TO THE EDITOR:
Bispecific antibodies (BsAbs) have significantly enhanced the treatment landscape of relapsed/refractory (R/R) large B-cell lymphoma (LBCL). The pivotal phase 2 study of glofitamab in R/R diffuse large B-cell lymphoma reported an overall response rate of 52% (39% complete) with cytokine release syndrome (CRS) in 63% (4% grade, ≥ 3) and immune effector cell–associated neurotoxicity syndrome (ICANS) in 8% (3% grade ≥ 3).1 Dexamethasone pretreatment has been associated with a reduction in CRS/ICANS rates with BsAbs.2,3 Given the relatively predictable kinetics of BsAb-associated CRS/ICANS, we hypothesized that extended posttreatment steroid prophylaxis in LBCL during periods of highest CRS/ICANS risk would be safe and effective, along with institutional toxicity management guidelines, and could ultimately facilitate entirely outpatient monitoring and management in low-risk patients.
We prospectively identified 31 consecutive adult patients with R/R LBCL treated with glofitamab at Mass General Hospital (MGH) from August 2023 to January 2025 using an institutional review board–approved patient registry. Eligible patients received at least 2 glofitamab doses prior to 30 January 2025. This prospective observational study was powered to detect a 30% reduction in the published CRS rate1 (33% from 63%) with 95% power and 3.3% alpha (<15 events). Prophylactic dexamethasone (4 mg) was administered after glofitamab infusion and twice daily for 3 additional days after cycle 1 day 8 (C1D8), C1D15, and C2D1 glofitamab doses. Patients were admitted for 24 hours of observation after the C1D8 glofitamab dose per package labeling.4 Abbreviated glofitamab ramp-up5 was permitted if clinically indicated. Institutional BsAb guidelines were used to manage CRS/ICANS, prioritizing outpatient management and steroids for grade 1 CRS when appropriate, and reserving tocilizumab for grade ≥2 CRS. All patients were assessed for safety. Response-evaluable patients received at least 2 glofitamab doses and underwent restaging imaging. Patient and disease characteristics were summarized using descriptive statistics. CRS/ICANS and other safety outcomes were graded per the American Society for Transplantation and Cellular Therapy6 and Common Terminology Criteria for Adverse Events, version 5, respectively. Response assessments were by Lugano7 and reported as proportions with exact binomial 95% confidence intervals (CI) values. Overall survival was time from treatment initiation to death from any cause and censored for patients alive at last contact. Progression-free survival (PFS) was the time from treatment initiation to the first of progression or death from any cause and censored for patients alive and progression-free at last contact. Survival curves were estimated via Kaplan-Meier, and groups were compared using log-rank tests. Univariable Cox regressions were fit for CRS, and models were summarized with hazard ratios, 95% CI, and Wald P values. Median follow-up time was estimated via the reverse Kaplan-Meier method.
At data cutoff (30 January 2025), 31 eligible patients had received at least 2 glofitamab doses (median 5, range 2-13) (Table 1). The median age was 76 years (range, 18-87). Most patients had diffuse large B-cell lymphoma (71% [22/31]). Synchronous systemic and central nervous system (CNS) lymphoma was present in 13% (4/31) at glofitamab initiation. The median number of prior therapies was 2 (range, 2-7). Most patients had received prior chimeric antigen receptor (CAR) T-cells (81% [25/31]) at a median of 104 days (range, 36-1348). Most (81% [25/31]) patients received glofitamab using standard step-up dosing; 6 underwent an accelerated ramp-up. All patients were admitted for the recommended 24 hours of observation after the first glofitamab dose (2.5 mg per C1D8) if not already admitted. Ten patients were additionally admitted for the 10 mg per C1D15 dose (3 for glofitamab monitoring, 7 for lymphoma complications).
Patient characteristics
| Characteristics . | N = 31 . |
|---|---|
| Age, median (range), y | 76 (18-87) |
| <80, n (%) | 23 (74) |
| ≥80, n (%) | 8 (26) |
| Sex, n (%) | |
| Female | 19 (61) |
| Male | 12 (39) |
| Race, n (%) | |
| White | 23 (74) |
| Asian | 5 (16) |
| Black | 1 (3) |
| Hispanic | 1 (3) |
| Not available | 1 (3) |
| Diagnosis, n (%) | |
| DLBCL, NOS | 22 (71) |
| High-grade BCL | 5 (16) |
| Intravascular LBCL | 2 (6) |
| Primary mediastinal large BCL | 1 (3) |
| TCHR LBCL | 1 (3) |
| Transformed disease, n (%) | |
| No | 24 (77) |
| Yes | 7 (23) |
| No. of prior lines, median (range) | 2 (2-7) |
| 2, n (%) | 19 (61) |
| 3, n (%) | 8 (26) |
| 4, n (%) | 3 (10) |
| 7, n (%) | 1 (3) |
| CNS disease at treatment with glofitamab, n (%) | |
| No | 27 (87) |
| Yes | 4 (13) |
| LDH, median (range) | 281 (112-8019) |
| Elevated LDH (>210 U/L) , n (%) | |
| No | 8 (26) |
| Yes | 23 (74) |
| Stage at glofitamab initiation, n (%) | |
| 1 | 1 (3) |
| 1E | 1 (3) |
| 2 | 0 (0) |
| 3 | 4 (13) |
| 4 | 25 (81) |
| Prior CAR T-cells, n (%) | |
| No | 6 (19) |
| Yes | 25 (81) |
| Refractory to CAR T-cells | |
| No | 14/25 (45) |
| Yes | 11/25 (35) |
| Before ASCT, n (%) | |
| No | 30 (97) |
| Yes | 1 (3) |
| Characteristics . | N = 31 . |
|---|---|
| Age, median (range), y | 76 (18-87) |
| <80, n (%) | 23 (74) |
| ≥80, n (%) | 8 (26) |
| Sex, n (%) | |
| Female | 19 (61) |
| Male | 12 (39) |
| Race, n (%) | |
| White | 23 (74) |
| Asian | 5 (16) |
| Black | 1 (3) |
| Hispanic | 1 (3) |
| Not available | 1 (3) |
| Diagnosis, n (%) | |
| DLBCL, NOS | 22 (71) |
| High-grade BCL | 5 (16) |
| Intravascular LBCL | 2 (6) |
| Primary mediastinal large BCL | 1 (3) |
| TCHR LBCL | 1 (3) |
| Transformed disease, n (%) | |
| No | 24 (77) |
| Yes | 7 (23) |
| No. of prior lines, median (range) | 2 (2-7) |
| 2, n (%) | 19 (61) |
| 3, n (%) | 8 (26) |
| 4, n (%) | 3 (10) |
| 7, n (%) | 1 (3) |
| CNS disease at treatment with glofitamab, n (%) | |
| No | 27 (87) |
| Yes | 4 (13) |
| LDH, median (range) | 281 (112-8019) |
| Elevated LDH (>210 U/L) , n (%) | |
| No | 8 (26) |
| Yes | 23 (74) |
| Stage at glofitamab initiation, n (%) | |
| 1 | 1 (3) |
| 1E | 1 (3) |
| 2 | 0 (0) |
| 3 | 4 (13) |
| 4 | 25 (81) |
| Prior CAR T-cells, n (%) | |
| No | 6 (19) |
| Yes | 25 (81) |
| Refractory to CAR T-cells | |
| No | 14/25 (45) |
| Yes | 11/25 (35) |
| Before ASCT, n (%) | |
| No | 30 (97) |
| Yes | 1 (3) |
ASCT, autologous stem cell transplant; DLBCL, diffuse large B-cell lymphoma; NOS, not otherwise specified; TCHR LBCL, T-cell histiocyte-rich LBCL.
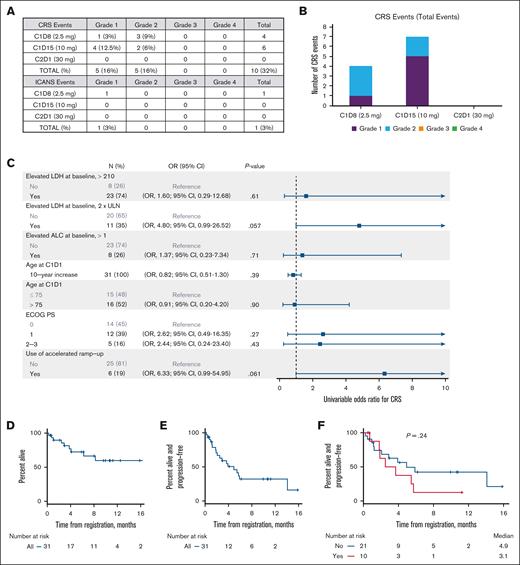
CRS occurred in 32% of patients (11 total events in 10 patients; 5 grade 1 events and 6 grade 2 events) (Figure 1A-B). All CRS occurred in cycle 1, with 4 events with C1D8 and 7 events with C1D15. Ten of the 11 CRS events occurred in patients who were already admitted either for monitoring or lymphoma complications. Four of the 6 accelerated ramp-up patients experienced CRS: 1 grade 2 event with C1D8, 1 grade 1 event with C1D15, and 2 grade 2 events with C1D15. The median time to onset of CRS was 19.0 hours (C1D8, 15.1 hours [range, 8.3-21.0]; C1D15, 28.25 hours [range, 2.7-146.0]). The median time to resolution of CRS was 7.3 hours (C1D8, 10.6 hours [range, 5.8-18.0]; C1D15, 3.3 hours [range, 1.0-36.5]). Grade 1 ICANS occurred in 1 patient (3%) 103.6 hours after the C1D8 glofitamab dose and lasted 5 hours (Figure 1A). Fifty percent (5/10) of patients with CRS or ICANS received at least 1 additional dexamethasone dose for CRS (n = 4) or ICANS (n = 1) management. Five patients (16% [5/31]) received 1 dose of tocilizumab (4 for grade 2 CRS, 1 for grade 1 CRS); 1 of these patients received both tocilizumab and siltuximab for prolonged grade 2 CRS. All CRS and ICANS were resolved fully and did not result in treatment discontinuation.
CRS/ICANS incidence, associations, and clinical outcomes. (A) Table of CRS and ICANS incidence by maximum grade and glofitamab dose. (B) Bar graph of CRS events by glofitamab dose. (C) Forest plot summarizing univariable Cox regressions for CRS (any grade) by baseline pretreatment characteristics. (D-F) Kaplan-Meier curves for PFS (D), overall survival (E), and PFS by CRS incidence (F). ALC, absolute lymphocyte count; C1D1, cycle 1 day 1; ECOG PS, Eastern Cooperative Oncology Group Performance Status; OR, odds ratio; ULN, upper limit of normal.
CRS/ICANS incidence, associations, and clinical outcomes. (A) Table of CRS and ICANS incidence by maximum grade and glofitamab dose. (B) Bar graph of CRS events by glofitamab dose. (C) Forest plot summarizing univariable Cox regressions for CRS (any grade) by baseline pretreatment characteristics. (D-F) Kaplan-Meier curves for PFS (D), overall survival (E), and PFS by CRS incidence (F). ALC, absolute lymphocyte count; C1D1, cycle 1 day 1; ECOG PS, Eastern Cooperative Oncology Group Performance Status; OR, odds ratio; ULN, upper limit of normal.
Baseline lactate dehydrogenase (LDH), lymphocyte count, performance status, age, prior CAR T-cells, or accelerated ramp-up did not predict for CRS (Figure 1C).
In response-evaluable patients (n = 25), the best overall response rate was 68% (95% CI, 46-85), and 28% were complete (95% CI, 12-49). With a median follow-up of 8.0 months, the median PFS was 4.9 months (range, 0.2-15.8), and the median overall survival was not reached (range, 0.2-17.2 months) (Figure 1D-F). Eighteen patients had PFS events, and 9 patients have died. The occurrence of CRS did not impact PFS (P = .24; Figure 1F).
In the 4 patients with synchronous CNS/systemic LBCL, 1 had a partial response, 1 had progressive disease, 1 died before restaging, and 1 has not yet been restaged. No concurrent patients with CNS lymphoma experienced ICANS.
All patients received pneumocystis and zoster prophylaxis, and there were no breakthrough infections. Approximately half (17/31 [55%]) of patients received allopurinol for tumor lysis syndrome prophylaxis. There were 2 tumor lysis syndrome events (both laboratory only and occurring at 2.5 mg of glofitamab) in patients receiving prophylactic allopurinol; no rasburicase was needed. Infections occurred in 12 of 31 patients (39%) at any time during glofitamab treatment (grade 2 [n =3], grade 3 [n =3], and grade 4 [n =3]). Three infections were COVID-19 and necessitated hospitalization. There were no deaths primarily attributed to infection.
This study met the primary end point of a reduction in CRS incidence by 30% and demonstrated relatively low rates of CRS and ICANS with a posttreatment prophylactic dexamethasone approach in a real-world R/R LBCL population. All CRS was low grade (grade 1 or 2) and fully resolved, and only 1 patient experienced recurrent CRS. ICANS was rare and was not enriched in patients with CNS lymphoma. Response rates were consistent with the pivotal trial.1 With anti-infective prophylaxis during steroid use, there were no pneumocystis or shingles infections. There were no infectious deaths; however, longer follow-up is needed to fully classify infection risk with this prophylactic steroid approach. There was no clear baseline predictor of CRS, although there was a trend toward increased CRS incidence with higher baseline LDH and accelerated ramp-up, findings which may become significant with additional patients. It is not surprising that tumor bulk (by surrogate of LDH) and use of accelerated ramp-up (typically clinically indicated for patients with rapidly progressive disease) may ultimately be associated with higher CRS risk. These data support the ongoing use of a prophylactic pretreatment dexamethasone strategy1 with incorporation of a prophylactic posttreatment dexamethasone strategy to further mitigate glofitamab CRS/ICANS risk. Given the relatively small number of patients in our study, these data require prospective validation, particularly in CAR T-refractory patients, to help further elucidate the impact of prophylactic dexamethasone on glofitamab safety and efficacy. Ultimately, these data will also be prospectively validated in low-risk patients appropriate for an entirely outpatient glofitamab administration with prophylactic dexamethasone support.
Acknowledgments: The authors thank the patients and their families who participated in this study, and the many caregivers across their institution who tirelessly provide excellent patient care.
J.E.H. is supported by the Lymphoma Research Foundation and the American Cancer Society. This work was made possible through the Mass General Cancer Center Lymphoma Translational Research and Biobanking Collaborative, supported by the Scott Nathan and Laura DeBonis Fund for Clinical Research (J.S.A.).
Contribution: J.E.H. and M.M.L. designed the study, identified eligible patients, provided clinical care, conducted the research, analyzed the data, and wrote the manuscript; R.A.R. performed statistical analyses; J.S.A. provided clinical care and supervised study design, data analysis, and manuscript preparation; J.D.S. provided clinical care and supervised the clinical database; J.A.B., E.P.D., E.P.H., P.C.J., R.W.T., J.I.W., E.C.W., T.L.H., C.D.L., P.L., J.M.L., E.N.P., J.E.M., B.M.M., K.A.T., A.O.S., U.Y.L., M.N.S., and L.B.W. provided clinical care; and C.C., J.F., and R.N. helped identify eligible patients and managed the clinical database.
Conflict-of-interest disclosure: J.E.H. received consulting fees, honoraria, and research support for investigator-initiated trials paid to the institution from Genmab. M.M.L. received consulting fees from Eli Lilly, C4XD, Sanofi, AstraZeneca, Genentech, Genmab, and MJH Life Sciences. P.C.J. received consulting fees from Bristol Myers Squibb (BMS), AbbVie, AstraZeneca, ADC Therapeutics, Incyte, Seagen, Novartis Research-Incyte, Novartis, Medically Home, and Leuko. M.N.S. received researching funding from Secura Bio and Daiichi Sankyo; and consulting fees from The Dedham Group. J.I.W. received consulting fees from AbbVie and BMS. J.D.S. received consulting fees from AstraZeneca, BeiGene, BMS, Genentech/Roche, and Loxo@Lilly; and research support for investigator-initiated trials paid to the institution from Adaptive Biotechnologies, BeiGene, BostonGene, Genentech/Roche, GlaxoSmithKline, Moderna, Takeda, and TG Therapeutics. J.S.A. received consulting fees from Genentech and Roche. E.P.H. has equity in Leuko. The remaining authors declare no competing financial interests.
Correspondence: J. Erika Haydu, Mass General Cancer Center, Yawkey 9A, 55 Fruit St, Boston, MA 02114; email: julie_haydu@mgh.harvard.edu.
References
Author notes
J.E.H. and M.M.L. contributed equally to this study.
Original data are available on request from the corresponding author, J. Erika Haydu (julie_haydu@mgh.harvard.edu).