Key Points
The UK real-world evidence for caplacizumab in iTTP represents the largest international data collection outside of clinical trials to date.
The UK real-world evidence for caplacizumab is comparable with RCT outcomes and includes pediatric patients.
Abstract
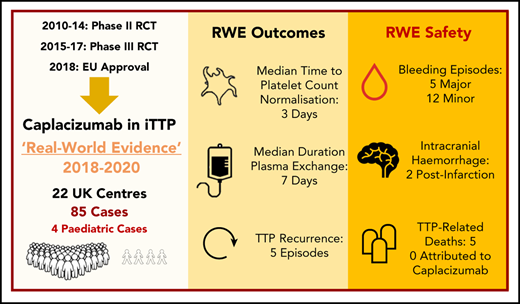
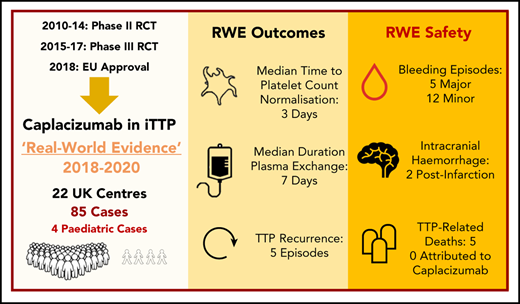
The cornerstone of life-saving therapy in immune-mediated thrombotic thrombocytopenic purpura (iTTP) has been plasma exchange (PEX) combined with immunomodulatory strategies. Caplacizumab, a novel anti–von Willebrand factor nanobody trialed in 2 multicenter randomized controlled trials (RCTs) leading to European Union and US Food and Drug Administration approval, has been available in the United Kingdom (UK) through a patient access scheme. Data were collected retrospectively from 2018 to 2020 for 85 patients (4 children) receiving caplacizumab from 22 UK hospitals. Patient characteristics and outcomes in the real-world clinical setting were compared with caplacizumab trial end points and historical outcomes in the precaplacizumab era. Eighty-four of 85 patients received steroid and rituximab alongside PEX; 26% required intubation. Median time to platelet count normalization (3 days), duration of PEX (7 days), and hospital stay (12 days) were comparable with RCT data. Median duration of PEX and time from PEX initiation to platelet count normalization were favorable compared with historical outcomes (P < .05). Thrombotic thrombocytopenic purpura (TTP) recurred in 5 of 85 patients; all had persistent ADAMTS13 activity < 5 IU/dL. Of 31 adverse events in 26 patients, 17 of 31 (55%) were bleeding episodes, and 5 of 31 (16%) were thrombotic events (2 unrelated to caplacizumab); mortality was 6% (5/85), with no deaths attributed to caplacizumab. In 4 of 5 deaths, caplacizumab was introduced >48 hours after PEX initiation (3-21 days). This real-world evidence represents the first and largest series of TTP patients, including pediatric patients, receiving caplacizumab outside of clinical trials. Representative of true clinical practice, the findings provide valuable information for clinicians treating TTP globally.
Introduction
Thrombotic thrombocytopenic purpura (TTP) results from a severe deficiency of von Willebrand factor–cleaving protease (ADAMTS13). In immune-mediated TTP (iTTP) there are immunoglobulin G autoantibodies against ADAMTS13.1 Until recently, management of iTTP focused on the replacement of ADAMTS13 and the removal of autoantibodies using plasma exchange (PEX) and immunosuppression.2-4 This approach has reduced the mortality of acute TTP from >90% to ∼10% to 20%.5
Caplacizumab is a humanized single-variable domain nanobody targeting the A1 domain of von Willebrand factor (vWF).6 It has been developed for the treatment of iTTP, and its novelty lies in its site of action, specifically inhibiting vWF-platelet interaction and, thereby, limiting platelet adhesion and microvascular thrombus formation.6,7
In August of 2018, caplacizumab was approved in the European Union (EU) for the treatment of iTTP following favorable results from the phase 2 TITAN and phase 3 HERCULES clinical trials, studying a total of 220 adult patients with acute iTTP. Patients who received caplacizumab, in addition to standard care, exhibited a significantly shorter time to platelet count normalization (P < .01) and reduction in duration of PEX and overall hospital stay compared with placebo.7,8 There were no deaths reported in those patients receiving caplacizumab during an acute TTP episode.
The use of caplacizumab was highly controlled in the clinical trials, subject to adherence to strict protocols for drug administration and discontinuation and limited to use in the trial-hosting centers. The unique value of real-world evidence lies in the inclusion of patients encountered in clinical practice where decisions are often influenced by everyday practice factors, such as location, patient compliance, concomitant treatments, and dynamic clinical factors (eg, refractory disease and relapse). Here, we describe 85 patients with iTTP who received caplacizumab in the United Kingdom (UK), following EU approval, through a patient access scheme. The objectives were to describe the iTTP patient population receiving caplacizumab in a real-world clinical setting, report their outcomes, including safety and tolerability, and compare with trial and historical outcome data. To our knowledge, this is the largest cohort of TTP patients who have received caplacizumab outside of a clinical trial to be reported in the literature and includes the only case series in the pediatric age group to date.
Methods
An invitation to participate in real-world evidence data collection was sent to all UK TTP Registry collaborators, indicating a final date for data submission. The data were retrospectively collected from consecutive eligible patients’ medical records from UK hospitals between May of 2018 and January of 2020. Inclusion criteria were patients of any age, who had received ≥1 dose of caplacizumab through the patient drug access scheme, following a confirmed diagnosis of acute TTP. There were no exclusion criteria. Patients received standard treatment, as per UK national guidance,2 and all patients included were recruited to the UK TTP Registry conferring consent, ethical approval, and permission for data collection. Sanofi confirmed the total number of patients for whom caplacizumab had been provided through the access scheme but had no other involvement in data collection or analysis.
Anonymized data were submitted by participating centers using password-protected standardized databases requesting specific patient characteristics and outcome data. Relevant outcome parameters were identified ahead of data collection, comparable with the caplacizumab randomized controlled trial (RCT) end points. These included patient demographics, ADAMTS13 serology, serological markers of organ injury, platelet count recovery, PEX, TTP recurrences, bleeding/thromboembolic complications, and mortality.
Characteristics and outcomes for patients receiving caplacizumab in the real-world setting were compared with outcome data from the HERCULES phase 3 RCT7 and a historical control group. The historical control group consisted of 39 consecutive cases from the UK TTP Registry from 2014 to 2018 prior to, and outside of, the caplacizumab clinical trials. The standard of care for the historical control group was per UK national guidance2 and included rituximab as a component of acute management.
For the real-world data collection, time to platelet count normalization was defined in line with the HERCULES RCT as the time from the first IV administration of caplacizumab to the normalization of platelet count (ie, platelet count ≥150 × 109/L, with discontinuation of PEX within 5 days thereafter). Recurrence of TTP was defined as a new decrease in platelet count after initial normalization, requiring PEX therapy to be reinitiated. A recurrence within 30 days after completion of PEX therapy was defined as an “exacerbation,” and a recurrence occurring >30 days after completion of PEX therapy was defined as a “relapse.” Refractory TTP refers to the progression of clinical symptoms or persistent thrombocytopenia despite PEX.
Descriptive statistical analyses were used to summarize the data. Quantitative variables are presented using median and interquartile range (IQR), and categorical variables are presented using counts and percentages. Between-group comparisons were undertaken using a Mann-Whitney U test for continuous data and Fisher’s exact test for binary variables. The Kaplan-Meier estimator was used to calculate median follow-up time. All analysis were carried out using SPSS software (version 25).
Results
Between May 2018 and January 2020, 115 patients (110 adult and 5 pediatric) from 25 UK hospitals received caplacizumab through the free drug patient access scheme. Data were provided from 22 hospitals for 85 of 115 patients. Participation in data submission was voluntary, and no data were received for the remaining 30 patients. Eighty-one patients were adults (≥18 years at the time of the first dose of caplacizumab), and 4 were pediatric (<18 years). Using the Kaplan-Meier estimator (with those patients who died included as censored observations), the median follow-up period (from date of initiation of caplacizumab to last documented clinical follow-up) was 80 days (IQR, 59-166).
Patients receiving caplacizumab covered a wide geographical area of England, Scotland, and Wales. The median number of cases treated per hospital was 1. The highest number of cases treated in 1 center was 30. In all pediatric cases, patient care was guided by an adult center.
Patient characteristics and outcomes
Presenting parameters and concomitant treatments are summarized in Table 1; patient outcomes are presented in Table 2. All 85 patients had ADAMTS13 activity at presentation <20 IU/dL, with 99% of patients (84/85) having ADAMTS13 activity <10 IU/dL, confirming a clinical diagnosis of acute TTP.
Eighty-five percent (72/85) of patients had a platelet count <30 × 109/L on admission, 76% (65/85) had increased troponin, and 66% (56/85) had neurological symptoms. Median presenting creatinine was 90 μmol/L (IQR, 71-135), and median presenting troponin on admission was 98 ng/mL (IQR, 31-317). Twenty-seven percent (23/85) had multiorgan failure consisting of increased troponin, acute kidney injury, and neurological symptoms.
Twenty-six percent (22/85) of patients required intubation and ventilation prior to transfer or following admission. The median duration of intubation was 4 days (IQR, 2-7); in all cases, the indication for intubation was neurological deterioration.
Time to initiate caplacizumab treatment
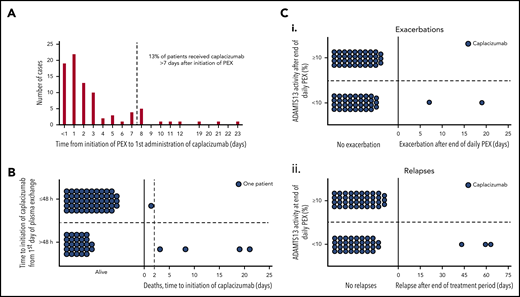
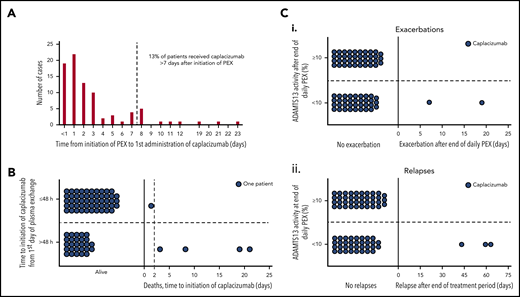
The median time for patients to receive the first dose of caplacizumab after initiation of PEX was 2 days (IQR, 1-3), and 87% (74/85) of patients received caplacizumab within 1 week of starting PEX (Figure 1A).
Drug initiation, mortality, and disease recurrence in relation to ADAMTS13 activity. (A) Time taken from initiation of PEX therapy to the first dose of caplacizumab (days). <1 day refers to the first administration of caplacizumab <24 hours following initiation of PEX. (B) Mortality according to caplacizumab initiation (≤48 hours vs >48 hours). Eighty percent of those who died had caplacizumab initiated >48 hours after the first PEX. (C) Recurrence status according to ADAMTS13 activity at completion of PEX. (i) Individual patient data with regard to exacerbation status. ADAMST13 activity after the end of daily PEX was available for 76 patients. Of these, 37 (48.7%) had ADAMST13 activity <10.0% (range, <1.0 to 5.2); 2 patients experienced exacerbations. The other 39 patients (51.3%) had ADAMST13 ≥10.0% (range, 10.2-107.2), without any exacerbations. (ii) Individual patient data with regard to relapse status. No patient with ADAMST13 activity ≥10.0% relapsed, whereas 3 of the 37 patients with ADAMST13 activity <10.0% did. Recurrences are termed exacerbations if they occur within 30 days of last PEX and are classified as relapses if they occur >30 days after last PEX.
Drug initiation, mortality, and disease recurrence in relation to ADAMTS13 activity. (A) Time taken from initiation of PEX therapy to the first dose of caplacizumab (days). <1 day refers to the first administration of caplacizumab <24 hours following initiation of PEX. (B) Mortality according to caplacizumab initiation (≤48 hours vs >48 hours). Eighty percent of those who died had caplacizumab initiated >48 hours after the first PEX. (C) Recurrence status according to ADAMTS13 activity at completion of PEX. (i) Individual patient data with regard to exacerbation status. ADAMST13 activity after the end of daily PEX was available for 76 patients. Of these, 37 (48.7%) had ADAMST13 activity <10.0% (range, <1.0 to 5.2); 2 patients experienced exacerbations. The other 39 patients (51.3%) had ADAMST13 ≥10.0% (range, 10.2-107.2), without any exacerbations. (ii) Individual patient data with regard to relapse status. No patient with ADAMST13 activity ≥10.0% relapsed, whereas 3 of the 37 patients with ADAMST13 activity <10.0% did. Recurrences are termed exacerbations if they occur within 30 days of last PEX and are classified as relapses if they occur >30 days after last PEX.
Median time to initiate caplacizumab was also compared for patients commenced on caplacizumab in the first 9 months of the data-collection period (May 2018 until February 2019, n = 24) with those who commenced treatment in the second 9 months (March to December 2019, n = 61) to evaluate any changes in familiarity and accessibility of the new therapy. The median time to initiate caplacizumab was 3 days (IQR, 2-7) for patients commencing caplacizumab in the first 9 months compared with 1 day (IQR, 0-3) for those who began it in the latter 9 months (P < .001).
Time to platelet count normalization, PEX, and hospital stay
Ninety-five percent of patients in the caplacizumab cohort achieved platelet normalization compared with 100% in the historical cohort (P = .31). In the 4 of 85 patients receiving caplacizumab whose platelet count did not normalize by 30 days post-PEX discontinuation, 1 case was due to a concomitant diagnosis of immune thrombocytopenia with a history of chronic low-grade thrombocytopenia, 1 case had a normal platelet count by day 43, and 2 cases had multiple organ failure resulting in death.
The median time from first PEX to platelet normalization was 4 days (IQR, 3-8) and 6 days (IQR, 4-10) in the caplacizumab cohort and the historical cohort, respectively (P = .011). The median duration of PEX was 7 days (IQR, 5-14) and 9 days (IQR, 8-16), respectively (P = .007). This is the total number of days delivered and includes cases in whom PEX was tapered or reintroduced (4/85). In 26% of cases (22/85), PEX was continued despite normalization of the platelet count for ≥48 hours.
The median hospital length of stay was 12 days (IQR, 8-24) and 14 days (IQR, 9-17), in the caplacizumab cohort and the historical cohort, respectively (P = .62). Death occurred in 6% and 0%, respectively (P = .32); however, 10% of patients in the historical group required intubation compared with 26% in the caplacizumab group, suggesting a more severe disease phenotype in the latter (Table 3).
Disease recurrence and ADAMTS13 activity recovery
Six percent (5/85) of patients were reported to have TTP recurrence: 2 of 5 were classified as an exacerbation and 3 of 5 were classified as relapse of TTP (supplemental Table 1, available on the Blood Web site). For this group, caplacizumab was commenced in all 5 cases within 3 days of starting PEX; in all cases, the time to platelet count normalization was ≤7 days.
Caplacizumab was interrupted in both cases of exacerbation: in 1 patient because of concurrent pulmonary embolism requiring anticoagulation and in the other patient as a result of patient noncompliance. In both cases, ADAMTS13 activity was <5 IU/dL at the time of discontinuing PEX.
For all patients, the median ADAMTS13 activity for specific time points was 12.4 IU/dL (IQR, 0.0-57.9) at the time of final PEX, 49.3 IU/dL (IQR, 16.3-72.7) at the time of caplacizumab discontinuation, and 58.3 IU/dL (IQR, 15.5-85.4) at 1 week post caplacizumab discontinuation. All patients with recurrent TTP had ADAMTS13 activity <10 IU/dL at the time of final PEX (Figure 1C). In addition, for 4 of 5 patients with recurrence, ADAMTS13 activity remained <5 IU/dL at all 3 time points. Time to relapse after stopping caplacizumab was 2, 10, and 14 days.
The duration of caplacizumab continuation post-PEX ranged from 0 to 92 days (median, 28 days), with 32% (27/85) of patients continuing caplacizumab beyond 30 days. Conversely, 55% of patients (47/85) discontinued caplacizumab ahead of the 30-day period; in almost half (23/47) of these cases, ADAMTS13 was >30 IU/dL.
Safety data
Adverse events
There were 31 adverse events reported in 26 patients. These were classified into bleeding or nonbleeding complications (Table 4).
Bleeding
Seventeen bleeding episodes were reported in the caplacizumab cohort compared with no reported bleeding episodes in the historical cohort. For the caplacizumab group, the most common bleeding complications were gum bleeding (6/17) and gastrointestinal (GI) bleeding (5/17). Six of 15 episodes resulted in interruption or discontinuation of caplacizumab. The 2 cases of intracranial bleeding were reported to be secondary hemorrhage following extensive cerebral infarction. One patient with intracranial bleeding died, but death was determined by the treating physician to not be attributable to caplacizumab; this complex case presented with dense hemiparesis following first PEX, with computed tomography (CT) brain imaging demonstrating acute infarct. Two doses of caplacizumab were administered before the patient became drowsy, and repeat CT imaging showed acute subarachnoid hemorrhage; consequently, caplacizumab was not given for 3 days. The patient continued to deteriorate with drowsiness/agitation, and a further CT brain scan showed progressive infarction (a single additional dose of caplacizumab was administered); the patient did not survive. The second case with intracranial hemorrhage survived; the patient had caplacizumab interrupted for 48 hours following imaging suggesting secondary hemorrhage of a cerebral infarct, serial imaging showed no extension of the suspected hemorrhage prior to/following reintroduction of caplacizumab. The patient experienced no further intracranial bleeding complications while receiving caplacizumab. The GI bleeding events included 2 upper GI complications and 3 lower GI complications. The lower GI bleeding events were all minor episodes of rectal bleeding with patients remaining hemodynamically stable. The upper GI bleeding events included 1 minor upper GI bleed and 1 major bleeding episode in a patient with a history of Barrett’s esophagus who experienced a drop in hemoglobin and hemodynamic instability with gastroscopy features of esophagitis/small ulcer. The patient was treated with high-dose IV omeprazole, received 7 units of red cell transfusion in total, and improved over the subsequent 5 days.
Nonbleeding
In the caplacizumab group, there were 14 episodes of complications other than bleeding: the most common of these was venous thromboembolism in 4 of 85 patients (compared with 2 of 39 patients in the historical group). Skin reaction/rashes related to caplacizumab injection and abnormal liver function tests were also reported. In 10 of 14 episodes, the complication resulted in interruption or discontinuation of caplacizumab; the decision to interrupt or stop therapy was based on the treating physician’s judgment.
Mortality
All 5 of 85 (6%) patient deaths were related to TTP, but not to caplacizumab. All cases had poor prognostic markers at presentation, and 4 of 5 patients required intubation (Table 5). Three of the 5 patients commenced caplacizumab >1 week after starting PEX (range, 8-21 days). The time to initiate caplacizumab was significantly longer in those patients who died compared with those who survived (Figure 1B; P = .007). Two of 5 patients did not achieve normal platelet counts, with 3 patients normalizing after ≥7 days (range, 7-18). One patient experienced hemorrhagic transformation of a cerebral infarct while on caplacizumab. All deaths were believed by the treating clinician to result from complications of severe/refractory TTP.
Pediatric subgroup analysis
Four patients were younger than 18 years (range, 3-17) of age at the time of administration of the first dose of caplacizumab. The dose of caplacizumab was modified according to weight, and ADAMTS13 activity was <5 IU/dL in all cases at presentation with a detectable inhibitor (range, 11 to >80 U/mL). One case experienced multiorgan failure and required intubation. All patients received steroid and rituximab; 1 patient also received mycophenolate mofetil (Table 6). The time between starting PEX and commencing caplacizumab ranged from 0 to 8 days. All patients achieved a normal platelet count. For 3 of 4 patients, the time to platelet count normalization was between 3 and 4 days; the remaining patient’s platelet count return to normal on day 25, having commenced caplacizumab 1 day after PEX. Caplacizumab was continued post-PEX for a median duration of 30.5 days, with no reports of recurrence at a median follow-up of 92 days (IQR, 61-130).
Discussion
It is widely acknowledged that a gap remains in the current therapeutic armory for acute TTP with regard to achieving a prompt and sustained normalization of the platelet count, reducing ongoing microvascular thrombosis, and preventing disease recurrence pending the action of immunomodulatory therapies. Presented is the largest international collection of real-world evidence describing the use of caplacizumab outside of formal clinical trials, and including pediatric cases, uncaptured within TITAN or HERCULES.7
Real-world evidence is crucial in rare disease. When evaluating the impact of new therapeutic molecules in the context of the natural course of the disease, it is valuable to consider complications associated with existing management. Severe treatment-related complications are relatively rare in acute TTP; in particular, bleeding complication rates are low.9,10 The mode of action of caplacizumab means that bleeding similar to a von Willebrand disease phenotype may occur related to its efficacy at high shear blood flow rates where the A1 domain of vWF is exposed.11 The historical control group did not demonstrate any further bleeding complications beyond cutaneous bleeding/bruising at presentation. For caplacizumab in the real-world setting, the primary bleeding event was gingival, similar to the pivotal trials.7,8 However, severe bleeding complications were observed in 6% of patients, with 2 cases of intracranial hemorrhage, 1 case of severe GI bleeding, 1 case of hemarthrosis, and 1 case of gingival bleeding requiring blood transfusion. In the 2 cases of intracranial bleeding, both occurred following an initial ischemic insult; interruption of caplacizumab was followed by further serial infarction and secondary hemorrhage. Cases complicated by acute ischemic stroke may represent a high-risk group for intracranial bleeding complications and will require a case-by-case evaluation of the risk vs benefit of drug initiation/continuation. Acute drug interruption itself may be associated with progressive microvascular thrombosis and disease exacerbation.
A post-hoc analysis of the HERCULES trial reported fewer thromboembolic complications in caplacizumab-treated patients.12 Here, 5 episodes of venous thromboembolism were reported; in 4 of 5 cases, caplacizumab was interrupted/discontinued to commence anticoagulation. In the UK, aspirin and low molecular weight heparin are typically introduced once the platelet count is >50 × 109/L; however, with concurrent caplacizumab therapy, the intuitive practice has been to withhold aspirin. Best practice regarding thromboprophylaxis or management of acute thrombosis remains unclear.
For rare conditions such as TTP, complex clinical trials often attract highly selected populations managed in environments with a capacity for strict adherence to study protocols. Further, it has been suggested that some patients with more severe disease have been underrepresented in clinical trials. Within this UK cohort, patients exhibited a severe disease phenotype, with a significant proportion demonstrating cardiac involvement and the need for assisted ventilation.
The median time to platelet count normalization from initiation of caplacizumab was comparable with data from the HERCULES trial, and all surviving patients had normal platelet counts (with the exception of 1 patient with a known history of immune thrombocytopenia). Registry data from 2009 to 2018 reveal that 56% of patients receiving standard care alone achieved a platelet count > 150 × 109/L within 1 week, compared with 88% of patients receiving caplacizumab.13 It could be postulated that an increase in rituximab use may have a bearing on this result; however, this has not been shown in previous studies of rituximab.14
A significant decrease in the median duration of PEX over time, from 14 days to 8 days, is observed in registry data.13 This is most likely due to earlier intervention with rituximab. Greater disease severity at presentation, including the need for intubation, may contribute to the prolonged duration of PEX to remission and the longer median duration of PEX seen within the caplacizumab cohort in this study (7 days) compared with the HERCULES study (5.8 days).7 Delayed initiation of caplacizumab, particularly in the initial months as hematologists became familiar with the treatment, may also have played a role.
It remains unclear whether early introduction of caplacizumab may limit microthrombotic complications long-term. Acute neurological recovery was observed in ∼60% of patients who received the drug, consistent with its proposed organ-protective properties.8 There are limited data on the recovery of organ damage in TTP; however, longer-term neurological complications have become an increasingly recognized feature, and a beneficial role of caplacizumab here is plausible.15-18
The summary of product characteristics information advises continuing caplacizumab for 30 days post discontinuation of PEX and extension as required thereafter.19 The UK TTP Forum previously agreed that caplacizumab could be discontinued when ADAMTS13 activity is >30 IU/dL, 7 days from discontinuation of PEX and confirmed on repeat sampling. This may have influenced the observed rate of early discontinuation. Discontinuation of caplacizumab once ADAMTS13 activity approaches >30 IU/dL could potentially limit drug exposure and additional drug costs. If there is delayed recovery of ADAMTS13, there is an argument to continue the drug to avoid a recurrence. The literature suggests that exacerbations can occur in up to 50% of patients despite treatment.20-23 Recurrent disease was seen in 5 of 85 (6%) patients in this study. Similar to HERCULES data, all patients experiencing a recurrence exhibited a persistently low ADAMTS13 activity.7 These real-world data support continuation of caplacizumab until an ADAMTS13 activity recovery in the region of 30 IU/dL is observed, recognizing that this may occur ahead of the advised 30-day drug-administration period.
The 6% mortality observed in this cohort is lower than the reported national mortality, and there has been little change in a mortality of 8% to 20% as a proportion of new diagnoses from the Office for National Statistics data for 2003 to 2013. In a recent multicenter retrospective study of patients with iTTP, Colling et al found a mortality of 3.7% per acute TTP episode, acknowledging that the patients in their study had been managed in large academic centers, potentially explaining the lower than usual mortality reported in the literature.10 In this study, 5 patients died while receiving caplacizumab compared with none in the caplacizumab arm of the HERCULES trial.7 All of those who died in our cohort had poor prognostic markers and severe disease characteristics at presentation. The utility of caplacizumab outside of the acute presenting event is unknown. Therefore, the outcomes for these patients are likely linked to disease severity, rather than failure of caplacizumab. The time to initiate caplacizumab was significantly longer in those patients who died than those who survived (P = .007), suggesting that caplacizumab may be more effective as a component of acute therapy. In the trials, caplacizumab was initiated within 24 hours of the first PEX.7 Logistical delays in obtaining caplacizumab in the absence of a previously established pathway may have contributed to the longer time taken to initiate therapy.
There are 2 case reports, both immune-mediated, in the literature describing the use of caplacizumab in children younger than 16 years of age: 1 had refractory disease, and the other had an acute episode of relapse. Both experienced platelet count normalization by day 3, with no reported adverse events or disease recurrence.24,25 Within our pediatric group there was no reported adverse events, including bleeding complications or mortality.
It is acknowledged that the data presented here are descriptive rather than the result of a randomized study. The validity of collection of real-world evidence was augmented by UK TTP registry data, which provided existing confirmation of the natural history of the disease and management in the UK. Reproducibility was optimized through participation from multiple health care settings completing standardized electronic database entry, increasing the uniformity and completeness of data. Although the demographics for the real-world group receiving caplacizumab and the historical control group were well matched, the limitations of using a historical unexposed control group are acknowledged; because of the number of patients, matching of cases and controls could not be undertaken.
Conclusions
This real-world evidence from the largest series of TTP patients receiving caplacizumab, outside of the pivotal studies, provides confirmation of the therapeutic benefits of caplacizumab and its inherent bleeding risk. The severe disease phenotype of patients presenting with acute iTTP is described, along with a reduced time to platelet count normalization and duration of PEX in patients receiving caplacizumab, which are comparable with clinical trial data. The wide use of caplacizumab in the UK represents current clinical practice and highlights important considerations when utilizing caplacizumab as a component of standard care in acute TTP.
For original data, please contact Tina Dutt (tina.dutt@liverpoolft.nhs.uk).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors give special thanks to Carol Powell for her administrative assistance.
Authorship
Contribution: T.D. and R.J.S. performed literature searches, designed the study, collected, analyzed, and interpreted data, created the figures, and wrote the manuscript; M. Stubbs and J.Y. collected and analyzed data and created figures; B.B., T.C., M.P.C., M.D., T.A.E., R.G., J.G., J. Hanley, J. Haughton, J. Hermans, Q.H., L.H., G.L., H.L., M.M., P.L.R.N., N.P., A.R., R.R., S.R., A.T., W.T., O.T., J.J.V.V., and C.-H.T. collected data and reviewed the manuscript; S.L. performed statistical analyses, interpreted data, and reviewed the manuscript; and M. Scully designed the study, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: T.D., M. Scully, R.G., J.J.V.V., and W.T have received honoraria from Sanofi for serving on advisory boards. T.D. and M. Scully have received speaker fees from Sanofi and Alexion. The remaining authors declare no competing financial interests.
Correspondence: Tina Dutt, Department of Haematology, Liverpool University Hospitals NHS Foundation Trust, Prescot St, Liverpool L7 8XP, United Kingdom; e-mail: tina.dutt@liverpoolft.nhs.uk.
REFERENCES
Author notes
T.D. and R.J.S. are joint first authors.