Key Points
At avapritinib trial enrollment, AdvSM disease type, previous therapy, and mutational profile affect features of clinical presentation.
mIWG organ damage is associated with BM mast cell burden, KIT D816V variant allele fraction, and the number of S/A/R mutated genes.
Visual Abstract
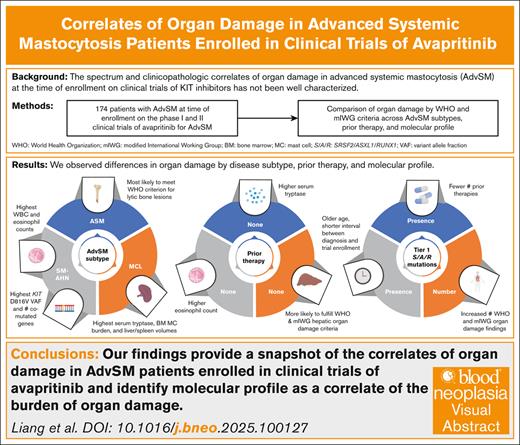
Organ damage in patients with advanced systemic mastocytosis (AdvSM) at the time of enrollment in clinical trials of KIT inhibitors has not been well characterized. We describe the spectrum and clinicopathologic correlates of organ damage as defined by World Health Organization (WHO) and modified International Working Group–Myeloproliferative Neoplasms Research and Treatment and European Competence Network on Mastocytosis (mIWG) criteria among patients with AdvSM enrolled in the phase 1 and 2 trials of the KIT D816V inhibitor avapritinib (n = 174; systemic mastocytosis [SM] with an associated hematologic neoplasm [SM-AHN], 68%; aggressive SM and mast cell leukemia [MCL], both 16%). Notably, 47% of patients received previous midostaurin. Patients with SM-AHN had the highest absolute monocyte and eosinophil counts, whereas those with MCL had the highest serum tryptase level, bone marrow (BM) mast cell burden, and spleen/liver volumes. Treatment-naïve patients were more likely to fulfill WHO and mIWG criteria for hepatic organ damage. The presence of tier 1 SRSF2, ASXL1, and/or RUNX1 mutations was associated with older age, shorter interval between diagnosis and enrollment, and fewer number of previous therapies, including midostaurin. AdvSM subtype, the presence and number of additional comutated genes beyond KIT D816V, BM mast cell burden, and KIT D816V variant allele fraction were associated with the presence and/or number of WHO/mIWG organ damage findings. Our study provides a snapshot of the correlates of organ damage in patients enrolled in clinical trials of avapritinib and identifies a key association between molecular profile and burden of organ damage.
Introduction
Systemic mastocytosis (SM) is characterized by the accumulation of neoplastic mast cells in ≥1 organs, including the bone marrow (BM), spleen, liver, and gastrointestinal tract.1 Advanced SM (AdvSM) comprises 3 subtypes: aggressive SM (ASM), SM with an associated hematologic neoplasm (SM-AHN), and mast cell leukemia (MCL). Although SM is driven by the KIT D816V mutation in most cases, KIT D816V-negative cases can occur, especially in MCL, where a lower proportion of patients (70%-80%) carry the canonical mutation.1,2 Historically, advanced forms of SM have carried a poor prognosis with survival ranging from <6 months to 4 years; however, outcomes are improving with the increasing use of KIT inhibitors such as avapritinib compared with conventional therapies.3,4 Multiagent acute myeloid leukemia-type induction chemotherapy and allogeneic stem cell transplantation are sometimes used for refractory cases involving the SM and/or AHN component.5,6
Individuals with AdvSM often exhibit hematologic and/or nonhematologic mast cell–related organ damage. Mast cell–related organ damage in AdvSM has been defined by World Health Organization (WHO) “C-findings,” which include cytopenias; palpable hepatomegaly with liver dysfunction, ascites, and/or portal hypertension; palpable splenomegaly with hypersplenism; malabsorption with hypoalbuminemia and/or weight loss; and large (>2 cm) osteolytic lesions with or without pathologic fractures.7 The definitions of organ damage were subsequently modified to reflect more clinically relevant and quantitative thresholds for enrollment of patients with AdvSM in clinical trials, especially with the emergence of KIT inhibitors. For example, the International Working Group-Myeloproliferative Neoplasms Research and Treatment and European Competence Network on Mastocytosis (IWG) specifies hematologic and nonhematologic eligible organ damage for anemia and thrombocytopenia, red blood cell and platelet transfusion dependence, ascites and/or pleural effusions requiring diuretics or paracenteses/thoracenteses, liver function test abnormalities, and symptomatic splenomegaly (>5 cm below the costal margin; supplemental Table 1).8 When published in 2013, the IWG criteria required that quantifiable organ damage reflect at least grade 2 organ dysfunction per the National Cancer Institute Common Toxicity Criteria. Modified IWG (mIWG) criteria were subsequently generated, which include a revision of the splenomegaly criterion (from >5 cm to ≥5 cm below the costal margin and irrespective of the presence of symptoms), and additional minor changes in definitions of organ damage response (supplemental Table 1).9
Certain types of organ damage, such as anemia, thrombocytopenia, and hypoalbuminemia, have been associated with worse survival in AdvSM and already constitute WHO C-findings.10 Other variables, such as older age, higher tryptase level, increased alkaline phosphatase, and presence of ≥1 mutation in SRSF2, ASXL1, and/or RUNX1 (S/A/R), also predict worse survival in AdvSM and have been incorporated into prognostic scoring systems such as the international prognostic scoring system for mastocytosis, mutation-adjusted risk score, global prognostic score for SM, and Mayo alliance prognostic system.11-14
Clinicopathologic and molecular features likely influence the phenotypic presentation of organ damage in patients with AdvSM.15-17 Although the KIT D816V mutation is found in most patients with SM, pathogenic mutations in other myeloid genes, such as TET2, CBL, RAS, JAK2, SRSF2, ASXL1, and RUNX1, are enriched in patients with SM-AHN.18 Mutations in the S/A/R genes have been associated with worse overall survival12-14,19,20; however, it is unclear whether and how the presence of additional mutations beyond KIT D816V is associated with differences in the presentation of organ damage, particularly at the time of screening of patients for clinical trials of selective KIT inhibitors such as avapritinib.
Given the increasing use of midostaurin and avapritinib for AdvSM, defining the clinicopathologic correlates of organ damage can improve our understanding of the phenotypic spectrum of patients with AdvSM enrolled in trials of KIT inhibitors. In this retrospective analysis, we describe the distribution and frequency of organ damage as defined by WHO and mIWG criteria among patients with AdvSM who underwent screening for the phase 1 and 2 registrational trials of the highly selective KIT D816V inhibitor avapritinib that led to the drug’s approval by the US Food and Drug Administration for patients with AdvSM in 2021. In turn, the presence and extent of organ damage were correlated with mast cell burden, previous therapy, and molecular profile. This analysis does not describe the outcomes of the phase 1 and 2 studies of avapritinib, nor baseline correlates of response, which have been previously reported.21,22
Methods
Study design
Patients with AdvSM from the phase 1 EXPLORER (ClinicalTrials.gov identifier: NCT02561988)21 and phase 2 PATHFINDER (ClinicalTrials.gov identifier: NCT03580655)22 clinical trials of avapritinib (Blueprint Medicines Corporation, Cambridge, MA) in AdvSM were included in this retrospective study, which was approved by the Stanford University Institutional Review Board. Patients were enrolled in the EXPLORER and PATHFINDER trials after an informed consent was obtained.
Data collection
Blueprint Medicines provided deidentified clinical, laboratory, histopathology, and molecular data from the screening period from patients enrolled at all sites for EXPLORER and PATHFINDER trials. Organ damage was strictly defined by both WHO (C-findings) and mIWG criteria (supplemental Table 1). All patients underwent next-generation sequencing from whole blood for myeloid gene mutations using the 54-gene TruSight Myeloid Panel (Illumina, San Diego, CA) at ICON/MolecularMD (Dublin, Ireland). KIT D816V variant allele frequency (VAF) was detected from whole blood by digital droplet polymerase chain reaction performed at ICON/MolecularMD.
Statistical analyses
For descriptive reporting, continuous variables were summarized by median and quartiles or range; categorical variables were described by counts and relative frequencies or percentages. In univariate analyses, continuous and categorical variables were compared across AdvSM subtypes using the Wilcoxon rank-sum test and Fisher exact test, respectively. Organ damage was compared by previous cytoreductive therapy and mutational profile using logistic regression. Given the limited sample size, risk of overfitting, and the exploratory nature of this study, multivariate analyses were not performed for primary analyses. We performed multivariate analyses using logistic regression with various sets of covariates solely for the purpose of sensitivity analyses. All analyses were performed using R version 4.2.2 (R Core Team, Vienna, Austria).
Results
Patient demographics
The full cohort consisted of 174 patients with AdvSM; 69 (40%) were treated in EXPLORER (enrolled from March 2016 to March 2020) and 105 (60%) in PATHFINDER (enrolled from November 2018 to January 2021). Notably, 27 (16%), 119 (68%), and 28 (16%) had ASM, SM-AHN, and MCL, respectively, which were adjudicated by central pathology at ARUP Laboratories (Salt Lake City, UT) (Table 1). The median age was 68 years (range, 31-88), and 102 (59%) were male. The median time between initial diagnosis and clinical trial screening was 11 months (range, 0-638). In the 110 previously treated patients (63%), the median number of previous cytoreductive therapies was 1 (range, 0-6), with 81 patients (47%) having received midostaurin. The most common last therapies before screening were midostaurin (n = 64 [58%]), cladribine (n = 12 [11%]), and interferon (n = 8 [7%]). The median time between the last therapy and trial screening was 1.41 months (range, 0-242). The median numbers of WHO and mIWG organ damage findings were both 1 (WHO, range, 0-5; mIWG, range, 0-6).
Demographics of the study cohort
| Characteristic . | N = 174 . |
|---|---|
| Diagnosis, n (%) | |
| ASM | 27 (16) |
| SM-AHN | 119 (68) |
| MCL | 28 (16) |
| Age | |
| Median (IQR) | 68 (61-73) |
| Range | 31-88 |
| Sex, n (%) | |
| Male | 102 (59) |
| Female | 72 (41) |
| Race, n (%) | |
| White | 150 (86) |
| Black or African American | 1 (1) |
| Asian | 3 (2) |
| Other/unknown | 20 (11) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 2 (1) |
| Not Hispanic or Latino | 156 (90) |
| Unknown | 16 (9) |
| Months between initial diagnosis and clinical trial screening | |
| Median (IQR) | 11 (4-30) |
| Range | 0-638 |
| Unknown | 2 |
| Months between last therapy and clinical trial screening | |
| Median (IQR) | 1.41 (0.82-3.78) |
| Range | 0-242 |
| No. of previous therapies | |
| Median (IQR) | 1 (0-2) |
| Range | 0-6 |
| Previous treatment with midostaurin, n (%) | 81 (47) |
| Serum tryptase, ng/mL | |
| Median (IQR) | 210 (125-432) |
| Range | 12-1600 |
| BM mast cell burden, % | |
| Median (IQR) | 40 (20-70) |
| Range | 1-95 |
| Unknown | 6 |
| No. of WHO organ damage findings | |
| Median (IQR) | 1 (1-2) |
| Range | 0-5 |
| No. of mIWG organ damage findings | |
| Median (IQR) | 1 (0-3) |
| Range | 0-6 |
| Characteristic . | N = 174 . |
|---|---|
| Diagnosis, n (%) | |
| ASM | 27 (16) |
| SM-AHN | 119 (68) |
| MCL | 28 (16) |
| Age | |
| Median (IQR) | 68 (61-73) |
| Range | 31-88 |
| Sex, n (%) | |
| Male | 102 (59) |
| Female | 72 (41) |
| Race, n (%) | |
| White | 150 (86) |
| Black or African American | 1 (1) |
| Asian | 3 (2) |
| Other/unknown | 20 (11) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 2 (1) |
| Not Hispanic or Latino | 156 (90) |
| Unknown | 16 (9) |
| Months between initial diagnosis and clinical trial screening | |
| Median (IQR) | 11 (4-30) |
| Range | 0-638 |
| Unknown | 2 |
| Months between last therapy and clinical trial screening | |
| Median (IQR) | 1.41 (0.82-3.78) |
| Range | 0-242 |
| No. of previous therapies | |
| Median (IQR) | 1 (0-2) |
| Range | 0-6 |
| Previous treatment with midostaurin, n (%) | 81 (47) |
| Serum tryptase, ng/mL | |
| Median (IQR) | 210 (125-432) |
| Range | 12-1600 |
| BM mast cell burden, % | |
| Median (IQR) | 40 (20-70) |
| Range | 1-95 |
| Unknown | 6 |
| No. of WHO organ damage findings | |
| Median (IQR) | 1 (1-2) |
| Range | 0-5 |
| No. of mIWG organ damage findings | |
| Median (IQR) | 1 (0-3) |
| Range | 0-6 |
IQR, interquartile range.
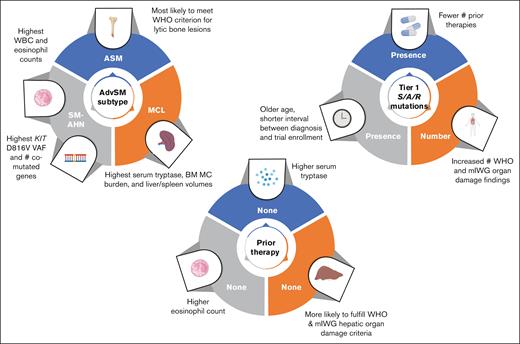
Differences in clinicopathologic features by AdvSM subtype
Laboratory and imaging findings among AdvSM subtypes are presented in Table 2 and summarized in Figure 1. The highest absolute neutrophil, monocyte, and eosinophil counts were observed in patients with SM-AHN, whereas the highest serum tryptase, BM mast cell burden, and spleen and liver volumes were found in patients with MCL. The proportion of patients fulfilling WHO and mIWG organ damage criteria among the entire cohort and by AdvSM subtype is presented in supplemental Table 2. In the entire cohort, the most common WHO C-findings were anemia (n = 65 [37%]), thrombocytopenia (n = 64 [37%]), and palpable splenomegaly with hypersplenism (n = 43 [25%]); the most common mIWG organ damage findings were anemia (n = 59 [34%]), palpable splenomegaly ≥5 cm (n = 59 [34%]), and liver function abnormalities (n = 43 [25%]).
Major laboratory and imaging findings among the AdvSM subtypes
| Laboratory or imaging finding . | ASM . | SM-AHN . | MCL . | P value . |
|---|---|---|---|---|
| ANC (× 109/L), median (range) | 3.57 (1.15-6.67) | 6.08 (0.32-97.8), n = 1 unknown | 3.73 (0.60-14.6) | .002 |
| AEC (× 109/L), median (range) | 0.10 (0-1.36) | 0.20 (0-24.2) | 0.06 (0-6.7), n = 1 unknown | .01 |
| AMC (× 109/L), median (range) | 0.36 (0.06-0.97) | 1.21 (0-8.44), n = 1 unknown | 0.35 (0.03-1.50) | <.001 |
| Serum tryptase, median (range), ng/mL | 227 (19.9-1410) | 172 (12.4-1600) | 378 (31-1600) | <.001 |
| BM mast cell burden, median (range), % | 40 (10-95), n = 1 unknown | 30 (1-90), n = 5 unknown | 80 (20-95) | <.001 |
| Liver volume by imaging, median (range), cm3 | 2100 (1020-3860) | 2350 (1010-3710) | 2860 (1370-4020) | .014 |
| Spleen volume by imaging, median (range), cm3 | 600 (40-2900), n = 1 unknown | 920 (150-2800), n = 3 unknown | 1170 (260-2270), n = 1 unknown | .027 |
| KIT D816V VAF, median (range), % | 3.88 (0-39.9) | 19.6 (0-80.1), n = 1 unknown | 15.8 (0-42.1), n = 3 unknown | .003 |
| Laboratory or imaging finding . | ASM . | SM-AHN . | MCL . | P value . |
|---|---|---|---|---|
| ANC (× 109/L), median (range) | 3.57 (1.15-6.67) | 6.08 (0.32-97.8), n = 1 unknown | 3.73 (0.60-14.6) | .002 |
| AEC (× 109/L), median (range) | 0.10 (0-1.36) | 0.20 (0-24.2) | 0.06 (0-6.7), n = 1 unknown | .01 |
| AMC (× 109/L), median (range) | 0.36 (0.06-0.97) | 1.21 (0-8.44), n = 1 unknown | 0.35 (0.03-1.50) | <.001 |
| Serum tryptase, median (range), ng/mL | 227 (19.9-1410) | 172 (12.4-1600) | 378 (31-1600) | <.001 |
| BM mast cell burden, median (range), % | 40 (10-95), n = 1 unknown | 30 (1-90), n = 5 unknown | 80 (20-95) | <.001 |
| Liver volume by imaging, median (range), cm3 | 2100 (1020-3860) | 2350 (1010-3710) | 2860 (1370-4020) | .014 |
| Spleen volume by imaging, median (range), cm3 | 600 (40-2900), n = 1 unknown | 920 (150-2800), n = 3 unknown | 1170 (260-2270), n = 1 unknown | .027 |
| KIT D816V VAF, median (range), % | 3.88 (0-39.9) | 19.6 (0-80.1), n = 1 unknown | 15.8 (0-42.1), n = 3 unknown | .003 |
AEC, absolute eosinophil count; AMC, absolute monocyte count; ANC, absolute neutrophil count.
Differences in clinicopathologic features by disease subtype, previous therapy, and molecular profile. MC, mast cell; WBC, white blood cell; #, number of.
Differences in clinicopathologic features by disease subtype, previous therapy, and molecular profile. MC, mast cell; WBC, white blood cell; #, number of.
When evaluating for statistically significant differences in the percentage of patients with a particular AdvSM subtype demonstrating organ damage, patients with SM-AHN were most likely to meet the WHO thrombocytopenia criterion (44%; P = .023) and the mIWG criterion for ascites and pleural effusions (24%; P = .021), whereas patients with ASM were most likely to meet the WHO criterion for large (>2 cm) lytic bone lesions (30%; P < .001). Patients with SM-AHN had the highest KIT D816V VAF (19.6% [range, 0-81]; P = .003).
Differences in clinicopathologic features by previous cytoreductive therapy
Differences in clinicopathologic features by previous cytoreductive therapy are summarized in Figure 1. Compared with patients without previous therapy, those who received ≥1 previous therapy had a significantly longer median time between initial diagnosis and clinical trial screening (20 vs 3 months; P < .001). In addition, they exhibited significantly higher tryptase levels at screening: 270 ng/mL (range, 19.9-1600) vs 166 ng/mL (range, 12.4-1340) (P = .029). Conversely, patients without previous therapy had a significantly higher absolute eosinophil count (0.32 × 109/L [range, 0-24.2] vs 0.10 × 109/L [range, 0-15.5]; P = .007) and alkaline phosphatase (232 U/L [range, 67.7-1120] vs 141 [range, 23-1260]; P = .002). Patients who had not received previous therapy were more likely to fulfill the WHO criterion for palpable hepatomegaly with elevated liver enzymes, ascites, and/or portal hypertension (31% vs 16%; P = .035) and, similarly, the mIWG criterion for liver function test abnormalities (36% vs 18%; P = .011).
Compared with patients who never received midostaurin, those who previously received midostaurin exhibited a lower median absolute eosinophil count (0.10 × 109/L [range, 0-15.5] vs 0.20 × 109/L [range, 0-24.2]; P = .015) and lower median serum albumin level (3.80 g/dL [range, 1.2-5.0] vs 4.1 [range, 2.3-5.0]; P = .008), although both fall within normal range. Interestingly, we did not observe an association between previous receipt of midostaurin and absolute monocyte count (received midostaurin, median, 0.810 × 109/L [range, 0.03-5.90]; no previous midostaurin, median, 0.90 × 109/L [range, 0-8.44]; P = .32). Patients who never received midostaurin were also more likely to fulfill the WHO criterion for palpable hepatomegaly with elevated liver enzymes, ascites, and/or portal hypertension (28% vs 15%; P = .043).
Differences in clinicopathologic features by molecular profile
Forty-six patients (26%) had a KIT D816V mutation only (ASM, n = 15, 33%; SM-AHN, n = 19, 41%; MCL, n = 12, 26%). Nine patients (5%) were KIT D816V negative (ASM, n = 3, 33%; SM-AHN, n = 4, 44%; MCL, n = 2, 22%). The remaining 119 patients (68%) had KIT D816V mutations and at least 1 additional tier 1 mutation in a non-KIT gene (ASM, n = 9, 8%; SM-AHN, n = 96, 81%; MCL, n = 14, 12%).
The most commonly mutated non-KIT genes (tier 1 or variants of uncertain significance) were TET2 (n = 107; 61%), SRSF2 (n = 64; 37%), GATA2 (n = 36; 21%), BCOR (n = 33; 19%), CUX1 (n = 33; 19%), and ASXL1 (n = 30, 17%). When restricted to tier 1 pathogenic mutations, the most commonly mutated non-KIT genes were TET2 (n = 44; 25%), SRSF2 (n = 28; 16%), CUX1 (n = 17; 10%), DNMT3A (n = 16; 9%), ASXL1 (n = 14; 8%), and CBL (n = 13; 7%). The median KIT D816V VAF was 14.5% (range, 0-80.1).
In univariate analyses, the presence of tier 1 S/A/R mutations was associated with male sex, older age at the time of clinical trial screening, shorter time between diagnosis and clinical trial screening, absence of previous midostaurin or previous therapies, and lower number of previous therapies (supplemental Table 3; Figure 1). Patients with ASM were less likely to have tier 1 S/A/R mutations.
Clinicopathologic features that were significantly associated with the total number of comutated genes beyond KIT D816V in univariate analyses are presented in supplemental Table 4. Notably, lower KIT D816V VAF, lower BM mast cell burden, and shorter time between diagnosis and clinical trial screening were associated with a greater number of comutated genes beyond KIT D816V. In contrast, a diagnosis of ASM (reference: SM-AHN) was inversely associated with the number of comutated genes.
Factors associated with WHO and mIWG organ damage findings
We next evaluated together patient demographics, disease subtypes, previous therapy, and molecular features and their association with WHO/mIWG organ damage findings in univariate and multivariable analyses. Significant associations in univariate and multivariable analyses at P < .05 are presented in Table 3 and supplemental Table 5. Factors associated with the presence and/or number of WHO/mIWG organ damage findings in univariate analyses included AdvSM subtype, the presence and number of additional comutated genes beyond KIT D816V (including S/A/R mutations), BM mast cell burden, and KIT D816V VAF.
Univariate and multivariable analyses of clinicopathologic features associated with the number of WHO and mIWG organ damage findings
| Organ damage . | Variable . | Univariate . | Multivariable . | ||
|---|---|---|---|---|---|
| Estimate . | P value . | Estimate . | P value . | ||
| No. of WHO hematologic organ damage findings | Presence of any S/A/R mutations | 0.57 | .0014 | 0.34 | .27 |
| Number of mutated S/A/R genes | 0.29 | .0013 | 0.10 | .53 | |
| Presence of tier 1 S/A/R mutations | 0.40 | .028 | 0.017 | .94 | |
| Number of additional comutated genes | 0.070 | .016 | 0.029 | .44 | |
| No. of WHO nonhematologic organ damage findings | Previous therapy | −0.35 | .046 | −0.091 | .72 |
| Previous midostaurin | −0.37 | .041 | −0.22 | .39 | |
| Presence of any S/A/R mutations | 0.45 | .013 | 0.26 | .41 | |
| Number of mutated S/A/R genes | 0.22 | .017 | 0.077 | .64 | |
| Total number of WHO organ damage findings | Presence of any S/A/R mutations | 0.51 | <.0001 | 0.31 | .19 |
| Number of mutated S/A/R genes | 0.26 | <.0001 | 0.14 | .30 | |
| Presence of tier 1 S/A/R mutations | 0.35 | .0068 | 0.20 | .61 | |
| Number of tier 1 mutated S/A/R genes | 0.25 | .013 | −0.19 | .55 | |
| Number of additional comutated genes | 0.049 | .031 | 0.0012 | .97 | |
| No. of mIWG hematologic organ damage findings | BM mast cell burden (%) | 0.0086 | .023 | 0.012 | .003∗ |
| Presence of any S/A/R mutations | 0.49 | .016 | 0.22 | .53 | |
| Number of mutated S/A/R genes | 0.28 | .0077 | 0.27 | .17 | |
| No. of mIWG nonhematologic organ damage findings | Diagnosis of MCL† (reference: SM-AHN) | −0.50 | .042 | −0.70 | .012 |
| BM mast cell burden (%) | 0.006 | .039 | 0.013 | .00016∗ | |
| KIT D816V VAF (%) | 0.011 | .010 | 0.0093 | .071 | |
| Presence of any S/A/R mutations | 0.45 | .0041 | 0.098 | .76 | |
| Number of mutated S/A/R genes | 0.24 | .0032 | 0.20 | .30 | |
| Presence of tier 1 S/A/R mutations | 0.37 | .020 | 0.010 | .98 | |
| Number of tier 1 mutated S/A/R genes | 0.29 | .016 | 0.086 | .83 | |
| Total number of mIWG organ damage findings | Diagnosis of ASM‡ (reference: SM-AHN) | −0.41 | .046 | −0.19 | .34 |
| BM mast cell burden (%) | 0.007 | .0043 | 0.012 | <.001∗ | |
| Presence of any S/A/R mutations | 0.46 | .00033 | 0.074 | .75 | |
| Number of mutated S/A/R genes | 0.25 | .00015 | 0.27 | .052 | |
| Presence of tier 1 S/A/R mutations | 0.33 | .017 | 0.34 | .40 | |
| Number of tier 1 mutated S/A/R genes | 0.22 | .040 | −0.26 | .44 | |
| Organ damage . | Variable . | Univariate . | Multivariable . | ||
|---|---|---|---|---|---|
| Estimate . | P value . | Estimate . | P value . | ||
| No. of WHO hematologic organ damage findings | Presence of any S/A/R mutations | 0.57 | .0014 | 0.34 | .27 |
| Number of mutated S/A/R genes | 0.29 | .0013 | 0.10 | .53 | |
| Presence of tier 1 S/A/R mutations | 0.40 | .028 | 0.017 | .94 | |
| Number of additional comutated genes | 0.070 | .016 | 0.029 | .44 | |
| No. of WHO nonhematologic organ damage findings | Previous therapy | −0.35 | .046 | −0.091 | .72 |
| Previous midostaurin | −0.37 | .041 | −0.22 | .39 | |
| Presence of any S/A/R mutations | 0.45 | .013 | 0.26 | .41 | |
| Number of mutated S/A/R genes | 0.22 | .017 | 0.077 | .64 | |
| Total number of WHO organ damage findings | Presence of any S/A/R mutations | 0.51 | <.0001 | 0.31 | .19 |
| Number of mutated S/A/R genes | 0.26 | <.0001 | 0.14 | .30 | |
| Presence of tier 1 S/A/R mutations | 0.35 | .0068 | 0.20 | .61 | |
| Number of tier 1 mutated S/A/R genes | 0.25 | .013 | −0.19 | .55 | |
| Number of additional comutated genes | 0.049 | .031 | 0.0012 | .97 | |
| No. of mIWG hematologic organ damage findings | BM mast cell burden (%) | 0.0086 | .023 | 0.012 | .003∗ |
| Presence of any S/A/R mutations | 0.49 | .016 | 0.22 | .53 | |
| Number of mutated S/A/R genes | 0.28 | .0077 | 0.27 | .17 | |
| No. of mIWG nonhematologic organ damage findings | Diagnosis of MCL† (reference: SM-AHN) | −0.50 | .042 | −0.70 | .012 |
| BM mast cell burden (%) | 0.006 | .039 | 0.013 | .00016∗ | |
| KIT D816V VAF (%) | 0.011 | .010 | 0.0093 | .071 | |
| Presence of any S/A/R mutations | 0.45 | .0041 | 0.098 | .76 | |
| Number of mutated S/A/R genes | 0.24 | .0032 | 0.20 | .30 | |
| Presence of tier 1 S/A/R mutations | 0.37 | .020 | 0.010 | .98 | |
| Number of tier 1 mutated S/A/R genes | 0.29 | .016 | 0.086 | .83 | |
| Total number of mIWG organ damage findings | Diagnosis of ASM‡ (reference: SM-AHN) | −0.41 | .046 | −0.19 | .34 |
| BM mast cell burden (%) | 0.007 | .0043 | 0.012 | <.001∗ | |
| Presence of any S/A/R mutations | 0.46 | .00033 | 0.074 | .75 | |
| Number of mutated S/A/R genes | 0.25 | .00015 | 0.27 | .052 | |
| Presence of tier 1 S/A/R mutations | 0.33 | .017 | 0.34 | .40 | |
| Number of tier 1 mutated S/A/R genes | 0.22 | .040 | −0.26 | .44 | |
Including univariate associations significant at the P < .05 level. Negative estimates refer to an inverse relationship between variables. All predictor variables evaluated were AdvSM subtype, age at diagnosis, previous therapy, number of previous therapies, previous midostaurin, months between diagnosis and clinical trial screening, serum tryptase level, BM mast cell burden, KIT D816V VAF, presence of any S/A/R mutations, number of mutated S/A/R genes, presence of tier 1 S/A/R mutations, number of tier 1 mutated S/A/R genes, presence of any additional comutations beyond KIT D816V, number of additional comutations, presence of tier 1 additional comutations beyond KIT D816V, and number of additional tier 1 comutations. There were no significant associations at the univariate level for the following outcome variables: any WHO nonhematologic organ damage, any mIWG nonhematologic organ damage, and any mIWG organ damage.
Statistically significant at P < .05.
Diagnosis of ASM was also included in multivariable model fitting.
Diagnosis of MCL was also included in multivariable model fitting.
In multivariable analyses, only BM mast cell burden remained significantly associated with the number of hematologic, nonhematologic, and total mIWG organ damage findings and the presence of any hematologic mIWG organ damage; only BM mast cell burden and KIT D816V VAF remained significantly associated with the presence of any mIWG organ damage findings and any nonhematologic mIWG organ damage findings. No predictors remained independently associated with WHO organ damage findings in multivariable analyses. KIT D816V VAF (P = .071) and the number of mutated S/A/R genes (P = .052) exhibited borderline statistical significance with the number of mIWG nonhematologic organ damage findings and the number of total mIWG organ damage findings, respectively.
Discussion
Although organ damage criteria are used in enrollment and monitoring of response in clinical trials for AdvSM, the spectrum of organ damage across AdvSM subtypes at the time of clinical trial enrollment in the modern KIT inhibitor era has not been well characterized. To address this knowledge gap, we sought to describe the presenting features of organ damage during screening for the avapritinib registrational trials that have underpinned the drug’s regulatory approval. We conducted a multidimensional analysis of the clinical and histopathologic correlates associated with WHO- and mIWG-defined organ damage, including AdvSM subtype, receipt of previous therapy, and mutational profile.
The landscape of KIT inhibitor therapy has dynamically evolved since the approval of avapritinib as frontline therapy for AdvSM in 2021 (and approval by the European Medicines Agency for use after 1 systemic therapy). For example, phase 2 trial evaluation of the selective KIT D816V inhibitor bezuclastinib is ongoing.23 Subjects enrolled in the AdvSM trial of bezuclastinib are permitted to receive previous treatment with not only midostaurin but also avapritinib. Therefore, the presenting organ damage in such individuals is anticipated to be different from those patients enrolled in the registrational trials of avapritinib.
In the current analysis of patients screened and enrolled in the avapritinib registrational trials, we found that the proportion of patients fulfilling WHO and mIWG organ damage criteria differed across AdvSM subtypes, with patients with SM-AHN more likely to meet the WHO organ damage criterion for thrombocytopenia and the mIWG criterion for ascites and pleural effusions. Patients with SM-AHN pose many challenges, including (1) a higher frequency of liver-associated organ damage (as described herein), (2) an increased number of mutations besides KIT D816V, (3) an increased baseline propensity for transformation to AML, and (4) reduced overall survival compared with pure ASM or MCL treated with KIT inhibition, as demonstrated in 3-year follow-up of avapritinib monotherapy.4 Therefore, selected patients with SM-AHN may require combination approaches with both KIT D816V-directed and AHN-targeted therapy to overcome this nexus of higher-risk features, including a potential higher burden of hepatic damage, which may be caused by the SM or AHN component or a combination of the 2.
Not unexpectedly, patients with MCL exhibited the highest median serum tryptase level, BM mast cell burden, and spleen and liver volumes, indicating a higher burden of disease. Patients with SM-AHN demonstrated the highest KIT D816V VAF and number of additional non-KIT D816V-mutated genes, reflecting that SM-AHN is often a multimutated neoplasm and KIT D816V may be found in both mast cell and/or non–mast cell lineages.18,24,25 Compared with patients with SM-AHN, those with MCL exhibited a relatively lower KIT D816V VAF despite these patients having the highest BM mast cell burden. We also found that patients who had received previous therapy, including midostaurin, had less severe organ damage as evidenced by decreased frequency of WHO and mIWG criteria–defined liver damage, which may in part reflect midostaurin’s activity in AdvSM.21,26
We also explored the landscape of mutation profile by evaluating associations among all non-KIT D816V myeloid mutations, tier 1 pathogenic non-KIT D816V mutations, the presence of S/A/R mutations, and the number of non-KIT mutated genes. We found that KIT D816V VAF was inversely associated with the number of non-KIT D816V-mutated genes, which could be related to a competition between clones bearing the KIT mutation and clones without the KIT mutation. However, this is likely more complicated given that the KIT D816V mutation can be found in cells with other myeloid mutations. How multimutated clones arise and dynamically change under the pressure of KIT inhibition in individual AdvSM cases is the subject of several ongoing translational studies, including single-cell DNA and RNA sequencing analyses.27 It is currently not well understood how variations in the clonal landscape affect the phenotypic presentation of organ damage in AdvSM.
In multivariable analyses, only BM mast cell burden and KIT D816V VAF were independently associated with mIWG organ damage, thus highlighting the linkage among the clonal burden of KIT D816V, morphologic expression of mast cell burden, and overt organ damage. The association between the presence and number of S/A/R mutations with the total number of mIWG organ damage findings is consistent with previous associations attributed to S/A/R mutations, which independently predict poor prognosis in patients with AdvSM and are incorporated into current scoring systems.10,12-14,18-20 Ultimately, we did not observe an impact of previous therapy on the presence and/or number of WHO or mIWG organ damage findings in multivariable analysis despite the association of hepatic organ damage with receipt of previous therapy, including midostaurin.
A significant strength of our study is the inclusion of patients with AdvSM treated in registrational clinical trials of avapritinib, which contained very similar eligibility criteria and central committee adjudication of organ damage at screening. To the best of our knowledge, there have been no studies evaluating correlates of organ damage as defined by the mIWG criteria, which are used by the regulatory agencies for drug approval. Analysis of this screening cohort from the avapritinib registrational trials will permit future comparisons with patient cohorts screened for future KIT inhibitor registrational trials (eg, bezuclastinib) and other agents being evaluated for regulatory approval. Limitations of our study include the retrospective nature and relatively small sample size of the relatively rare AdvSM subtypes, ASM and MCL, which is reflected in the small point estimates in the multivariable sensitivity analyses.
In summary, we performed a comprehensive analysis of organ damage in patients with AdvSM at the time of screening in the phase 1 and 2 EXPLORER and PATHFINDER trials for avapritinib in AdvSM. We found that disease subtype, previous therapy, and molecular profile, in particular, were associated with the pattern and/or burden of organ damage. These clinicopathologic associations help frame the genotype-phenotype interactions underpinning the presentations of patients with AdvSM enrolling in trials in the KIT inhibitor era.
Acknowledgments
This work was supported by the American Society of Hematology Hematology Opportunities for the Next Generation of Research Scientists Award (2021; E.C.L.) and the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (5T32HL007093; E.C.L.) and partially supported by the Biostatistics Shared Resource of the NIH–funded Stanford Cancer Institute (P30CA124435).
Authorship
Contribution: E.C.L. conceived of and designed the study, analyzed and interpreted data, and wrote the manuscript; C.P. collected, analyzed, and interpreted data and edited the manuscript; R.L. analyzed and interpreted data and edited the manuscript; H.S. collected data; J.G. conceived of and designed the study, analyzed and interpreted data, reviewed and edited the manuscript, and provided critical oversight; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: H.S. and S.D. are employees and/or shareholders of Blueprint Medicines Corporation. W.S. received consultancy and institutional research funding for conduct of clinical trials from Incyte and Blueprint Medicines. J.G. received institutional research funding for conduct of clinical trials from, received honoraria from, and served as chair of the steering committee and central response review committee for Blueprint Medicines and Cogent Biosciences. The remaining authors declare no competing financial interests.
Correspondence: Jason Gotlib, Stanford Cancer Institute/Stanford University School of Medicine, 875 Blake Wilbur Dr, Stanford, CA 94305-6661; email: jason.gotlib@stanford.edu.
References
Author notes
Data are available on reasonable request from the corresponding author, Jason Gotlib (jason.gotlib@stanford.edu).
The full-text version of this article contains a data supplement.