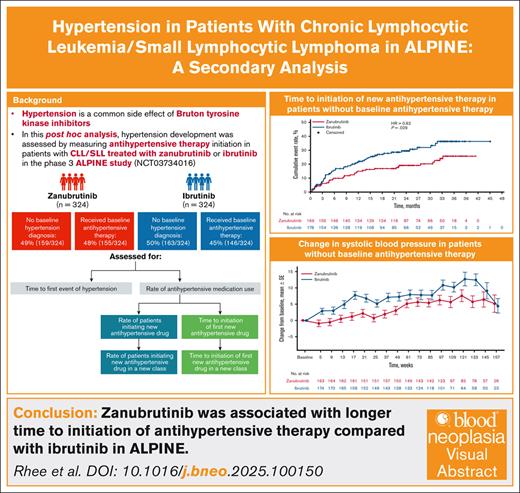
Key Points
In the ALPINE trial, unlike the ASPEN trial, hypertension rates were comparable for zanubrutinib vs ibrutinib.
Initiation of antihypertensive therapy occurred later with zanubrutinib vs ibrutinib, providing context for hypertension rates in ALPINE.
Visual Abstract
Hypertension is a common side effect of Bruton tyrosine kinase inhibitors (BTKis). The second-generation BTKi zanubrutinib has high BTK selectivity, which may minimize off-target effects. A phase 3 trial (ALPINE) demonstrated improved efficacy and safety of zanubrutinib vs ibrutinib in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). To better understand hypertension risk with zanubrutinib vs ibrutinib in ALPINE, this post hoc analysis evaluated hypertension development by measuring antihypertensive therapy initiation. Eligible adults with R/R CLL/SLL were randomized to zanubrutinib 160 mg twice-a-day or ibrutinib 420 mg daily until disease progression/unacceptable toxicity. Differences in treatment-emergent hypertension, antihypertensive therapy use and changes in blood pressure were evaluated. Of 648 patients (zanubrutinib, n = 324; ibrutinib, n = 324), nearly half used antihypertensive therapy at baseline (zanubrutinib, 48%; ibrutinib, 45%). With zanubrutinib vs ibrutinib, initiation of a new antihypertensive agent (28% vs 32%) or new antihypertensive class (24% vs 29%) were comparable. In patients without baseline antihypertensive therapy, 21% vs 29% with zanubrutinib vs ibrutinib, respectively, initiated new antihypertensive therapy. In all patients, time-to-initiation of a new antihypertensive class was longer with zanubrutinib vs ibrutinib; in those without baseline antihypertensive therapy, time-to-initiation of a new antihypertensive agent was also longer. Mean systolic blood pressure changes were lower with zanubrutinib vs ibrutinib. In conclusion, zanubrutinib was associated with longer time to initiation of antihypertensive therapy compared with ibrutinib in ALPINE. These findings could be of clinical importance when initiating BTKi therapy in patients with CLL/SLL. This trial was registered at www.ClinicalTrials.gov as #NCT03734016.
Introduction
Hypertension and adverse cardiovascular events commonly occur after treatment with the first-generation Bruton tyrosine kinase (BTK) inhibitor ibrutinib which is indicated for various hematologic malignancies.1-3 Ibrutinib-associated hypertension and cardiovascular toxicity can occur due to off-target effects, such as nitric oxide synthesis inhibition, vascular endothelial growth factor receptor 2 inhibition, and downregulation of the phosphatidylinositol 3-kinase (PI3K)–Akt pathway.4 A large proportion of patients with B-cell malignancies treated with ibrutinib have been reported to develop hypertension, ranging from 23% to 72% of patients, with the prevalence increasing over time.5-7 In one retrospective analysis, development of new or worsened hypertension in patients treated with ibrutinib was associated with a more than twofold increase in the risk of major adverse cardiovascular events, including arrhythmia, myocardial infarction, stroke, heart failure, and cardiovascular death.7 Next-generation BTK inhibitors including, acalabrutinib, pirtobrutinib, and zanubrutinib, that have been subsequently approved by the US Food and Drug Administration8-10 have shown higher specificity for BTK and may have improved cardiovascular safety profiles compared with ibrutinib.1,8-11
Zanubrutinib is a potent, highly selective, and irreversible second-generation BTK inhibitor designed to provide complete and sustained BTK occupancy while minimizing off-target binding.8,12,13 Compared with ibrutinib, zanubrutinib has greater potency for BTK (IC50: zanubrutinib, 0.5 nM; ibrutinib, 1.5 nM) and greater selectivity for BTK relative to tyrosine kinases expressed in carcinoma (vs tyrosine protein kinase TEC; fold selectivity: zanubrutinib, 88; ibrutinib, 6.7 and vs epidermal growth factor receptor; fold selectivity: zanubrutinib, 42; ibrutinib, 3.5).12,14 Overall, the high selectivity of zanubrutinib may reduce off-target cardiovascular events when used to treat patients with B-cell malignancies.8,15 A pooled retrospective analysis of 10 clinical studies of zanubrutinib supported this concept, showing lower rates of atrial fibrillation, symptomatic ventricular arrhythmias, and hypertension with zanubrutinib than with ibrutinib.16
ALPINE was a multinational, randomized, open-label phase 3 trial of zanubrutinib vs ibrutinib in patients with relapsed or refractory (R/R) chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).17,18 Results from ALPINE demonstrated that zanubrutinib was superior to ibrutinib with respect to overall response and progression-free survival and had a more favorable cardiovascular safety profile.17,18 Specifically, fewer adverse cardiovascular events, primarily atrial fibrillation, led to treatment discontinuation with zanubrutinib compared with ibrutinib (0.3% vs 4.3%, respectively).17 Although ASPEN, a trial conducted in patients with Waldenström's macroglobulinemia, demonstrated a significantly reduced exposure-adjusted incidence rate of hypertension per 100 person-months with zanubrutinib (0.43; n = 101) vs ibrutinib (0.89; n = 98), the rate of hypertension in ALPINE did not differ between the zanubrutinib (1.04; n = 324) and ibrutinib (1.17; n = 324) treatment groups.16
It is not currently known what underlies the differences in hypertension rates between ALPINE and ASPEN, but differences in study design (ASPEN did not exclude patients with uncontrolled hypertension), the B-cell malignancy (CLL/SLL vs Waldenström's macroglobulinemia), or use of antihypertensive therapy at baseline are possible explanatory factors. Previous studies have shown that initiation of antihypertensive therapies as an indication of new-onset or worsened hypertension is a more robust parameter than the reporting of hypertension event rates in both clinical studies and administrative data analyses.19-21 Differences in the rate of antihypertensive therapy initiation between treatment groups may have played a role in the equipoise of rates of hypertension seen in ALPINE. Hence, the purpose of the present analysis is to further investigate rates of new-onset or worsened hypertension in ALPINE, as reflected by the use and time to initiation of antihypertensive therapies, in patients treated with zanubrutinib vs ibrutinib in this 3-year study.
Methods
Study design and patients
ALPINE (ClinicalTrials.gov identifier: NCT03734016) was an open-label, phase 3, randomized, multicenter trial to directly compare the efficacy, safety, and tolerability of zanubrutinib with those of ibrutinib in patients with R/R CLL or SLL.17 Detailed methods for ALPINE have been previously published.17 Briefly, patients were randomized 1:1 to zanubrutinib 160 mg orally twice daily or ibrutinib 420 mg orally once daily until disease progression or unacceptable toxicity. Eligible patients were adults (≥18 years of age) with a confirmed diagnosis of CLL or SLL that met International Workshop on CLL criteria,22 required treatment, was R/R to ≥1 prior line of therapy, and was measurable via imaging. Patients who had previously received BTK-inhibitor treatment or had a history of bleeding disorders, active infections, stroke or intracranial hemorrhage, recent previous cancer, or major surgery were excluded. Patients with uncontrolled hypertension as indicated by ≥2 consecutive blood pressure measurements showing systolic blood pressure of >170 mm Hg and diastolic blood pressure of >105 mm Hg at screening were also ineligible for the study. The trial was approved by the institutional review board or independent ethics committee at each trial site and conducted in accordance with the principles of the Declaration of Helsinki, the International Council for Harmonisation Good Clinical Practice guidelines, and all applicable regulatory requirements. All patients provided written informed consent.
Assessments and case definitions
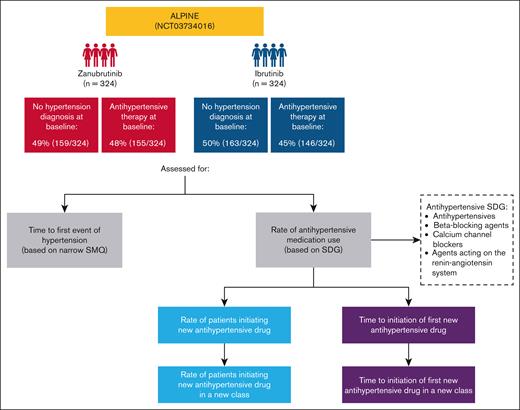
This post hoc analysis of ALPINE investigated rates of diagnoses of treatment-emergent hypertension, rates of and time to initiating or modifying antihypertensive agents, and changes from baseline in blood pressure. Systolic and diastolic blood pressure were measured in the clinic at each study visit. Hypertension included any event coded as hypertension per Standardized Medical Dictionary of Regulatory Activities Query narrow terms (version 24.0). In this analysis, the definition of hypertension was also based on the use of antihypertensive medication, and changes in antihypertensive medication were used to interpret the risk of hypertensive events. Concomitant antihypertensive medications were defined based on Standardized Drug Grouping and adjudicated by an independent cardiologist (author W.B.W.) blinded to study drug assignment according to the following general rules: 1) loop diuretics were excluded as they are more likely to be used for heart failure or edema than hypertension; 2) similarly, aldosterone antagonists (spironolactone and eplerenone) were excluded as they are more commonly prescribed for heart failure or liver failure than hypertension; and 3) vasoactive drugs for glaucoma and for erectile dysfunction/pulmonary hypertension were excluded. All fixed-dose combination products indicated for the treatment of hypertension were included. A new antihypertensive therapy was defined as any new medication indicated for hypertension taken after the first dose of study drug and is a surrogate marker for new-onset hypertension in patients without antihypertensive therapy at baseline.19-21 A new class of antihypertensive therapy was defined as any new therapy in a new therapeutic class indicated for hypertension taken after the first dose of study drug and is a surrogate marker for worsening hypertension control.19-21 Any changes in concomitant antihypertensive therapy due to causes other than hypertension were excluded from the analysis.
Statistical analyses
Descriptive statistics were used to evaluate baseline demographics and clinical characteristics. The exposure-adjusted incidence rate, an average event count per unit of person-time, was calculated as the number of patients with treatment-emergent adverse event of interest divided by the total exposure time. Total exposure time was the time from first dose to first event (or last dose + 30 days, if there was no event) converted to 100 person-months unit of time. The Poisson regression model was used to compare exposure-adjusted incidence rates between treatment arms, with the number of patients with events as the dependent variable and log (exposure time) as the offset.
The incidence of initiating a new antihypertensive drug or a new class of antihypertensive drug was analyzed by chi-square test. The time to initiating new antihypertensive therapy or a new class of antihypertensive therapy was assessed using the Kaplan-Meier method, which evaluates the cumulative event rates for time to initiation. Cumulative event rates for time to initiation assesses both the incidences of initiating a new (or new class) of antihypertensive medication and the time it takes between the start of the study and the initiation of a new antihypertensive medication23; lower cumulative events for time to initiation represents a longer delay until a new antihypertensive medication is needed to be prescribed to the patient. Comparisons of the time-to-onset end points were analyzed based on the log-rank test. Changes from baseline in clinical systolic and diastolic blood pressure were calculated at varying time points during the trial and provided as the mean and standard error of the mean. Inferential analysis of comparisons of blood pressure changes between treatment arms was conducted via longitudinal modeling adjusting for the following covariates as fixed effects: baseline systolic blood pressure, treatment group, region, race, sex, and age group; a random intercept was included in the model. Comparisons of blood pressure changes between treatment arms were performed via a linear contrast with P values reported based on the F test.
Results
Patient population
In ALPINE, 324 patients with R/R CLL/SLL received zanubrutinib and 324 received ibrutinib (Figure 1). Patient characteristics were generally balanced between the zanubrutinib and ibrutinib arms at baseline (Table 1). The median age was 67 years in the zanubrutinib arm and 68 years in the ibrutinib arm; most patients were men and the majority were White. At baseline, 51% of patients in the zanubrutinib arm and 50% in the ibrutinib arm had medical history of hypertension at baseline and nearly half of patients were receiving antihypertensive therapy (zanubrutinib, 48%; ibrutinib, 45%).
Study design and assessments of incident hypertension in ALPINE. Hypertension diagnosis at baseline was determined via medical history, which would include during study entry. SDG, Standardized Drug Grouping; SMQ, Standardized Medical Dictionary for Regulatory Activities Query.
Study design and assessments of incident hypertension in ALPINE. Hypertension diagnosis at baseline was determined via medical history, which would include during study entry. SDG, Standardized Drug Grouping; SMQ, Standardized Medical Dictionary for Regulatory Activities Query.
Baseline demographics and clinical characteristics
| . | Zanubrutinib (n = 324) . | Ibrutinib (n = 324) . |
|---|---|---|
| Age, median (range), y | 67.0 (35-90) | 67.5 (35-89) |
| <65, n (%) | 126 (38.9) | 125 (38.6) |
| ≥65 to <75, n (%) | 124 (38.3) | 130 (40.1) |
| ≥75, n (%) | 74 (22.8) | 69 (21.3) |
| Sex, n (%) | ||
| Men | 212 (65.4) | 231 (71.3) |
| Women | 112 (34.6) | 93 (28.7) |
| Race, n (%) | ||
| White | 261 (80.6) | 264 (81.5) |
| Asian | 45 (13.9) | 44 (13.6) |
| Black or African American | 4 (1.2) | 2 (0.6) |
| Not reported | 8 (2.5) | 12 (3.7) |
| Other | 6 (1.9) | 2 (0.6) |
| BMI, mean (SD), kg/m2 | 27.2 (4.9) | 27.1 (4.6) |
| <18.5, n (%) | 4 (1.2) | 2 (0.6) |
| ≥18.5 to <25, n (%) | 111 (34.3) | 112 (34.6) |
| ≥25 to <30 kg/m2, n (%) | 115 (35.5) | 131 (40.4) |
| ≥30 kg/m2, n (%) | 89 (27.5) | 74 (22.8) |
| Missing | 5 (1.5) | 5 (1.5) |
| Key hematologic parameters, n (%) | ||
| Hemoglobin-low | ||
| G1-G2 | 145 (44.8) | 160 (49.4) |
| G3-G4 | 9 (2.8) | 9 (2.8) |
| Neutrophil count-low | ||
| G1-G2 | 38 (11.7) | 45 (13.9) |
| G3-G4 | 13 (4.0) | 16 (4.9) |
| Platelet count-low | ||
| G1-G2 | 181 (55.9) | 178 (54.9) |
| G3-G4 | 9 (2.8) | 14 (4.3) |
| eGFR, mean (SD), mL/min/1.73 m2 | 72.8 (17.8) | 70.2 (17.8) |
| <30, n (%) | 1 (0.3) | 4 (1.2) |
| ≥30 to <60, n (%) | 85 (26.2) | 95 (29.3) |
| ≥60, n (%) | 238 (73.5) | 225 (69.4) |
| Type 2 diabetes, n (%) | 67 (20.7) | 63 (19.4) |
| Hyperlipidemia, n (%) | 95 (29.3) | 80 (24.7) |
| Heart rate, mean (SD), beats per min | 76.9 (10.7) | 76.2 (11.7) |
| Hypertension diagnosis at baseline, n (%)∗ | 165 (50.9) | 162 (50.0) |
| Systolic blood pressure at baseline, mean (SD), mm Hg | 128.9 (16.0) | 127.3 (15.1) |
| <120, n (%) | 96 (29.6) | 99 (30.6) |
| 120-129, n (%) | 78 (24.1) | 78 (24.1) |
| 130-139, n (%) | 64 (19.8) | 72 (22.2) |
| ≥140, n (%) | 86 (26.5) | 75 (23.1) |
| Antihypertensive drug use at baseline, n (%) | ||
| No | 169 (52.2) | 178 (54.9) |
| Yes | 155 (47.8) | 146 (45.1) |
| . | Zanubrutinib (n = 324) . | Ibrutinib (n = 324) . |
|---|---|---|
| Age, median (range), y | 67.0 (35-90) | 67.5 (35-89) |
| <65, n (%) | 126 (38.9) | 125 (38.6) |
| ≥65 to <75, n (%) | 124 (38.3) | 130 (40.1) |
| ≥75, n (%) | 74 (22.8) | 69 (21.3) |
| Sex, n (%) | ||
| Men | 212 (65.4) | 231 (71.3) |
| Women | 112 (34.6) | 93 (28.7) |
| Race, n (%) | ||
| White | 261 (80.6) | 264 (81.5) |
| Asian | 45 (13.9) | 44 (13.6) |
| Black or African American | 4 (1.2) | 2 (0.6) |
| Not reported | 8 (2.5) | 12 (3.7) |
| Other | 6 (1.9) | 2 (0.6) |
| BMI, mean (SD), kg/m2 | 27.2 (4.9) | 27.1 (4.6) |
| <18.5, n (%) | 4 (1.2) | 2 (0.6) |
| ≥18.5 to <25, n (%) | 111 (34.3) | 112 (34.6) |
| ≥25 to <30 kg/m2, n (%) | 115 (35.5) | 131 (40.4) |
| ≥30 kg/m2, n (%) | 89 (27.5) | 74 (22.8) |
| Missing | 5 (1.5) | 5 (1.5) |
| Key hematologic parameters, n (%) | ||
| Hemoglobin-low | ||
| G1-G2 | 145 (44.8) | 160 (49.4) |
| G3-G4 | 9 (2.8) | 9 (2.8) |
| Neutrophil count-low | ||
| G1-G2 | 38 (11.7) | 45 (13.9) |
| G3-G4 | 13 (4.0) | 16 (4.9) |
| Platelet count-low | ||
| G1-G2 | 181 (55.9) | 178 (54.9) |
| G3-G4 | 9 (2.8) | 14 (4.3) |
| eGFR, mean (SD), mL/min/1.73 m2 | 72.8 (17.8) | 70.2 (17.8) |
| <30, n (%) | 1 (0.3) | 4 (1.2) |
| ≥30 to <60, n (%) | 85 (26.2) | 95 (29.3) |
| ≥60, n (%) | 238 (73.5) | 225 (69.4) |
| Type 2 diabetes, n (%) | 67 (20.7) | 63 (19.4) |
| Hyperlipidemia, n (%) | 95 (29.3) | 80 (24.7) |
| Heart rate, mean (SD), beats per min | 76.9 (10.7) | 76.2 (11.7) |
| Hypertension diagnosis at baseline, n (%)∗ | 165 (50.9) | 162 (50.0) |
| Systolic blood pressure at baseline, mean (SD), mm Hg | 128.9 (16.0) | 127.3 (15.1) |
| <120, n (%) | 96 (29.6) | 99 (30.6) |
| 120-129, n (%) | 78 (24.1) | 78 (24.1) |
| 130-139, n (%) | 64 (19.8) | 72 (22.2) |
| ≥140, n (%) | 86 (26.5) | 75 (23.1) |
| Antihypertensive drug use at baseline, n (%) | ||
| No | 169 (52.2) | 178 (54.9) |
| Yes | 155 (47.8) | 146 (45.1) |
BMI, body mass index; eGFR, estimated glomerular filtration rate; G, grade; SD, standard deviation.
Based on narrow Standardized Medical Dictionary for Regulatory Activities Query and was determined via medical history.
Rates of hypertension
The median duration of treatment for CLL/SLL in ALPINE was 28.4 and 24.3 months in the zanubrutinib and ibrutinib arms, respectively.17 The investigator-reported cumulative event rates of hypertension were comparable between treatment arms, ranging from 9% in the zanubrutinib arm and 12% in the ibrutinib arm at 6 months to 28% and 29%, respectively, at 36 months (Figure 2).
Cumulative event rate of hypertension over time. The vertical axis represents the cumulative event rate percentage, and the horizontal axis represents data grouped by months of treatment.
Cumulative event rate of hypertension over time. The vertical axis represents the cumulative event rate percentage, and the horizontal axis represents data grouped by months of treatment.
Rates of antihypertensive therapy use
Across all patients treated in ALPINE, a comparable percentage of patients treated with zanubrutinib vs ibrutinib initiated a new antihypertensive therapy (92/324 [28%] vs 105/324 [32%]; P = .267) or a new class of antihypertensive therapy (78/324 [24%] vs 95/324 [29%]; P = .131) (Figure 3A). Among patients without baseline antihypertensive therapy (347/648 [54%]), 21% (35/169) of zanubrutinib-treated patients vs 29% (51/178) of ibrutinib-treated patients were initiated on a new antihypertensive therapy during the trial (Figure 3B); a difference that was not statistically significant (P = .087). Among patients taking antihypertensive therapy at baseline, a similar proportion in both treatment arms initiated a new antihypertensive therapy (zanubrutinib, 57/155 [37%]; ibrutinib, 54/146 [37%]; P = .970) or a new class of antihypertensive therapy (zanubrutinib, 43/155 [28%]; ibrutinib, 44/146 [30%]; P = .647) (Figure 3C).
Incidence of initiating a new antihypertensive therapy or new class of antihypertensive therapy. Incidence rates for initiation are shown for (A) all patients, (B) patients without baseline antihypertensive therapy, and (C) patients with baseline antihypertensive therapy. For zanubrutinib vs ibrutinib all populations are P > .05; chi-square test.
Incidence of initiating a new antihypertensive therapy or new class of antihypertensive therapy. Incidence rates for initiation are shown for (A) all patients, (B) patients without baseline antihypertensive therapy, and (C) patients with baseline antihypertensive therapy. For zanubrutinib vs ibrutinib all populations are P > .05; chi-square test.
Time to initiation of antihypertensive therapy
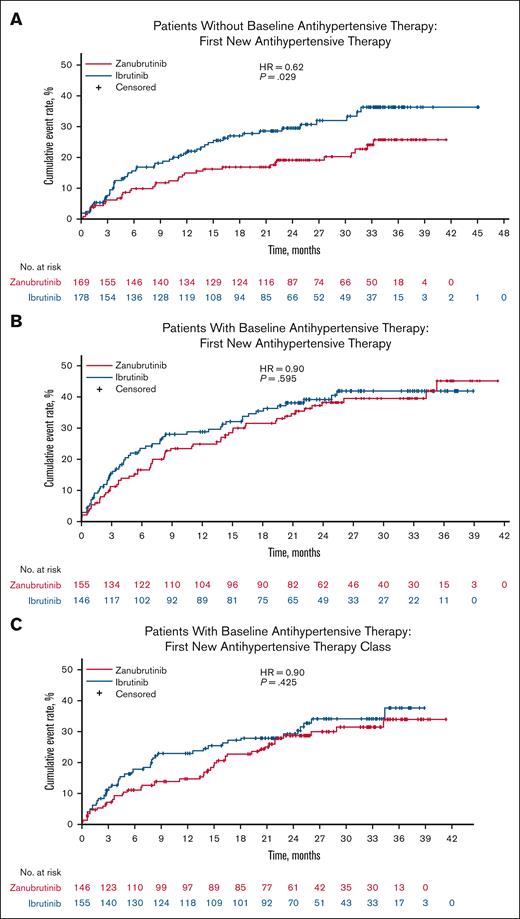
Among all patients, differences in cumulative event rates for time to initiation of a new antihypertensive therapy in the zanubrutinib arm vs ibrutinib arm did not reach statistical significance (hazard ratio [HR] = 0.77, P = .071; Figure 4A). The cumulative event rates for time to initiation of a new class of antihypertensive therapy were significantly lower with zanubrutinib compared with ibrutinib in all patients in ALPINE (HR = 0.72, P = .034; Figure 4B), representing a delay in the time for zanubrutinib. In patients without baseline antihypertensive therapy, the cumulative event rates for time to initiation of a new antihypertensive therapy were significantly lower in the zanubrutinib arm vs ibrutinib arm (HR = 0.62, P = .029; Figure 5A). In contrast, in patients with baseline antihypertensive therapy, the cumulative event rates for time to initiation were comparable between zanubrutinib and ibrutinib for both new antihypertensive therapy (HR = 0.90, P = .595) (Figure 5B) as well as new antihypertensive therapy class (HR = 0.84, P = .425) (Figure 5C).
Time to initiation of a new antihypertensive therapy or new class of antihypertensive therapy in all patients. Cumulative event rates for time to initiation are shown for (A) a new antihypertensive therapy in all patients and (B) a new antihypertensive therapy class in all patients. HR, hazard ratio.
Time to initiation of a new antihypertensive therapy or new class of antihypertensive therapy in all patients. Cumulative event rates for time to initiation are shown for (A) a new antihypertensive therapy in all patients and (B) a new antihypertensive therapy class in all patients. HR, hazard ratio.
Time to initiation of a new antihypertensive therapy in patients without or in patients with antihypertensive therapy at baseline. Cumulative event rates for time to initiation are shown for (A) a new antihypertensive therapy in patients without baseline antihypertensive therapy, (B) a new antihypertensive therapy in patients with baseline antihypertensive therapy, and (C) a new antihypertensive therapy class in patients with baseline antihypertensive therapy. HR, hazard ratio.
Time to initiation of a new antihypertensive therapy in patients without or in patients with antihypertensive therapy at baseline. Cumulative event rates for time to initiation are shown for (A) a new antihypertensive therapy in patients without baseline antihypertensive therapy, (B) a new antihypertensive therapy in patients with baseline antihypertensive therapy, and (C) a new antihypertensive therapy class in patients with baseline antihypertensive therapy. HR, hazard ratio.
Absolute changes in blood pressure
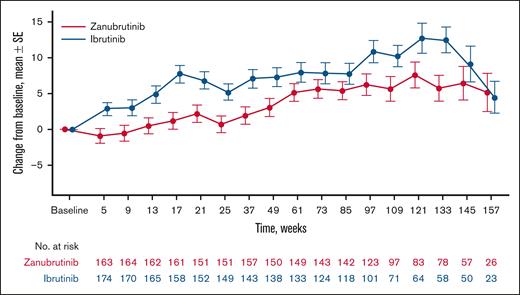
Mean changes from baseline in systolic blood pressure were generally lower in patients treated with zanubrutinib vs ibrutinib in the overall ALPINE population, with the largest treatment difference between zanubrutinib and ibrutinib at week 17 (mean change from baseline at week 17, zanubrutinib: +3.0; ibrutinib: +7.6; supplemental Table 1). Similarly, in patients without baseline antihypertensive therapy, mean changes from baseline in systolic blood pressure were generally lower in the zanubrutinib arm compared with the ibrutinib arm during the study, with the largest treatment difference at week 133 (mean change from baseline at week 133: zanubrutinib, +5.8; ibrutinib, +12.4]; Figure 6); differences in changes in systolic blood pressure were statistically significant (P = .0002). Changes in systolic blood pressure were comparable between treatment groups in patients with baseline antihypertensive therapy (supplemental Figure 1), as were changes in diastolic blood pressure between treatment groups in all 3 populations (supplemental Figure 2). No apparent differences were noted in available blood pressure readings between treatment groups at 3 years of treatment.
Change in systolic blood pressure in patients without baseline antihypertensive therapy. The mean (SE) change from baseline is shown by weeks of treatment. For zanubrutinib vs ibrutinib P = .0002; longitudinal modeling. SE, standard error.
Change in systolic blood pressure in patients without baseline antihypertensive therapy. The mean (SE) change from baseline is shown by weeks of treatment. For zanubrutinib vs ibrutinib P = .0002; longitudinal modeling. SE, standard error.
Discussion
This post hoc analysis of the ALPINE study used initiation of antihypertensive therapy as a surrogate for new-onset or worsened hypertension, a measure that is more sensitive than relying solely on reported hypertension-associated adverse event rates. Among patients without baseline antihypertensive therapy, time-to-initiation of a new antihypertensive therapy was significantly longer in the zanubrutinib arm compared to the ibrutinib arm over the course of the study. Furthermore, across all patients, time to initiation of a new class of antihypertensive therapy, potentially indicative of worsening or more treatment-resistant hypertension, was also significantly longer with zanubrutinib than with ibrutinib.
Among patients without baseline antihypertensive therapy, a smaller percentage of patients treated with zanubrutinib compared with ibrutinib were initiated on a new antihypertensive therapy (21% vs 29%, respectively), although the difference was not statistically significant. The analysis also indicated that changes in systolic blood pressure were generally lower with zanubrutinib compared with ibrutinib, which was more pronounced among patients without baseline antihypertensive therapy. These results align well with a previous pooled analysis of the cardiovascular profile of zanubrutinib that included trials with ibrutinib treatment arms.16 The observed differences in systolic blood pressure between treatments are considered clinically meaningful. A meta-analysis of patient-level data suggested that a 3-mm Hg reduction in systolic blood pressure can be associated with an ∼10% reduction in cardiovascular event rates in a middle-aged or older population.24 Although average changes in blood pressure are associated with worse cardiovascular outcomes, this may underestimate cardiovascular risks, particularly in patients with greater cardiovascular vulnerability.19-21 Overall, the delayed initiation of antihypertensive therapy suggests that zanubrutinib may be less likely than ibrutinib to induce clinically significant elevations in blood pressure, as observed in both the overall ALPINE population and those without baseline antihypertensive therapy.
In a previous report on the ALPINE trial, 23.5% and 22.8% of patients in the zanubrutinib and ibrutinib arms, respectively, were reported to have had hypertension as an adverse event, with exposure-adjusted incidence rates of hypertension being 1.04 for zanubrutinib and 1.17 for ibrutinib arms.16 In ALPINE, 15.1% and 13.6% of patients in the zanubrutinib and ibrutinib arms, respectively, experienced grade ≥3 hypertension adverse events.17 However, based on the present 3-year post hoc analysis, these comparable rates of developing clinically recognized hypertension as an adverse event were confounded by a higher antihypertensive therapy burden in patients treated with ibrutinib vs zanubrutinib. In contrast to ALPINE, differential rates of hypertension were reported in another trial with these 2 BTK inhibitors.16 In the phase 3 ASPEN trial, the exposure-adjusted incidence rate of hypertension-related adverse events was significantly lower with zanubrutinib (0.43 events per 100 person-months) compared with ibrutinib (0.89 events per 100 person-months), with hypertension rates of 16.8% and 25.5% in the zanubrutinib and ibrutinib arms, respectively.16 To provide further context for the reported hypertension rates, the rates of hypertension adverse events with zanubrutinib were also lower in other clinical trials compared with that in ALPINE. In a pooled analysis of 10 trials in 1550 patients with B-cell malignancies treated with zanubrutinib monotherapy, 16.7% of patients were reported to have had a hypertension adverse event.16 Additionally, a comparable analysis of the exposure-adjusted incidence rate of hypertension adverse events across clinical trials with zanubrutinib reported a range of 0.27 to 0.62 persons per 100 person-months, whereas the rate in ALPINE was 1.04 persons per 100 person-months with zanubrutinib.16 The higher exposure–associated incidence rate of hypertension in ALPINE compared with other zanubrutinib studies was originally thought to be due to differences in study designs. In our more detailed analysis of ALPINE, using the initiation of antihypertensive therapy rather than reported adverse events of hypertension as a more robust hypertension end point, we have shown an overall lower rate of off-target BTK-inhibitor–induced hypertension and a significantly delayed time to initiation of a new antihypertensive therapy and new antihypertensive class with zanubrutinib compared with ibrutinib. Initiation of a new antihypertensive therapy is associated with new-onset hypertension, while initiating a new class of antihypertensive therapy would be indicative of worsening and potentially more difficult to treat hypertension.19-21,25 Additionally, these factors may have played a role in confounding the rates of hypertension adverse events seen in ALPINE compared with other studies with zanubrutinib.
In addition to these results, the incidence rates of atrial arrhythmias (including atrial fibrillation), myocardial infarction, and other cardiac disorders were reported to be lower with zanubrutinib than with ibrutinib in ALPINE.17 These cardiac adverse events are associated with hypertension, particularly in older patients.3,26-28 A potential factor impacting the occurrence of hypertension and adverse cardiovascular events is the difference in BTK-inhibitor mechanisms of action. Ibrutinib-associated cardiovascular toxicity is thought to occur due to off-target effects, as ibrutinib modulates many cardiovascular targets including vascular endothelial growth factor receptor 2, TEC, Src, and the PI3K-Akt pathway.3,4,29 Ibrutinib’s increased incidence of atrial fibrillation compared with zanubrutinib has been reported to be due to off-target inhibition of C-terminal Src kinase (CSK), and ibrutinib has greater inhibition of CSK compared with zanubrutinib.17,30 CSK and Src have also been implicated in blood pressure regulation.31 Although the exact mechanisms detailing ibrutinib’s association with increased hypertension are not fully understood, another possible explanation is off-target inhibition of PI3K and the PI3K-Akt pathway, which is in part modulated by TEC kinases.7,32 The enhanced selectivity of zanubrutinib compared with ibrutinib has been demonstrated by reduced in vitro binding to epidermal growth factor receptor, Janus kinase 3, Src-family kinases, and TEC tyrosine kinases.12,33 Importantly, hypertension is not the only factor associated with adverse cardiovascular events29; and thus, despite the higher rates of hypertension observed with zanubrutinib in ALPINE compared with other trials, the limited off-target effects of zanubrutinib likely contribute to the overall reduced risk of cardiovascular events observed with zanubrutinib compared with ibrutinib.16,17
The impact of BTK-inhibitor treatment on hypertension is important when selecting treatment, as hypertension is a well-established risk factor for stroke34 and has been associated with increased adverse cardiovascular events, such as arrhythmias, myocardial infarction, heart failure, and cardiovascular death.3 Additionally, hypertension increases the risk of atrial fibrillation by 50% in men and 40% in women, and atrial fibrillation in turn increases the risk of stroke, heart failure, and all-cause mortality by fivefold, threefold, and twofold, respectively.35 Identifying hypertension can be challenging in some cases as patients can experience asymptomatic hypertension, leading to delayed treatment.36 Antihypertensive therapy initiation is recommended no later than 4 weeks following a hypertension diagnosis,37 and of adults in the United States with hypertension, the use of antihypertensive medication increases with age, from 75.7% in those aged 40 to 59 years to 83.6% in those aged ≥60 years.38 Clinicians managing patients on BTK inhibitors should be aware of our findings and appropriately manage hypertension, which may develop during long-term therapy for CLL and other hematologic malignancies.
A strength of our analysis is the utilization of antihypertensive medication initiation to detect the adverse event of hypertension. The rationale for this approach is that hypertension events are typically underreported in nonhypertension studies, whereas prescription data for antihypertensive therapy are much more robust and detectable in studies in noncardiovascular populations.19-21 Additionally, in cancer patients, physiological and psychological (eg, pain and anxiety) factors may increase inaccuracies in blood pressure measurements and confound hypertension diagnoses; hence, other surrogate markers of hypertension may provide greater clinical utility and predictive power.39 Analyses using administrative claims data indicated that prescription drug records provided a high degree of validity in distinguishing patients who developed hypertension while on drugs that cause this off-target side effect.19,20
A limitation of this study is that antihypertensive medications may be used for indications other than hypertension (eg, migraine headaches, heart failure, or coronary disease). However, we prospectively excluded antihypertensive drugs that were more likely to be used for these other indications than for hypertension. An additional limitation is that detailed information on drug dosages was not available due to the post hoc nature of the analysis, which limited analysis to initiation of new antihypertensive drugs or drug classes and not changes in antihypertensive drug doses. Additionally, while zanubrutinib and ibrutinib share similar drug-drug interaction profiles,1,8,13 this study had limited pharmacokinetic sampling across sites, which precluded any drug-drug pharmacokinetic interactions between the BTK inhibitors and antihypertensive medications. However, the drug-drug interactions would be similar for zanubrutinib- and ibrutinib-treated patients and would not expect to impact the overall results of these analyses.
Conclusion
As BTK inhibitors have been associated with hypertension,2 the results of these findings from ALPINE provide additional context for understanding the rate of hypertension adverse events reported with zanubrutinib and ibrutinib. In ALPINE, initiation of a new antihypertensive therapy and a new class of antihypertensive therapy occurred at a later time point with zanubrutinib compared with ibrutinib. Changes in systolic blood pressure were generally lower with zanubrutinib vs ibrutinib and these differences may be clinically meaningful. In addition, there were fewer cardiovascular adverse events and discontinuations of therapy due to cardiac events reported with zanubrutinib vs ibrutinib in ALPINE, supporting the greater selectivity and reduced off-target effects associated with this agent compared with ibrutinib. Nevertheless, it is important to counsel all patients with CLL/SLL, with or without elevated cardiovascular risk factors, regarding the potential for increased hypertension when initiating any BTK-inhibitor therapy.
Acknowledgments
The authors thank the patients and their families, investigators, coinvestigators, and the study teams at each of the participating centers. This study was sponsored by BeOne Medicines Ltd. Medical writing was provided by Kendall Foote, and Adam Ruth, of Nucleus Global, an Inizio company, and supported by BeOne Medicines Ltd.
Authorship
Contribution: J.-W.R., N.L., L.C., D.R., and W.B.W. analyzed and interpreted the data; W.A. and J.Z. acquired, analyzed, and interpreted the data; and all authors participated in writing/reviewing the manuscript and approved the manuscript for publication.
Conflict-of-interest disclosure: J.-W.R. received research funding from Pfizer. N.L. provided consultancy for AbbVie, AstraZeneca, BeOne Medicines Ltd, Lilly/Loxo, Genentech, Janssen, and Pharmacyclics; and received research funding from AbbVie, AstraZeneca, BeOne Medicines Ltd, Lilly/Loxo, Genentech, Octapharma, Oncternal, MingSight, and TG Therapeutics. W.A. and D.R. reported employment, leadership, and stock or other ownership of BeOne Medicines Ltd. L.C. is currently employed at and is a current equity holder in the publicly traded company, BeOne Medicines Ltd. J.Z. is currently employed at BeOne Medicines Ltd. W.B.W. reported consultancy for BeOne Medicines Ltd.
Correspondence: June-Wha Rhee, Division of Cardiology, Department of Medicine, City of Hope Comprehensive Cancer Center, 1500 E Duarte Rd, Duarte, CA 91010; email: jrhee@coh.org.
References
Author notes
J.-W.R. and N.L. are joint first authors.
On request, and subject to certain criteria, conditions, and exceptions, BeOne Medicines Ltd, will provide access to individual deidentified participant data from BeOne Medicines Ltd–sponsored global interventional clinical studies conducted (1) for indications that have been approved based on the BeOne Medicines Ltd data sharing policy, or (2) in programs that have been terminated. BeOne Medicines Ltd shares data only when permitted by applicable data privacy and security laws and regulations, shares when it is feasible to do so without compromising the privacy of the study participants and other considerations. Data requests may be submitted to ClinicalTrials@BeOneMedicines.com.
The full-text version of this article contains a data supplement.