunostaining for F-actin (yellow, phalloidin) and MKs (red, CD41). The arrows indicate proplatelet formation. Scale bar, 10 μm. Right, assessment of proplatelet formation in MKs from patients with ITP and healthy controls (n = 6). (B) MKs were isolated from WT and YAP1+/− mice to observe the cytoskeleton and proplatelet formation. Right: proplatelet numbers were calculated for WT and YAP1+/− mice (n = 10). (C) Confocal images of MKs from the YAP1 upregulation or reduction group after immunostaining for actin/stress fibers (yellow, phalloidin) and MKs (red, CD41). Scale bar, 10 μm. (D) The number of proplatelet formation (top) was analyzed and percentage of proplatelet formation (bottom) were assessed by flow cytometry (n ≥ 10). (E) Flow cytometry analysis of the percentage of CD41+ MKs and CD41+CD42+ MKs after XMU-MP-1 and blebbistatin treatment in D7, D10, and D12 (n = 3). (F-G) Measurement for adhered (F) and migrated (G) MKs in the untreated group and YAP1 activation (XMU-MP-1) or inhibition (Peptide 17) groups after blebbistatin (5 μM), cytochalasin D (500 nM), and narciclasine (50 nM) treatment (n ≥ 3). (H-J) Confocal images of MK thrombopoiesis in the untreated group (H) and YAP1 activation (XMU-MP-1; I) or inhibition (Peptide 17; J) groups after blebbistatin, cytochalasin D, and narciclasine treatment, after immunostaining for F-actin/stress fibers (yellow, phalloidin) and MKs (red, CD41). Scale bar, 10 μm. (K-L) Measurement of proplatelet formation in YAP1 activation (XMU-MP-1; K) or inhibition (Peptide 17; L) groups after blebbistatin, cytochalasin D, and narciclasine treatment (n ≥ 5). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. B, blebbistatin; XB, XMU-MP-1 + blebbistatin; Cy, cytochalasin D; N, narciclasine.
We first uncovered direct binding between YAP1 and MYH9, implicated in cytoskeletal activation. Next, we explored the potential role of MYH9 in YAP1-regulated thrombopoiesis by examining MK differentiation (D7, D10, and D12), mobility, and proplatelet formation. Notably, treatment with the specific MYH9 inhibitor, blebbistatin (B), significantly compromised YAP1-stimulated (XMU-MP-1 group) MK differentiation (Figure 4E). Considering the association of MYH9 with F-actin for force generation in cell motility,29 the colocalization of YAP1 and actin was validated through coimmunoprecipitation and confocal imaging (supplemental Figure 5D-E). Thus, we used blebbistatin, the actin polymerization inhibitor cytochalasin D (Cy), and the actin polymerization activator narciclasine (N) in MKs pretreated with the YAP1 activator (XMU-MP-1) or inhibitor (peptide 17). Suppression of blebbistatin and cytochalasin D on YAP1-mediated adhesion and migration was observed, whereas narciclasine rescued MK motility defects induced by YAP1 inhibition (Figure 4F; supplemental Figure 5G-H). Mechanistically, blebbistatin and cytochalasin D impeded actin activation downstream of YAP1 in MKs (Figure 4H-J, yellow), diminishing augmented proplatelet formation. Narciclasine reversed the actin disruption caused by YAP1 inhibition, counteracting defects in proplatelet formation (Figure 4K-L). In summary, YAP1-MYH9 interactions orchestrate actin regulation, driving MK thrombopoiesis.
Megakaryocytic YAP1 functions in thrombopoiesis during ITP
To investigate the role of YAP1 in platelet recovery, we first conducted BM transplantation (BMT) between WT and YAP1+/− mice, and ITP was induced after recovery (Figure 5A). Remarkably, WT mice that received YAP1+/− BM cells exhibited submaximal platelet recovery compared with those that received WT BM cells (Figure 5B). Further analysis uncovered a considerable decline in total MKs and mature MKs around the sinusoids in both groups receiving YAP1+/− BM, regardless of their genetic background (Figure 5C). Additionally, we isolated MKs from the BM of both WT and YAP1+/− recipients and showed that reduced YAP1 expression led to impaired cytoskeletal activation, resulting in hindered proplatelet formation (Figure 5D), suggesting a crucial role of YAP1 in regulating the MK cytoskeleton and further thrombopoiesis.
Megakaryocytic YAP1 facilitates thrombopoiesis during ITP development. (A) Sketch map of BMT mouse model. (B) Changes in peripheral platelet levels in mice with anti-CD41 antibody administration after BMT recovery (n ≥ 5). (C) Confocal images of BM sections from transplanted WT and YAP1+/− mice after immunostaining for YAP1 (green, YAP1), vascular sinusoids (yellow, laminin A), and MKs (red, CD41). Scale bar, 100 μm (n ≥ 10). Analysis of the number of MKs in the field (top) and the number of MKs located around the sinusoids (bottom; n ≥ 10). (D) Confocal images of BM MKs from WT and YAP1+/− mice after immunostaining for YAP1 (green, YAP1), F-actin (yellow, phalloidin), and MKs (red, CD41) and proplatelet formation were observed. Scale bar, 20 μm. (E) Sketch map of optimized BMT model. (F) Changes in the peripheral platelet levels in mice after optimized BMT. (G) Confocal images of BM sections from different groups of mice after immunostaining for YAP1 (green, YAP1) and MKs (red, CD41). Scale bar, 100 μm. Analysis of MK numbers (top) and number of MKs located around the sinusoids (bottom; n ≥ 10). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. D, donor; MNC, mononuclear cell; R, recipient; W3, WT D3-MK; W8, WT D8-MK; W H, WT HSC; Y 3, YAP1+/− D3-MK; Y 8, YAP1+/− D3-MK; Y H, YAP1+/− HSC.
Megakaryocytic YAP1 facilitates thrombopoiesis during ITP development. (A) Sketch map of BMT mouse model. (B) Changes in peripheral platelet levels in mice with anti-CD41 antibody administration after BMT recovery (n ≥ 5). (C) Confocal images of BM sections from transplanted WT and YAP1+/− mice after immunostaining for YAP1 (green, YAP1), vascular sinusoids (yellow, laminin A), and MKs (red, CD41). Scale bar, 100 μm (n ≥ 10). Analysis of the number of MKs in the field (top) and the number of MKs located around the sinusoids (bottom; n ≥ 10). (D) Confocal images of BM MKs from WT and YAP1+/− mice after immunostaining for YAP1 (green, YAP1), F-actin (yellow, phalloidin), and MKs (red, CD41) and proplatelet formation were observed. Scale bar, 20 μm. (E) Sketch map of optimized BMT model. (F) Changes in the peripheral platelet levels in mice after optimized BMT. (G) Confocal images of BM sections from different groups of mice after immunostaining for YAP1 (green, YAP1) and MKs (red, CD41). Scale bar, 100 μm. Analysis of MK numbers (top) and number of MKs located around the sinusoids (bottom; n ≥ 10). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. D, donor; MNC, mononuclear cell; R, recipient; W3, WT D3-MK; W8, WT D8-MK; W H, WT HSC; Y 3, YAP1+/− D3-MK; Y 8, YAP1+/− D3-MK; Y H, YAP1+/− HSC.
Subsequently, to further elucidate the role of megakaryocytic YAP1 in thrombopoiesis, we established optimized BMT models using mouse HSCs (ckit+ HSCs), manipulated early (MKs cultured for 3 days [D3-MKs]) and late (MKs cultured for 8 days [D8-MKs]) stages of MKs (Figure 5E). Distinctly, platelet recovery in WT donors outpaced that of YAP1+/− donors, with the late-stage MKs (D8-MKs) from WT mice exhibiting the fastest recovery, followed by the early-stage MK (D3-MKs) group and HSC group from WT mice. In contrast, the slowest recovery was observed in the HSC group from YAP1+/− mice (Figure 5F). This pattern aligns with the typical BM reconstruction timeline, which typically commences on the seventh day after transplantation. At this time point, the transplanted D8-MKs differentiated into mature MKs and actively produced proplatelets. MKs in the early MK group (D3-MKs), as well as in the HSC group, have not yet fully matured, limiting thrombopoiesis. Late MK group (D8-MKs) from WT mice exhibited the highest MK population in the BM, followed by the early MK group (D3-MKs) from WT mice via immunofluorescence. Conversely, the HSC group of YAP1+/− mice displayed the lowest density of MKs (Figure 5G). Taken together, these findings demonstrate that megakaryocytic YAP1, especially in late MKs, is crucial for the efficient restoration of platelets in ITP mice.
Targeting YAP1 is sufficient to rescue thrombopoiesis in YAP1+/− ITP mice and patients with ITP
As an important transcription factor upstream of YAP1, GATA1 binds to the promoter of YAP1, regulating its transcription and promoting MK maturation. Moreover, decreased YAP1 and GATA1 expression correlates closely with differentiation defects in ITP MKs, suggesting a critical role of the GATA1-YAP1 axis in megakaryopoiesis. To address this, BMT mouse models using GATA1 knockdown BMs were established, followed by restoring YAP1 expression using the YAP1 activator XMU-MP-1 in GATA1-knockdown mice (Figure 6A; supplemental Figure 6A). These results showed that thrombopoietic defects triggered by GATA1 deficiency were rescued by YAP1 administration. In details, both platelet counts (Figure 6B) and MK distribution (Figure 6C) in the BM were restored after YAP1 treatment, thereby highlighting the pivotal role of YAP1 in mediating the function of GATA1 in megakaryopoiesis and hematopoietic homeostasis.
Targeting YAP1 is sufficient to rescue thrombopoiesis in YAP1+/− ITP mice and patients with ITP. (A) Comprehensive schematic overview of the YAP1 rescue mouse model. ckit+ HSCs were isolated from WT mice, and further conducted GATA1 knockdown HSCs using short hairpin RNA. Subsequently, BMT mouse models using GATA1 knockdown BM were established. Seven days after BMT, XMU-MP-1 was administrated once per day for the following 3 days. (B) Changes in the peripheral platelet levels in mice during BMT reconstruction (n ≥ 5). (C) Confocal images of BM sections from WT mice in groups after immunostaining for vascular sinusoids (yellow, laminin A) and MKs (red, CD41). Scale bar, 100 μm. Analysis of MK numbers (top) and the number of MKs located around the sinusoids (bottom; n ≥ 10). (D) The anti-CD41 antibody–induced ITP mouse model was established for 7 days, and XMU-MP-1 was administrated once per day for the following 3 days. Changes in peripheral platelet levels in mice during antibody administration and recovery (n ≥ 6). (E) Confocal images of BM sections from WT and YAP1+/− mice after immunostaining for YAP1 (green, YAP1), vascular sinusoids (yellow, laminin A), and MKs (red, CD41). Scale bar, 50 μm. Analysis of MK numbers (middle) and number of MKs located around the sinusoids (right; n ≥ 10). (F) Confocal images of spreading and cytoskeletal organization in MKs from WT and YAP1+/− mice in groups after immunostaining for YAP1 (green, YAP1), F-actin (yellow, phalloidin), and MKs (red, CD41). Scale bar, 10 μm. (G) XMU-MP-1 (300 nM for 24 hours) treatment facilitated proplatelet formation in MKs from patients with refractory ITP (ITP1) and chronic ITP (ITP2). (H) Proposed model. YAP1 regulates thrombopoiesis by binding to MYH9 in the context of ITP. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. G sh1, GATA1sh1; G sh2, GATA1sh2; sh, short hairpin; Sh1 X, GATA1sh1 + XMU; Sh2 X, GATA1sh2 + XMU; W, WT.
Targeting YAP1 is sufficient to rescue thrombopoiesis in YAP1+/− ITP mice and patients with ITP. (A) Comprehensive schematic overview of the YAP1 rescue mouse model. ckit+ HSCs were isolated from WT mice, and further conducted GATA1 knockdown HSCs using short hairpin RNA. Subsequently, BMT mouse models using GATA1 knockdown BM were established. Seven days after BMT, XMU-MP-1 was administrated once per day for the following 3 days. (B) Changes in the peripheral platelet levels in mice during BMT reconstruction (n ≥ 5). (C) Confocal images of BM sections from WT mice in groups after immunostaining for vascular sinusoids (yellow, laminin A) and MKs (red, CD41). Scale bar, 100 μm. Analysis of MK numbers (top) and the number of MKs located around the sinusoids (bottom; n ≥ 10). (D) The anti-CD41 antibody–induced ITP mouse model was established for 7 days, and XMU-MP-1 was administrated once per day for the following 3 days. Changes in peripheral platelet levels in mice during antibody administration and recovery (n ≥ 6). (E) Confocal images of BM sections from WT and YAP1+/− mice after immunostaining for YAP1 (green, YAP1), vascular sinusoids (yellow, laminin A), and MKs (red, CD41). Scale bar, 50 μm. Analysis of MK numbers (middle) and number of MKs located around the sinusoids (right; n ≥ 10). (F) Confocal images of spreading and cytoskeletal organization in MKs from WT and YAP1+/− mice in groups after immunostaining for YAP1 (green, YAP1), F-actin (yellow, phalloidin), and MKs (red, CD41). Scale bar, 10 μm. (G) XMU-MP-1 (300 nM for 24 hours) treatment facilitated proplatelet formation in MKs from patients with refractory ITP (ITP1) and chronic ITP (ITP2). (H) Proposed model. YAP1 regulates thrombopoiesis by binding to MYH9 in the context of ITP. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. G sh1, GATA1sh1; G sh2, GATA1sh2; sh, short hairpin; Sh1 X, GATA1sh1 + XMU; Sh2 X, GATA1sh2 + XMU; W, WT.
Given the connection between reduced YAP1 expression and disrupted MK cytoskeleton with impaired thrombopoiesis, we aimed to determine whether the functional defects observed in YAP1+/− MKs and patients with ITP could be corrected by pharmacological administration of YAP1. Thus, we administered XMU-MP-1 intraperitoneally to YAP1+/− mice for 3 consecutive days after ITP induction (supplemental Figure 6B). As expected, YAP1 administration restored platelet counts in YAP1+/− mice after ITP induction to levels comparable with those in WT mice (Figure 6D) and augmented the total MK count, especially around marrow sinusoids (Figure 6E). Moreover, YAP1 injection corrected cytoskeletal defects and enhanced MK spreading (Figure 6F; supplemental Figure 6C-D) in YAP1+/− mice. We further studied the impact of XMU-MP-1 on thrombopoiesis of MKs from patients with refractory ITP and chronic ITP and revealed that YAP1 administration improved cytoskeletal organization and stress fiber formation after XMU-MP-1 treatment (supplemental Figure 6E). Furthermore, more proplatelets were formed in MKs from refractory ITP and chronic ITP after XMU-MP-1 treatment (Figure 6G), suggesting a fundamental regulatory role of YAP1 in thrombopoiesis. In conclusion, these results indicate that the administration of YAP1 effectively corrects MK cytoskeletal dysregulation resulting from reduced YAP1 expression during ITP development.
Discussion
Dysregulation of megakaryopoiesis, which can be derived from cell-extrinsic immune stresses and cell-intrinsic regulation in response to immune attack, has been recognized as the pathogenesis of ITP.5 Here, we identified a novel mechanistic connection between the hematopoietic regulatory functions of YAP1 and ITP pathogenesis. First, YAP1 expression was reduced in ITP MKs, which delayed MK maturation. Moreover, reduced YAP1 expression weakened the interactions between P-YAP1 and the cytoskeletal protein MYH9, impairing downstream cytoskeletal actin organization and resulting in thrombocytopenia (Figure 6H). Contrary to previous reports implicating a suppressive role of YAP1 in the differentiation and proplatelet formation of the megakaryocytic cell line MEG-01,21 our investigation used HSCs to emulate primary megakaryopoiesis and demonstrated that YAP1 promotes thrombopoiesis, thus sidestepping artifacts of cell-specific phenotypes that deviate from authentic MK maturation dynamics and proplatelet production. Divergent findings across various research platforms, methodologies, and focuses expose disparities. By adopting a novel perspective, our findings differ from the conventional focus on proliferation and embrace a deeper exploration of intracellular mechanisms during MK maturation, particularly exploring the interactions between YAP1 and cytoskeletal-related proteins and their roles in orchestrating cytoskeletal rearrangements to facilitate thrombopoiesis.
MYH9 regulates proplatelet formation in MKs by tethering F-actin and affecting its organization.30,31 Our study unveiled a novel interaction between YAP1 and MYH9, crucial for cytoskeletal regulation. Specifically, we identified the WW2 domain as a precise binding site for the YAP1-MYH9 interaction. However, considering the nonspecificity of Blebbi as an MYH9 inhibitor, MK-specific MYH9 knockout mice can be generated and used to further confirm the YAP1-MYH9 interaction in future studies.
We observed that decreased YAP1 was closely related to defects in differentiation and thrombopoiesis in ITP MKs. Impaired MK maturation and platelet production in response to autoimmune attack have been considered as one of the important pathogenesis mechanisms of ITP.32,33 However, the precise mechanisms by which immunity diminishes YAP1 expression remain unidentified. To address this, we used anti-CD41 antibody (supplemental Figure 7A-C), BM supernatant from ITP (supplemental Figure 7D-G), and CTLs (supplemental Figure 7H-J) to coculture with MKs and demonstrated that CTLs, instead of humoral immune responses, resulted in YAP1 reduction. Thus, dysregulation of cellular immune responses, leading to downregulation of YAP1, ultimately contributes to persistent MK maturation defects and thrombocytopenia. Our observations across both patients with ITP and animal models consistently link a reduced YAP1 expression to a reduction in peripheral platelet counts. However, further investigation is warranted to unravel the specific mechanisms.
Single-cell RNA sequencing revealed that YAP1 is essential for thrombopoiesis. YAP1 knockdown impeded cytoskeletal activation, resulting in thrombocytopenia, which was further verified in primary MKs and mouse models. We identified 2 distinct MK subsets and demonstrated that a reduction in YAP1 leads to reductions in both early and late MK subpopulations, especially in the late MKs associated with thrombopoiesis, exhibiting a more severe impairment. Given the functional enrichment analysis of these subsets, YAP1 emerges as pivotal not only in thrombopoiesis but also in immunoregulation during ITP pathogenesis. However, several limitations should be noted in our study. First, our observations were obtained on day 11 after ITP establishment, which remains the greatest differences in platelet levels. Collection at additional time points (such as days 7 and 14) may provide more comprehensive insights into the pathogenic process of ITP. Second, bioinformatics analysis of differentially expressed genes was performed using only 2 MK subsets. Further investigations into the various functional MK subsets implicated in YAP1-associated ITP are necessary. Last but not least, the passive ITP model has contributed significantly to elucidating the pathophysiology of ITP but incompletely recapitulates chronic ITP.6 Thrombocytopenia correlates with CTL expansion and exacerbates immune damage. Therefore, although the passive ITP model does not directly address CTL-mediated autoimmune responses, thrombocytopenia-induced cellular immune responses indirectly contribute to its pathogenesis.
In conclusion, we demonstrated that YAP1 upregulation via the binding of GATA1 to its promoter promotes MK maturation. P-YAP1 promotes cytoskeletal activation by binding its WW2 domain to MYH9, facilitating thrombopoiesis. Conversely, during ITP development, YAP1 expression is decreased in MKs in response to autoimmune responses, impairing thrombopoiesis and leading to the deterioration of ITP. Our work provides novel insights into the YAP1-mediated thrombopoiesis mechanism involving the cytoskeleton, suggesting potential for the development of diagnostic markers and therapeutic strategies for ITP.
Acknowledgments
The authors thank the Tissue Specimen Library of the Hematology Department at The First Affiliated Hospital of Soochow University for providing the clinical samples for this study.
This work was supported by grants from the National Key Research and Development Program (2022YFC2502700) (Y.X.), the National Natural Science Foundation of China (82000140 [S.H.], 81730003 and 82020108003 [D.W.], 81870120 and 82070187 [Y.X.], 82170466 [L.Z.]), the Natural Science Foundation of Jiangsu Province (BK20200197) (S.H.), the Social Development Project of Jiangsu Province (BE2019655) (Y.X.), the Jiangsu Province Key R&D Program (BE2019798) (D.W.), the Suzhou Science Project (SKY2021104) (Y.X.), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (D.W.).
Authorship
Contribution: S.H., Y. Liu, X.Z., X.W., M.C., and Y. Li performed the experiments and analyzed the data; S.H., Y. Liu, Y.X., and L.Z. designed the research, interpreted the results, and wrote the manuscript; J.Y. and Y. Li collected the samples; C.R., L.Z., Y.X., and D.W. provided overall guidance; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Depei Wu, The First Affiliated Hospital of Soochow University, Soochow University, Suzhou 215123; email: wudepei@suda.edu.cn; and Yang Xu, The First Affiliated Hospital of Soochow University, Soochow University, Suzhou 215123; email: yangxu@suda.edu.cn.
References
Author notes
S.H. and Y. Liu contributed equally to this study.
All extended data may be found in a data supplement available with the online version of this article. Original data are available on request from the corresponding author, Yang Xu (yangxu@suda.edu.cn).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.