Key Points
Two compounds targeting p38γ lipid-binding site enhance olfactory transduction with unique combinations of ORs.
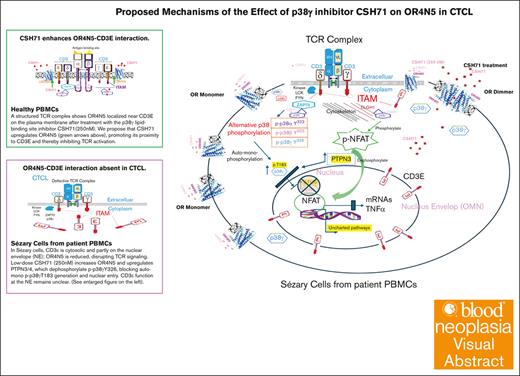
OR4N5 is identified as an exemplar tumor suppressor in CTCL, possibly modulating TCR activation through interaction with CD3E.
Visual Abstract
Our previous observations revealed significant overexpression of p38γ in cutaneous T-cell lymphoma (CTCL), small molecule inhibitors of the lipid-binding site of p38γ, CSH18 and CSH71 exhibited strong cytotoxicity against CTCL cells while sparing healthy cells. We report here that both compounds significantly enhanced the activity of the olfactory transduction pathway, and each induces a unique combination of olfactory receptors (ORs). CSH71 increased gene expression of OR4N5, an OR that functions as a tumor suppressor CTCL Hut78 cells; its suppression is associated with accelerated cell proliferation. The study elucidates the potential mechanism wherein the targeting of the p38γ lipid-binding site affects the alternative p38 phosphorylation via ζ-chain–associated protein kinase 70, thereby T-cell receptor (TCR) activation. The expression of OR4N5 and CD3E is reduced in CTCL; scattered aberrant CD3Es are in the cytosol and surrounds the nuclear envelope. Membranous OR4N5 no longer interacts with CD3E in CTCL cells, which sets TCR signaling transduction on the loose via depending on other mechanisms such as the DLGH1-p38γ–nuclear factor of activated T-cells 1 axis. Analysis of public data sets shows that most ORs are significantly downregulated in cancer, suggesting their grassroot tumor suppressors role when a network emerged as they teamed together against cancer.
Introduction
The p38 MAPK family in humans, which includes the isoforms p38α, p38β, p38γ, and p38δ, is integral to a range of cellular operations, oscillating between the promotion of cell proliferation and the induction of cell death. Specifically, p38γ has been implicated as an oncogenic promoter,1 in contrast to the tumor-suppressing activities attributed to p38α.2 Importantly, the significant overexpression of p38γ in cutaneous T-cell lymphoma (CTCL) compared with its absence in normal T cells positions it as an attractive therapeutic target.3
Our previous study identified that p38γ is a driver in CTCL and discovered a small-molecule inhibitor targeting its adenosine triphosphate (ATP)-binding site with strong therapeutic potential.3 To improve specificity, we used virtual screening on the p38γ lipid-binding site (LBS), identifying compounds CSH18 and CSH71 from the National Institutes of Health Developmental Therapeutics Program library, which exhibited potent cytotoxicity against CTCL cells while sparing healthy cells.4 β-Octyl glucoside helped reveal this LBS, and nuclear magnetic resonance (NMR) analysis confirmed that CSH71 binding induced conformational changes in key regions, including the histidine-arginine-aspartate motif, L16 helix, and residue Y326.4 Notably, Y326, phosphorylated by ζ-chain–associated protein kinase 70 (ZAP70), links the p38γ and T-cell receptor (TCR) pathways, highlighting an alternative p38 phosphorylation5-7 contrasting with the classical dual phosphorylation (Thr-x-Tyr), which is essential for TCR activation.8
We also observed p38 activation in Hut78 cells, paralleled by an increase in nuclear factor of activated T-cells 1 and tumor necrosis factor α expression in CTCL cells, as well as a significant upregulation of the olfactory transduction pathways by CSH71,4 underscoring the compelling link between olfactory receptors (ORs) in nonolfactory tissues and the implication in the abrogation of malignant T-cell growth.
The functions of ORs in nonolfactory tissues and their prospective roles in modulating cancer has been studied.9-11 In line with these findings, this study uncovered the role of p38γ in CTCL cells and elucidated its molecular dynamics that impinge on ORs within the framework of the TCR signaling pathway via 2 small molecules CSH71 and CSH18 that target p38γ’s LBS.
Materials and methods
Samples from a patient with SS
The patient with Sézary syndrome (SS) described in the text is in stage VI and exhibits skin involvement tumor stage 3 (T3), nodal involvement node stage 2 (N2, indicating positive lymph nodes), and blood involvement. All patients included in this study have a detectable population of Sézary cells, defined as CD4+CD26− cells exceeding 40%, as assessed by flow cytometry.
NMR and competition STD experiment
p38γ (inactive form, unphosphorylated) was expressed in Escherichia coli and purified as previously described.4 For competition saturation transfer difference (STD) experiment and NMR analysis, please refer to the supplemental Methods.
Microarray analysis
The Affymetrix GeneChip Human Gene 1.0-ST array (Affymetrix, Santa Clara, CA) was used to define gene expression profiles from the samples. Synthesis and labeling of complementary DNA targets, hybridization, and scanning of GeneChips were carried out by the Integrative Genomics Core Facility at the City of Hope. Briefly, complementary RNA was generated according to the manufacturer’s protocol by using Affymetrix's GeneChip Whole Transcript Sense Target Labeling Assay Hybridization cocktails containing 5.5 μg of fragmented, end-labeled complementary DNA were prepared and applied to GeneChip Human Gene 1.0 ST arrays. Hybridization was performed for 16 hours, and the arrays were washed and stained with the GeneChip Fluidics Station 450 using FS450_0007 script. Arrays were scanned at 5-μm resolution using the Affymetrix GCS 3000 7G.
Raw intensity measurements of all probe sets were background corrected, normalized, and converted into expression measurements using the Affymetrix’s Expression Console version 1.1.1. The Bioconductor “ArrayTools” package was then used to identify the genes differentially expressed between the treated and untreated samples. Significant genes were selected with a cutoff of adjusted P < .05 and fold change of 2. DAVID functional annotation tool was then used to identify the modulated Kyoto Encyclopedia of Genes and Genomes signaling pathways.
Cell transduction and viability
A total of 20 000 Hut78 cells in 500 μL media (40 000 cells per mL) were treated with 8 μg/mL polybrene and transduced with 20 μL (low titer) or 80 μL (high titer) competent replication–deficient lentiviral particles control short hairpin RNA (shRNA) containing scramble sequence (Sigma), shOR4N5, or shOR10K2 (Sigma). Virus stocks were 1:1 mixture of 2 clones (TRCN0000203629 and TRCN0000204445 for shOR4N5, and TRCN0000061221 and TRCN0000061222 for shOR10K2). After 72 hours of incubation, transduced cells were selected with 0.5 μg/mL puromycin. Cells were counted at 1, 4, 7, and 10 days after selection using an automated cell counter and trypan blue. All viral particles were purchased from Sigma (St Louis, MO), at 106 transducing units per mL.
Western blot (WB) analysis
Thirty micrograms each of protein samples of CTCL cells treated and untreated with selected drugs were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subsequently transferred onto nitrocellulose membranes. Blots were visualized using enhanced chemiluminescence solutions and subsequent X-ray film exposure.
Primary antibodies purchased from Cell Signaling Technology were used at the following dilutions: p38α (catalog no. 9218, 1:1000), p38β (catalog no. 2339, 1:1000), p38δ (catalog no. 2308, 1:1000), p38γ (catalog no. 2307, 1:1000), phosphorylated p-38 (p-p38) Thr180Tyr182 (catalog no. 4511, 1:1000), glyceraldehyde 3-phosphate dehydrogenase (catalog no. 2118, 1:5000), and anti–stress-activated protein kinase 3 (ab205926) were purchased from Abcam and used at 1:1000 dilution. P-p38 Tyr323 antibody (catalog no. 12322-1) was purchased from Signalway Antibody and used at 1 μg/mL. Antibodies targeting OR proteins were used at the following dilutions: OR2H2 (Antibodies Online, catalog no. ABIN3186062, 1:1000), OR6C68 (Antibodies Online, catalog no. ABIN3186177, 1:1000), OR2L2 (ThermoFisher, catalog no. PA5-67766, 1:1000), OR4N5 (ThermoFisher, catalog no. PA5-71184, 1:1000), OR10K2 (Abcam, catalog no. ab182488, 1 μg/mL), and OR4K17 (Abcam, catalog no. ab129962, 1:1000). Goat anti-rabbit horseradish peroxidase–linked antibody (GE, catalog no. NA934, 1:2500) was used as secondary antibody.
Kinase assay with p38 isoforms in vitro
Described as previously reported.3 Briefly, to characterize the selected small molecules that inhibited p38γ (CSH18 and CSH71), we performed kinase assays in vitro using an adenosine diphosphate (ADP)-Glo kit (Promega), as described previously. Briefly, the active kinase forms of p38 isoforms (α, β, γ, and δ) were obtained from SignalChem, and incubated with increasing doses of CSH18 and CSH71. A synthetic peptide substrate (IPTTPITTTYFFFKKK) was added to the mixture at a final concentration of 0.2 μg/μL, followed by an addition of 10 μM ATP. The luminescent ADP-Glo kinase assay measures ADP produced from ATP consumption in the reaction. Staurosporine was used as a positive control. Luminescence was monitored at an integration time of 0.5 seconds using an automated BMG PHERAstar plate reader (BMG Labtech). Each experiment was performed in triplicate.
Confocal immunofluorescence microscopy
Described as previously reported.4 Briefly, with antibodies information: primary antibodies purchased from Cell Signaling Technology were used at the following dilutions: p38γ (catalog no. 2307, 1:100), and p-p38 Thr180Tyr182 (catalog no. 4511, 1:100). Anti–p38γ (H6300-M04) was purchased from Abnova, Anti–p38γ (ab205926) was purchased from Abcam and used at 1:100 dilution. p-p38 Tyr323 (PA5-40258) and CD3z (PA5-98304) antibodies were purchased from ThermoFisher and used at 10 μg/mL. Antibodies targeting OR proteins were used at the following dilutions OR4N5 (ThermoFisher, catalog no. PA5-71184, 1:100) and OR10K2 (Aviva Systems Biology, catalog no. ARP71282_P050, 1:100). Goat anti-mouse Alexa 594 (ThermoFisher, catalog no. A32744) and goat anti-rabbit Alexa 647 (ThermoFisher, catalog no. A32795) were used as secondary antibodies at 1:200 dilutions. In addition, fluorescein isothiocyanate–conjugated CD3 (BD Biosciences, catalog no. 349201) and CD20 (ThermoFisher, catalog no. 11-0209-42) antibodies were used at 1:20 dilutions.
Statistical analysis
All experimental data are presented as mean ± standard error of the mean, unless otherwise indicated. Statistical significance of differences, such as in-cell viability assays and messenger RNA (mRNA) expression of target genes, was assessed using the Student t test (SPSS; IBM, Armonk, NY) or 1-way analysis of variance (GraphPad PRISM, version 3.0, GraphPad). Differences were considered significant at P < .05.
Results
Impact of CSH71 and CSH18 on olfactory transduction pathways in Hut78 cells
Previously, we showed that CSH71 (250 nM) strongly upregulates the olfactory transduction pathway in Hut78 cells (CD4+, SS; American Type Culture Collection) via RNA microarray.4 Here, we confirm this pathway as the top upregulated target with CSH18 (2 μM) treatment (Table 1).
Top upregulated pathway of CSH71 and CSH18 by RNA microarray and DE analysis
| Pathway . | Source . | P value . | q value . | Overlap . |
|---|---|---|---|---|
| CSH71 Olfactory transduction, Homo sapiens (human) | KEGG | 8.47E−10 | 2.05E−07 | OR5B21; OR52N1; OR52J3; OR56A3; OR4N5; OR1S1; OR11H1; OR2L2; OR10K2; OR6N1; OR8K3; OR2B3; OR6Y1; OR5W2; OR2L8; OR5A1 |
| CSH18 Olfactory transduction, Homo sapiens (human) | KEGG | 8.97E−11 | 1.61E−08 | OR51F1; OR6C70; OR51D1; OR4C15; OR1F1; OR2L2; OR2H2; OR5D14; OR52N4; OR4Q3; OR8B12; OR8K5; OR2K2; OR52A1; OR6C68; OR2M5; OR6C2; OR6C6; OR51I1; OR2A7; OR10K2; OR5R1; OR56B1; OR4K14; OR4K17 |
| Pathway . | Source . | P value . | q value . | Overlap . |
|---|---|---|---|---|
| CSH71 Olfactory transduction, Homo sapiens (human) | KEGG | 8.47E−10 | 2.05E−07 | OR5B21; OR52N1; OR52J3; OR56A3; OR4N5; OR1S1; OR11H1; OR2L2; OR10K2; OR6N1; OR8K3; OR2B3; OR6Y1; OR5W2; OR2L8; OR5A1 |
| CSH18 Olfactory transduction, Homo sapiens (human) | KEGG | 8.97E−11 | 1.61E−08 | OR51F1; OR6C70; OR51D1; OR4C15; OR1F1; OR2L2; OR2H2; OR5D14; OR52N4; OR4Q3; OR8B12; OR8K5; OR2K2; OR52A1; OR6C68; OR2M5; OR6C2; OR6C6; OR51I1; OR2A7; OR10K2; OR5R1; OR56B1; OR4K14; OR4K17 |
Olfactory transduction pathway as the top upregulated pathways in Hut78 cells treated with CSH71 and CSH18, as identified by RNA microarray and DE analysis. The treatment with CSH71 (250 nM) and CSH18 (2 μM) was for 48 hours. Both compounds upregulated the olfactory transduction pathway, with each inducing a unique combination of ORs.
DE, differential expression; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Additionally, distinct patterns of ORs upregulation were observed between the 2 treatments. Although there was a convergence on the activation of 2 common ORs, OR10K2 and OR2L2, each compound predominantly affected different combination of ORs (as listed in Table 1) after CSH71 or CSH18 treatment.
CSH71 binds both ATP and LBSs of p38γ.12,13 Similarly, CSH18 preferentially targets the lipid-binding domain, supported by Glide docking scores (Figure 1A-B) and in vitro binding with >1 site by 1-dimensional NMR and a competition STD experiment (Figure 1C; supplemental Figure 1A; Table 2).
Compound CSH18 binds to p38γ. (A) CSH18 is predicted to bind to the LBS of p38γ, with a Glide XP docking score of −10.9 kcal/mol. (B) Chemical structure of compound CSH18. (C) Mass spectrometry data: 1-dimensional spectra of the 1H NMR aromatic region of compound CSH18 at 25°C; (i) molar ratio between CSH18 and p38γ is 50:1 and (ii) free CSH18 with a concentration of 50 μM. (D) Substitution in CSH18: CSH18 has a Br group, which might act as an alkylator, potentially causing nonspecific binding. To reduce this risk, we replaced Br with CN in CSH18 to create CSH18CN. This substitution maintains a similar conformation in the lipid-binding domain of p38γ without functioning as an alkylator. (E) Top chemical shift residues of p38γ in complex with CSH18/CN by 2-dimensional NMR (1H-15N). Many residues around the ATP binding sites exhibit chemical shifts, indicating that CSH18CN likely binds at the ATP site of p38γ. (F) 3-Dimensional (3D) structure of the ATP pocket of p38γ. The 3D structure of p38γ with its inhibitor SU005 is calculated based on the docking pose of 6 inhibitors to the p38γ and p38α (PMID: 27431267). The differences of ATP pockets’ residues in p38γ and p38α listed at the top corner of the figure. SU005 demonstrates the highest specificity for p38γ in the ATP pocket among the compounds tested (SU001-SU006). This inhibitor preferentially binds to the p38γ isoform because of its unique structural interactions with residues in the ATP binding pocket, specifically M109, F111, T114, and K118. CSP, chemical shift perturbation; β-OG, n-octyl-β-D-glucoside.
Compound CSH18 binds to p38γ. (A) CSH18 is predicted to bind to the LBS of p38γ, with a Glide XP docking score of −10.9 kcal/mol. (B) Chemical structure of compound CSH18. (C) Mass spectrometry data: 1-dimensional spectra of the 1H NMR aromatic region of compound CSH18 at 25°C; (i) molar ratio between CSH18 and p38γ is 50:1 and (ii) free CSH18 with a concentration of 50 μM. (D) Substitution in CSH18: CSH18 has a Br group, which might act as an alkylator, potentially causing nonspecific binding. To reduce this risk, we replaced Br with CN in CSH18 to create CSH18CN. This substitution maintains a similar conformation in the lipid-binding domain of p38γ without functioning as an alkylator. (E) Top chemical shift residues of p38γ in complex with CSH18/CN by 2-dimensional NMR (1H-15N). Many residues around the ATP binding sites exhibit chemical shifts, indicating that CSH18CN likely binds at the ATP site of p38γ. (F) 3-Dimensional (3D) structure of the ATP pocket of p38γ. The 3D structure of p38γ with its inhibitor SU005 is calculated based on the docking pose of 6 inhibitors to the p38γ and p38α (PMID: 27431267). The differences of ATP pockets’ residues in p38γ and p38α listed at the top corner of the figure. SU005 demonstrates the highest specificity for p38γ in the ATP pocket among the compounds tested (SU001-SU006). This inhibitor preferentially binds to the p38γ isoform because of its unique structural interactions with residues in the ATP binding pocket, specifically M109, F111, T114, and K118. CSP, chemical shift perturbation; β-OG, n-octyl-β-D-glucoside.
ATP STD values in complex with p38γ inactive with CSH18
| Proton . | 1H, ppm . | STD (%) ± error . | STD (%) ± error . | STD (%) ± error . |
|---|---|---|---|---|
| CSH18 = 4 μM . | CSH18 = 20 μM . | |||
| H8 | 8.51 | 1.07 ± 0.18 | 0.64 ± 0.18 | 2.02 ± 0.2 |
| H2 | 8.26 | 11.05 ± 0.36 | 9.77 ± 0.29 | 11.15 ± 0.25 |
| H1′ | 6.144 | 3.4 ± 0.34 | 1.89 ± 0.33 | 4.48 ± 0.34 |
| H1′ | 6.136 | 3.52 ± 0.33 | 2.28 ± 0.31 | 4.12 ± 0.32 |
| Proton . | 1H, ppm . | STD (%) ± error . | STD (%) ± error . | STD (%) ± error . |
|---|---|---|---|---|
| CSH18 = 4 μM . | CSH18 = 20 μM . | |||
| H8 | 8.51 | 1.07 ± 0.18 | 0.64 ± 0.18 | 2.02 ± 0.2 |
| H2 | 8.26 | 11.05 ± 0.36 | 9.77 ± 0.29 | 11.15 ± 0.25 |
| H1′ | 6.144 | 3.4 ± 0.34 | 1.89 ± 0.33 | 4.48 ± 0.34 |
| H1′ | 6.136 | 3.52 ± 0.33 | 2.28 ± 0.31 | 4.12 ± 0.32 |
STD values in the interaction of p38γ with compound CSH18. The compound competes with ATP at the ATP binding site. A competition STD experiment was conducted, measuring the STD values of 50 μM ATP in the presence of 1 μM inactive p38γ under 3 conditions: with 4 μM of compound 18, with 20 μM of compound 18, and no compound 18 added.
Notably, CSH18CN, a modified version of CSH18 with Br replaced by CN to reduce nonspecific alkylation, shares a nearly identical 3-dimensional structure and docking pose with CSH18 when bound to p38γ (Figure 1D). Two-dimensional NMR 15N spectra results reveal that CSH18CN primarily binds to the lipid-binding domain of p38γ, as shown in Figure 1E and supplemental Figure 1B, with most perturbations (red) localized to this site. Additionally, results of Figure 1F, which, based on a p38γ inhibitor SU005,14 support our observation that significant perturbations are seen at ATP site residues K57, F111, D115, and K118, which are critical and unique for the ATP pocket of p38γ.
We observed that CSH18 does not inhibit p38γ kinase activity toward its downstream targets (supplemental Figure 2A). However, at higher doses, CSH18 elevates p-p38 Y323 levels, indicating the activation of an alternative phosphorylation pathway for p38. Notably, p38γ protein levels decrease in a dose-dependent manner (supplemental Figure 2B).
CSH18 and CSH71 increased OR4N5 protein levels, suggesting enhanced dimerization of OR4N5
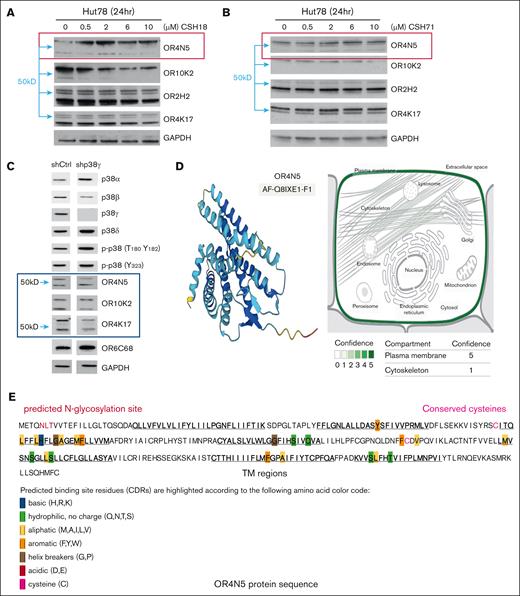
OR2H2, OR4N5, OR4K17, and OR10K2 were selected for further WB analysis. Changes in the levels of above OR proteins after treatment with compounds CSH18 and CSH71 were observed. Specifically, we noted an increase in the upper band (60 kDa), which corresponds to a dimer of OR4N5, after the administration of varying doses of CSH18 and CSH71 in Hut78 cells indicated by red rectangles (Figure 2A-B).
p38γ inhibitors increase olfactory transduction pathway activity. (A) WB analysis of Hut78 cells treated with 0.5 to 10 μM of CSH18. (B) WB analysis of Hut78 cells treated with 0.5 to 10 μM of CSH71. Both CSH18 and CSH71 increased OR4N5 protein levels. Other olfactory factors OR10K2, OR2H2, and OR4K17 were also assessed, with dimerization of OR4N5 observed based on molecular weight (blue arrows indicate 50 kDa). (C) WB assays for lentiviral expression of sh-p38γ vs sh-Ctrl. OR4N5 bands at ∼30 and 60 kDa correspond to the monomer and dimer, respectively (sh-Ctrl lane). (D) OR4N5 3D structure prediction by AlphaFold (AF-Q8IXE1-F1, left). Right: localization of OR4N5 in the plasma membrane (green, confidence score = 5). Slight presence in the cytoskeleton (confidence score = 1) according to Genecard.com. (E) In-depth analysis of the OR4N5 amino acid sequence identified 7 transmembrane domains (underlined) and 2 cysteines that may contribute to OR4N5 dimerization. CDR, Complementarity Determining Region; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hr, hour; sh-Ctrl, shRNAs targeting control; sh-p38γ, shRNAs targeting p38γ; TM, Transmembrane.
p38γ inhibitors increase olfactory transduction pathway activity. (A) WB analysis of Hut78 cells treated with 0.5 to 10 μM of CSH18. (B) WB analysis of Hut78 cells treated with 0.5 to 10 μM of CSH71. Both CSH18 and CSH71 increased OR4N5 protein levels. Other olfactory factors OR10K2, OR2H2, and OR4K17 were also assessed, with dimerization of OR4N5 observed based on molecular weight (blue arrows indicate 50 kDa). (C) WB assays for lentiviral expression of sh-p38γ vs sh-Ctrl. OR4N5 bands at ∼30 and 60 kDa correspond to the monomer and dimer, respectively (sh-Ctrl lane). (D) OR4N5 3D structure prediction by AlphaFold (AF-Q8IXE1-F1, left). Right: localization of OR4N5 in the plasma membrane (green, confidence score = 5). Slight presence in the cytoskeleton (confidence score = 1) according to Genecard.com. (E) In-depth analysis of the OR4N5 amino acid sequence identified 7 transmembrane domains (underlined) and 2 cysteines that may contribute to OR4N5 dimerization. CDR, Complementarity Determining Region; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hr, hour; sh-Ctrl, shRNAs targeting control; sh-p38γ, shRNAs targeting p38γ; TM, Transmembrane.
Typically, OR proteins exhibited 2 bands in the WB assays. OR4N5 with molecular weights of ∼30 kDa and ∼60 kDa respond to the monomer and dimer of OR4N5, respectively (Figure 2C, the line of short hairpin control), because dimerization is considered common in mammalian ORs.15 These results suggest that the actions of CSH18 and CSH71 affect the OR signaling pathway, with a particular emphasis on the upregulation of OR4N5. The expression level of the dimmer (60 kDa upper band) diminishes upon the knockdown of p38γ expression through shRNA in Hut78 cells, as illustrated in Figure 2C. OR4N5 is the only OR expressed in the cytoskeleton besides presence in plasma membrane (Figure 2D, right, confidence score = 1, in comparison with that in plasma membrane, score = 5, according to Genecard.com).
In-depth analysis into the OR4N5 amino acid sequence identified 2 cysteines (shown in Figure 2E) conserved across species, suggesting OR4N5 dimer formation via disulfide bonding. Furthermore, underlined amino acid sequences representing 7 transmembrane amino acid sequences (Figure 2E) and 3D structure (Figure 2D left, AlphaFold prediction), allude to the plasma membrane localization of OR4N5 as shown in Figure 2D (right).
Based on our observation in Figure 2A-B that OR4N5 increased at the protein level whereas OR10K2 decreased after CSH18 or CSH71 treatment although both increased in mRNA level, we emphasize the role of OR4N5 in CTCL throughout the remaining content.
The OR4N5 protein level is significantly reduced in samples from patients with SS
To investigate OR4N5’s role in CTCL, we performed confocal immunofluorescence microscopy on peripheral blood mononuclear cells (PBMCs) from a p38γ-positive female patient, using a healthy donor’s PBMCs as a control. In Figure 3A, OR4N5 (magenta) shows reduced expression in the patient, whereas CD3 (green) marks T cells, and DAPI (4′,6-diamidino-2-phenylindole) stains the nucleus. A zoomed-in region highlights individual color channels and merged views, showing colocalization of OR4N5, p38γ, and CD3E in healthy PBMCs after CSH71 treatment (250 nM, yellow arrow, Figure 3B, left).
OR4N5 is significantly reduced in CTCL. (A) Expression of OR4N5 (magenta), p38γ (red), and CD3 (green) was measured by immunofluorescence confocal microscopy in CTCL PBMC samples compared with normal healthy donors. Most CTCL cells showed weaker CD3 positivity (green) than control healthy PBMCs. (B) p38γ levels were measured by WB analysis (top). OR4N5 was significantly reduced in CTCL PBMC samples (patient [P1] with SS is shown), with statistical analysis of magenta expression presented (bottom) using Zen Software. GAPDH, glyceraldehyde-3-phosphate dehydrogenase, HD, healthy donor; P, patient.
OR4N5 is significantly reduced in CTCL. (A) Expression of OR4N5 (magenta), p38γ (red), and CD3 (green) was measured by immunofluorescence confocal microscopy in CTCL PBMC samples compared with normal healthy donors. Most CTCL cells showed weaker CD3 positivity (green) than control healthy PBMCs. (B) p38γ levels were measured by WB analysis (top). OR4N5 was significantly reduced in CTCL PBMC samples (patient [P1] with SS is shown), with statistical analysis of magenta expression presented (bottom) using Zen Software. GAPDH, glyceraldehyde-3-phosphate dehydrogenase, HD, healthy donor; P, patient.
Using Zen software, we analyzed OR4N5 fluorescence intensity across multiple images (Figure 3A), revealing a significant reduction in OR4N5 levels in a sample of a patients with CTCL compared with controls (Figure 3B, right). These findings suggest that higher OR4N5 expression in healthy individuals may play a protective role against cancer development.
Loss of OR4N5 promotes cell proliferation, indicating a potential tumor-suppressive role
To investigate OR4N5’s role in CTCL, we silenced its expression in Hut78 cells using lentiviral shRNA. Silencing OR4N5 significantly increased cell proliferation (green, Figure 4A), whereas silencing OR10K2 had modest effects (orange, Figure 4A). In contrast, scramble shRNA showed no impact on cell proliferation (colored purple, Figure 4A). Interestingly, high-titer shOR4N5 transduction enhanced proliferation more than low-titer transduction, a pattern not observed with OR10K2. Confocal immunofluorescence confirmed efficient OR4N5 and OR10K2 silencing at the protein level, 7 to 9 days after transduction (Figure 4B; supplemental Figure 3).
Gene silencing of either OR4N5 or OR10K2 promotes cell proliferation in Hut78 cells. (A) Cell viability assays were conducted on OR4N5- and OR10K2-silenced cells (sh-OR4N5 and sh-OR10K2) over a 10-day period after transduction to assess cell proliferation. Cell counts were measured on days 1 through 10 using the trypan blue exclusion method to distinguish viable cells. A scramble sequence shRNA served as the control in these assays. (B) Confocal immunofluorescence analysis confirmed the successful silencing of OR4N5 and OR10K2 proteins in Hut78 cells (left). The loss of OR expression in Hut78 cells correlates with increased cell proliferation 7 to 9 days after transduction. Statistical data are presented as mean (standard deviation). Cells were counted on days 1, 4, 7, and 10 after selection using an automated cell counter, with trypan blue exclusion to differentiate viable cells. sh-Ctrl, short hairpin control.
Gene silencing of either OR4N5 or OR10K2 promotes cell proliferation in Hut78 cells. (A) Cell viability assays were conducted on OR4N5- and OR10K2-silenced cells (sh-OR4N5 and sh-OR10K2) over a 10-day period after transduction to assess cell proliferation. Cell counts were measured on days 1 through 10 using the trypan blue exclusion method to distinguish viable cells. A scramble sequence shRNA served as the control in these assays. (B) Confocal immunofluorescence analysis confirmed the successful silencing of OR4N5 and OR10K2 proteins in Hut78 cells (left). The loss of OR expression in Hut78 cells correlates with increased cell proliferation 7 to 9 days after transduction. Statistical data are presented as mean (standard deviation). Cells were counted on days 1, 4, 7, and 10 after selection using an automated cell counter, with trypan blue exclusion to differentiate viable cells. sh-Ctrl, short hairpin control.
Using DepMap and BioGRID data sets,16-18 we explored cancer dependencies. A Chronos score of less than −1 indicates that a gene is essential for survival or normal function. Note that Chronos and DEMETER2 are gene-dependency scoring systems used in the DepMap project in cancer research: Chronos analyzes CRISPR-CRISPR–associated protein 9 screening data with a robust computational model, whereas DEMETER2 quantifies gene dependency from RNA interference screening data, excelling at handling RNA interference noise and occasionally applied to CRISPR data sets. Chronos and DEMETER2 dependency scores near 0 in cancer cell lines suggest that OR4N5 and OR10K2 are not essential for general cancer cell survival (supplemental Figure 4A). Although they are not drivers of cancer growth, gene knockdown assays (Figure 4A) indicate a potential growth-suppressive function, because their loss uniquely increases Hut78 cell proliferation in CTCL.
Interestingly, public data set analyses revealed that OR4N5 loss promotes proliferation in specific noncancerous cells, such as neural stem cells (HF6562-CRISPR–associated protein 9) and embryonic kidney cells (HEK293-ACE2-TMPRSS2; supplemental Figure 4B).19,20 Additionally, OR10K2 knockout enhanced proliferation in primary T cells when regulatory T cells (CD4+CD127lowCD25+)21 were present, suggesting a role in T-cell regulation. These findings align with the observation that OR4N5 and OR10K2 expression levels are typically lower in cancer cells compared with healthy tissue. Loss of OR4N5 may enable cell proliferation through pathways dependent on stem cell–like features in CTCL.
p38γ gains proximity to OR4N5 in the CD3 of TCR complex in CTCL cells by CSH71
To explore the molecular mechanisms of ORs in CTCL, we conducted confocal immunofluorescence microscopy to assess protein-protein interactions between key molecules in the OR pathway in Hut78 cells and samples from patients with CTCL SS.
The TCR complex, including CD3 subunits (CD3ε, CD3γ, CD3δ, and CD3ζ), is crucial for T-cell activation. CD3ζ, located on the inner plasma membrane, is critical for signal transduction, recruiting kinases such as ZAP70. In CTCL, TCR signaling is propagated via CD3ζ, with p38γ acting as a key mediator of aberrant T-cell activation and proliferation.
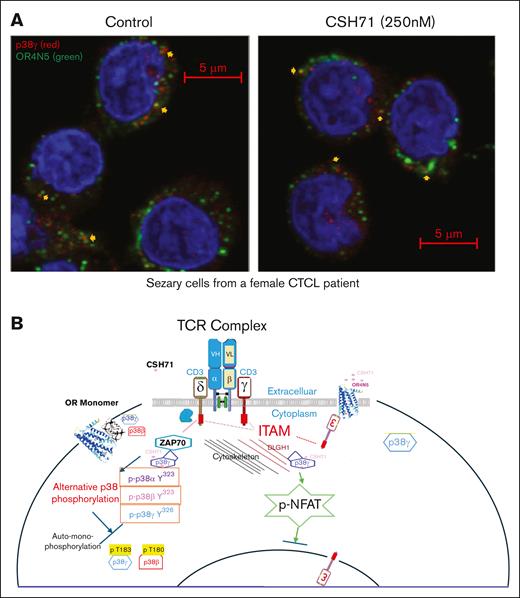
Our previous findings showed that CSH71 interacts with p38γ at its LBS, and 250 nM CSH71 treatment elevates OR4N5 mRNA levels.4 Confocal analysis revealed that p38γ and OR4N5 do not directly colocalize, likely because of the loss of CD3ε on the membrane, which anchors OR4N5 in CTCL Sézary cells (Figure 5A). We observed that p38γ, overexpressed in CTCL, gains proximity to OR4N5, likely at the TCR complex (yellow arrows, Figure 5A). OR4N5 may interact with CD3ε on the plasma membrane because of their transmembrane domains in healthy PBMCs (Figure 6A, yellow arrows) but not in CTCL cells (Figure 6B). In CTCL, p38γ likely interacts with immunoreceptor tyrosine-based activation motifs of CD3 on the inner membrane through ZAP70 and its activation of alternative p38 phosphorylation (cartoon, Figure 5B).
p38γ gains proximity to OR4N5 in the CD3 of the TCR complex in CTCL Sézary cells. (A) Sézary cells from a patient with CTCL were treated with CSH71 (250 nM, right) or untreated (control, left), followed by confocal immunofluorescence analysis. OR4N5 (red) and CD3E (green) colocalize, indicated by the merged yellow signal, suggesting an interaction between p38γ and OR4N5. (B) A summary of the involvement of these molecules in the signal transduction process of SS cells (malignant T cells). A cartoon diagram illustrates the proposed molecular mechanism by which p38γ may interfere with OR dimerization via protein-protein interactions within the TCR complex via alternative p38 activation. DLGH1, also known as DLG1, Discs Large MAGUK scaffold protein 1 (Human); ITAM, Immunoreceptor Tyrosine-based Activation Motif; p-NFAT, phosphorylated nuclear factor of activated T cells.
p38γ gains proximity to OR4N5 in the CD3 of the TCR complex in CTCL Sézary cells. (A) Sézary cells from a patient with CTCL were treated with CSH71 (250 nM, right) or untreated (control, left), followed by confocal immunofluorescence analysis. OR4N5 (red) and CD3E (green) colocalize, indicated by the merged yellow signal, suggesting an interaction between p38γ and OR4N5. (B) A summary of the involvement of these molecules in the signal transduction process of SS cells (malignant T cells). A cartoon diagram illustrates the proposed molecular mechanism by which p38γ may interfere with OR dimerization via protein-protein interactions within the TCR complex via alternative p38 activation. DLGH1, also known as DLG1, Discs Large MAGUK scaffold protein 1 (Human); ITAM, Immunoreceptor Tyrosine-based Activation Motif; p-NFAT, phosphorylated nuclear factor of activated T cells.
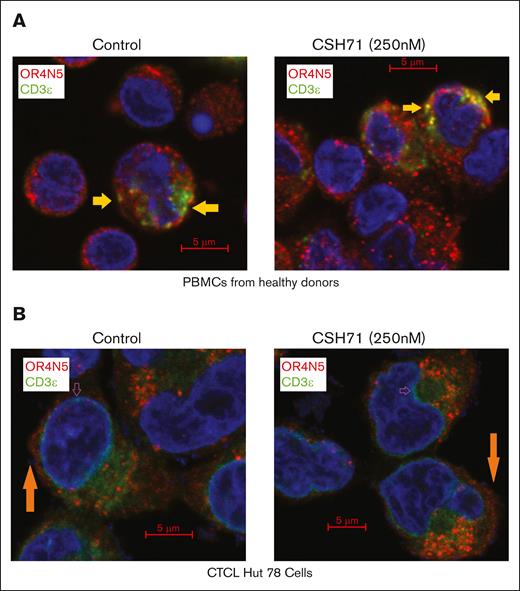
Colocalization of OR4N5 and CD3E, and colocalization of p38γ with CD3Z in TCR complexes using confocal immunofluorescence microscopy. (A) Colocalization of OR4N5 and CD3E in PBMCs from healthy donors. (B) Colocalization of OR4N5 and CD3E in CTCL Hut78 cells, both treated with CSH71 (250 nM) and untreated controls. In both panels A and B, the interaction between CD3E and OR4N5 was assessed, with colocalization indicated by the yellow signal in merged 2-color images.
Colocalization of OR4N5 and CD3E, and colocalization of p38γ with CD3Z in TCR complexes using confocal immunofluorescence microscopy. (A) Colocalization of OR4N5 and CD3E in PBMCs from healthy donors. (B) Colocalization of OR4N5 and CD3E in CTCL Hut78 cells, both treated with CSH71 (250 nM) and untreated controls. In both panels A and B, the interaction between CD3E and OR4N5 was assessed, with colocalization indicated by the yellow signal in merged 2-color images.
CTCL samples exhibit significantly reduced CD3ζ (Figure 6C-D) and dispersed CD3ε localization (Figure 6B). In Hut78 cells, CD3ζ is almost absent, and CD3ε is scattered in the cytosol and nuclear envelope, preventing colocalization with OR4N5 or p38γ (supplemental Figure 5B-C).
OR4N5 levels are markedly reduced in Hut78 cells, with minimal plasma membrane localization (orange arrows, Figure 6B). This lack of colocalization with CD3ε, regardless of treatment, reflects aberrant CD3 expression, characteristic of CTCL.22-24 These findings suggest that OR4N5 and p38γ interactions at the TCR complex are disrupted in CTCL, contributing to its pathogenesis.
Expression of OR4N5 and its role in cancer suppression
To investigate the role of ORs in cancer, particularly in CTCL, we examined OR4N5 as an exemplar. This receptor responded to CSH71, as shown by RNA sequencing4 and WB analysis (Figure 2). OR4N5 is expressed in the blood (Bgee database,25 supplemental Table 2), although its levels in healthy PBMCs are generally low. Tumor-educated blood platelets, instead, provide valuable insight into OR expression in cancer. Analysis of the Gene Expression Omnibus GSE-68086 data set26 revealed significantly reduced OR4N5 expression across 6 solid tumor types, breast, colorectal, glioblastoma, hepatobiliary, lung, and pancreatic cancers, compared with normal controls (supplemental Figure 6A).
Comparative analyses further demonstrated that both OR4N5 and OR10K2 were significantly downregulated across cancers (Table 3). OR4N5, along with OR2A7 and OR6C68, showed significant downregulation in tumor samples (P < .05, supplemental Figure 6A-B). Although most ORs were reduced in solid tumors, exceptions such as OR2L13 and OR2B6 exhibited mixed or increased expression in certain cancers, including glioblastoma and breast cancer (supplemental Table 3). The tumor-suppressive role of OR4N5 in CTCL may be enhanced through synergistic interactions with other ORs. In analyzing OR4N5’s interactions, we found it associates with 19 non-OR proteins and 22 other ORs based on the Gene Expression Omnibus GSE-68086 data set (Tables 4 and 5).
Gene expression of OR4N5, OR2A7, OR6C68, and OR10K2 in 6 cancers vs normal control
| Gene name . | Breast carcinoma . | Colorectal carcinoma . | Glioblastoma . | |||
|---|---|---|---|---|---|---|
| P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | |
| OR4N5 | 5.08E−05 | −3.3 | .000203805 | −3 | .037922805 | −1.5 |
| OR10K2 | 1.99E−16 | −5 | 1.61E−21 | −5.8 | 4.81E−06 | −2.6 |
| OR2A7 | 2.47E−19 | −4.7 | 8.42E−23 | −5.5 | 1.18E−05 | −2.2 |
| OR6C68 | 1.25E−06 | −3.4 | 9.69E−09 | −3.8 | .00392438 | −1.8 |
| Gene name | Hepatobiliary carcinoma | NSC lung carcinoma | Pancreatic adenocarcinoma | |||
| P value | log2FC | P value | log2FC | P value | log2FC | |
| OR4N5 | .04766668 | −1.9 | 5.79E−05 | −2.5 | 4.44E−05 | −3.4 |
| OR10K2 | .00153234 | −2.3 | 7.77E−11 | −3.7 | 1.18E−07 | −3.5 |
| OR2A7 | .0001452 | −2.7 | 6.51E−16 | −4.1 | 2.37E−07 | −3 |
| OR6C68 | .00590347 | −2.3 | 5.17E−06 | −2.6 | 1.46E−10 | −4.4 |
| Gene name . | Breast carcinoma . | Colorectal carcinoma . | Glioblastoma . | |||
|---|---|---|---|---|---|---|
| P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | |
| OR4N5 | 5.08E−05 | −3.3 | .000203805 | −3 | .037922805 | −1.5 |
| OR10K2 | 1.99E−16 | −5 | 1.61E−21 | −5.8 | 4.81E−06 | −2.6 |
| OR2A7 | 2.47E−19 | −4.7 | 8.42E−23 | −5.5 | 1.18E−05 | −2.2 |
| OR6C68 | 1.25E−06 | −3.4 | 9.69E−09 | −3.8 | .00392438 | −1.8 |
| Gene name | Hepatobiliary carcinoma | NSC lung carcinoma | Pancreatic adenocarcinoma | |||
| P value | log2FC | P value | log2FC | P value | log2FC | |
| OR4N5 | .04766668 | −1.9 | 5.79E−05 | −2.5 | 4.44E−05 | −3.4 |
| OR10K2 | .00153234 | −2.3 | 7.77E−11 | −3.7 | 1.18E−07 | −3.5 |
| OR2A7 | .0001452 | −2.7 | 6.51E−16 | −4.1 | 2.37E−07 | −3 |
| OR6C68 | .00590347 | −2.3 | 5.17E−06 | −2.6 | 1.46E−10 | −4.4 |
Gene expression levels of OR4N5, OR2A7, OR6C68, and OR10K2 in 6 types of cancer compared to normal controls, based on data from the E-GEOD-68086 data set (EMBL-EBI). OR4N5, OR2A7, and OR6C68 are predominantly expressed in blood tissues, whereas OR10K2, induced by CSH71, is included as a control. All data are presented as log2FC with P values <.05.
EMBL-EBI, European Molecular Biology Laboratory – European Bioinformatics Institute; FC, fold change; NSC, non-small cell.
Gene expression level of OR4N5-interacting ORs (STRINGS) in tumor-educated blood platelets of 6 cancers
| Gene ID . | Gene name . | Breast carcinoma . | Colorectal carcinoma . | Glioblastoma . | Hepatobiliary carcinoma . | NSC lung carcinoma . | Pancreatic adenocarcinoma . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | ||
| ENSG00000094661 | OR1I1 | 3.27E−15 | −5.1 | 2.63E−15 | −5.1 | .000447 | −2.1 | .002053 | −2.5 | .000197 | −2.2 | 1.39E−12 | −4.9 |
| ENSG00000188269 | OR7A5 | 2.05E−14 | −3.9 | 2.31E−14 | −4 | .000273 | −1.8 | .002215 | −2 | 5.76E−08 | −2.6 | 7.06E−12 | −3.8 |
| ENSG00000187857 | OR6C75 | 2.06E−14 | −4.5 | 2.73E−12 | −4.2 | .017369 | −1.3 | 3.20E−07 | −4.5 | .000246 | −2.1 | 2.02E−05 | −2.6 |
| ENSG00000205328 | OR6C65 | 2.93E−12 | −4.6 | 3.42E−08 | −3.6 | .000356 | −2.2 | .000828 | −2.7 | 5.54E−10 | −3.5 | 1.73E−09 | −4.1 |
| ENSG00000177535 | OR2B11 | 3.90E−10 | −4.3 | 3.15E−09 | −4 | .000181 | −2.4 | .000527 | −3 | 1.39E−07 | −3.2 | .00022 | −2.5 |
| ENSG00000184166 | OR1D2 | 2.30E−08 | −3.9 | .001177 | −2.3 | .022729 | −1.4 | .031772 | −1.8 | 3.44E−06 | −2.9 | 3.18E−07 | −3.7 |
| ENSG00000141194 | OR4D1 | 4.00E−08 | −3.6 | 1.35E−11 | −4.5 | .000113 | −2.2 | .003561 | −2.4 | 2.46E−09 | −3.4 | 3.63E−06 | −3.2 |
| ENSG00000124657 | OR2B6 | 7.88E−07 | 1.1 | 1.46E−06 | 1 | .806643 | 0.1 | 7.71E−06 | 1.6 | .000992 | 0.7 | 4.73E−07 | 1.3 |
| ENSG00000171942 | OR10H2 | 5.60E−05 | −3 | 9.10E−05 | −2.9 | .024594 | −1.5 | .130867 | −1.4 | 1.73E−05 | −2.8 | 3.93E−05 | −3.2 |
| ENSG00000196240 | OR2T2 | 6.35E−05 | −3 | 4.50E−05 | −2.9 | .002662 | −2 | .011559 | −2.4 | 1.82E−05 | −2.7 | 6.51E−05 | −3 |
| ENSG00000183024 | OR1G1 | .00011 | −2.9 | 2.67E−07 | −3.6 | .015671 | −1.5 | .136376 | −1.3 | 3.30E−06 | −2.7 | 7.50E−10 | −4.3 |
| ENSG00000175143 | OR2T1 | .000151 | −3.2 | .000201 | −3.2 | .0039 | −2.1 | .146061 | −1.5 | .000469 | −2.4 | .000778 | −3 |
| ENSG00000221989 | OR2A2 | .000586 | −2.6 | 2.44E−05 | −3.2 | .000131 | −2.6 | .026129 | −2 | .0008 | −2.1 | 1.22E−06 | −3.6 |
| ENSG00000254834 | OR5M10 | .001344 | −2.5 | .000185 | −2.7 | .110938 | −1.1 | .050194 | −1.8 | .01517 | −1.6 | 9.78E−07 | −4 |
| ENSG00000172188 | OR4C11 | .004885 | −2.2 | .008672 | −2 | .742986 | −0.2 | .899352 | −0.1 | .005355 | −1.8 | .002925 | −2.5 |
| ENSG00000237388 | OR4A47 | .21225 | −1.5 | .468567 | −0.9 | .347595 | −0.9 | NA | −0.8 | .922457 | −0.1 | .392475 | −1.1 |
| ENSG00000198283 | OR5B21 | .21835 | −1.5 | .306278 | −1.2 | .535776 | −0.6 | NA | −0.7 | .489942 | −0.6 | .506153 | −0.8 |
| ENSG00000280236 | OR12D2 | .240472 | −1.3 | .144644 | −1.5 | .094247 | −1.5 | .496986 | −0.8 | .043656 | −1.8 | .066428 | −2.1 |
| ENSG00000197125 | OR8B8 | .40181 | −1.1 | .491146 | −0.9 | .619077 | −0.5 | NA | −0.5 | .748128 | 0.3 | .609838 | −0.7 |
| ENSG00000180016 | OR1E1 | .411894 | −0.8 | .241953 | −1.3 | .29586 | −0.9 | .662732 | −0.5 | .27676 | −0.9 | .459317 | −0.8 |
| ENSG00000165202 | OR1Q1 | .48255 | −0.7 | .264031 | −1.3 | .726106 | 0.3 | NA | −0.5 | .853721 | −0.1 | .312266 | −1.1 |
| ENSG00000204700 | OR2J2 | NA | −0.5 | .896021 | −0.3 | NA | 0.2 | NA | 0.2 | .091244 | 1.5 | NA | −0.3 |
| Gene ID . | Gene name . | Breast carcinoma . | Colorectal carcinoma . | Glioblastoma . | Hepatobiliary carcinoma . | NSC lung carcinoma . | Pancreatic adenocarcinoma . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | ||
| ENSG00000094661 | OR1I1 | 3.27E−15 | −5.1 | 2.63E−15 | −5.1 | .000447 | −2.1 | .002053 | −2.5 | .000197 | −2.2 | 1.39E−12 | −4.9 |
| ENSG00000188269 | OR7A5 | 2.05E−14 | −3.9 | 2.31E−14 | −4 | .000273 | −1.8 | .002215 | −2 | 5.76E−08 | −2.6 | 7.06E−12 | −3.8 |
| ENSG00000187857 | OR6C75 | 2.06E−14 | −4.5 | 2.73E−12 | −4.2 | .017369 | −1.3 | 3.20E−07 | −4.5 | .000246 | −2.1 | 2.02E−05 | −2.6 |
| ENSG00000205328 | OR6C65 | 2.93E−12 | −4.6 | 3.42E−08 | −3.6 | .000356 | −2.2 | .000828 | −2.7 | 5.54E−10 | −3.5 | 1.73E−09 | −4.1 |
| ENSG00000177535 | OR2B11 | 3.90E−10 | −4.3 | 3.15E−09 | −4 | .000181 | −2.4 | .000527 | −3 | 1.39E−07 | −3.2 | .00022 | −2.5 |
| ENSG00000184166 | OR1D2 | 2.30E−08 | −3.9 | .001177 | −2.3 | .022729 | −1.4 | .031772 | −1.8 | 3.44E−06 | −2.9 | 3.18E−07 | −3.7 |
| ENSG00000141194 | OR4D1 | 4.00E−08 | −3.6 | 1.35E−11 | −4.5 | .000113 | −2.2 | .003561 | −2.4 | 2.46E−09 | −3.4 | 3.63E−06 | −3.2 |
| ENSG00000124657 | OR2B6 | 7.88E−07 | 1.1 | 1.46E−06 | 1 | .806643 | 0.1 | 7.71E−06 | 1.6 | .000992 | 0.7 | 4.73E−07 | 1.3 |
| ENSG00000171942 | OR10H2 | 5.60E−05 | −3 | 9.10E−05 | −2.9 | .024594 | −1.5 | .130867 | −1.4 | 1.73E−05 | −2.8 | 3.93E−05 | −3.2 |
| ENSG00000196240 | OR2T2 | 6.35E−05 | −3 | 4.50E−05 | −2.9 | .002662 | −2 | .011559 | −2.4 | 1.82E−05 | −2.7 | 6.51E−05 | −3 |
| ENSG00000183024 | OR1G1 | .00011 | −2.9 | 2.67E−07 | −3.6 | .015671 | −1.5 | .136376 | −1.3 | 3.30E−06 | −2.7 | 7.50E−10 | −4.3 |
| ENSG00000175143 | OR2T1 | .000151 | −3.2 | .000201 | −3.2 | .0039 | −2.1 | .146061 | −1.5 | .000469 | −2.4 | .000778 | −3 |
| ENSG00000221989 | OR2A2 | .000586 | −2.6 | 2.44E−05 | −3.2 | .000131 | −2.6 | .026129 | −2 | .0008 | −2.1 | 1.22E−06 | −3.6 |
| ENSG00000254834 | OR5M10 | .001344 | −2.5 | .000185 | −2.7 | .110938 | −1.1 | .050194 | −1.8 | .01517 | −1.6 | 9.78E−07 | −4 |
| ENSG00000172188 | OR4C11 | .004885 | −2.2 | .008672 | −2 | .742986 | −0.2 | .899352 | −0.1 | .005355 | −1.8 | .002925 | −2.5 |
| ENSG00000237388 | OR4A47 | .21225 | −1.5 | .468567 | −0.9 | .347595 | −0.9 | NA | −0.8 | .922457 | −0.1 | .392475 | −1.1 |
| ENSG00000198283 | OR5B21 | .21835 | −1.5 | .306278 | −1.2 | .535776 | −0.6 | NA | −0.7 | .489942 | −0.6 | .506153 | −0.8 |
| ENSG00000280236 | OR12D2 | .240472 | −1.3 | .144644 | −1.5 | .094247 | −1.5 | .496986 | −0.8 | .043656 | −1.8 | .066428 | −2.1 |
| ENSG00000197125 | OR8B8 | .40181 | −1.1 | .491146 | −0.9 | .619077 | −0.5 | NA | −0.5 | .748128 | 0.3 | .609838 | −0.7 |
| ENSG00000180016 | OR1E1 | .411894 | −0.8 | .241953 | −1.3 | .29586 | −0.9 | .662732 | −0.5 | .27676 | −0.9 | .459317 | −0.8 |
| ENSG00000165202 | OR1Q1 | .48255 | −0.7 | .264031 | −1.3 | .726106 | 0.3 | NA | −0.5 | .853721 | −0.1 | .312266 | −1.1 |
| ENSG00000204700 | OR2J2 | NA | −0.5 | .896021 | −0.3 | NA | 0.2 | NA | 0.2 | .091244 | 1.5 | NA | −0.3 |
Gene expression levels of OR4N5-interacting ORs in tumor-educated platelets across 6 cancer types. The listed ORs interact with OR4N5 according to STRING analysis. Yellow highlights indicate significant downregulation, whereas purple highlights indicate upregulation in all 6 cancer types.
FC, fold change; ID, identity; NSC, non-small cell; STRING, Search Tool for the Retrieval of Interacting Genes/Proteins, refers to Protein-Protein Interaction Networks Functional Enrichment Analysis.
Gene expression OR4N5-interacting proteins in tumor-educated blood platelets of 6 cancers
| Gene ID . | Gene name . | Breast carcinoma . | Colorectal carcinoma . | Glioblastoma . | Hepatobiliary carcinoma . | NSC lung carcinoma . | Pancreatic adenocarcinoma . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | ||
| ENSG00000168398 | BDKRB2 | 8.34E−06 | −2.5 | 2.01E−10 | −3.4 | .017445 | −1.3 | .029873 | −1.5 | 7.62E−05 | −1.9 | 8.29E−14 | −4.6 |
| ENSG00000170989 | S1PR1 | 4.25E−16 | −3 | 5.66E−23 | −4.1 | .000261 | −1.6 | 1.33E−06 | −2.7 | 1.29E−11 | −2.8 | 6.49E−26 | −4.3 |
| ENSG00000173578 | XCR1 | 1.42E−10 | −3.1 | 2.88E−12 | −3.6 | .001756 | −1.4 | .000563 | −2 | 4.14E−07 | −2.3 | 3.56E−15 | −4 |
| ENSG00000179934 | CCR8 | 9.95E−20 | −5.1 | 2.56E−09 | −3.5 | 6.89E−05 | −2.2 | .00036 | −2.5 | 4.48E−11 | −3.3 | 3.38E−10 | −3.8 |
| ENSG00000182162 | P2RY8 | 5.20E−21 | −3.7 | 2.77E−31 | −4.8 | .067366 | −0.8 | 1.80E−05 | −2.2 | 1.85E−08 | −2.3 | 1.98E−15 | −3.6 |
| ENSG00000126266 | FFAR1 | 2.97E−11 | −4.3 | 9.82E−10 | −3.9 | .000244 | −2.2 | .001767 | −2.5 | 7.43E−07 | −2.7 | 1.85E−06 | −3.4 |
| ENSG00000176136 | MC5R | 1.71E−09 | −3.8 | 4.73E−13 | −4.3 | .004435 | −1.6 | .000332 | −2.9 | 3.65E−06 | −2.5 | 5.71E−08 | −3.4 |
| ENSG00000078549 | ADCYAP1R1 | 2.72E−17 | −4.4 | 1.43E−07 | −2.8 | .00033 | −1.8 | .001806 | −2 | 1.63E−06 | −2.3 | 4.25E−08 | −3.1 |
| ENSG00000130368 | MAS1 | 4.58E−12 | −3 | 2.72E−10 | −2.9 | 1.92E−06 | −2.1 | .000103 | −2.2 | 2.63E−11 | −2.7 | 9.52E−11 | −3.2 |
| ENSG00000134443 | GRP | 1.12E−05 | −2.8 | 1.37E−06 | −3 | .004968 | −1.7 | .003538 | −2.5 | .000561 | −1.9 | 2.46E−05 | −2.8 |
| ENSG00000230164 | MAS1LP1 | .0016 | −2.8 | .00586 | −2.4 | .244462 | −0.9 | .05131 | −2.1 | .019567 | −1.6 | .004013 | −2.6 |
| ENSG00000232168 | P2RY10BP | .088036 | −1.8 | .125546 | −1.8 | .159953 | −1.2 | .479725 | −0.8 | .066438 | −1.6 | .07665 | −2.1 |
| ENSG00000179271 | GADD45GIP1 | .03062 | −0.7 | 1.13E−06 | −1.8 | .009743 | −1 | .040748 | −0.8 | .002364 | −1 | 5.01E−08 | −1.8 |
| ENSG00000204687 | MAS1L | .085128 | −1.9 | .181923 | −1.4 | .408644 | −0.7 | .433884 | −0.9 | .943563 | −0.1 | .199627 | −1.5 |
| ENSG00000185899 | TAS2R60 | .285055 | −1 | .315573 | −1 | .617987 | −0.4 | .684782 | −0.4 | .281859 | 0.8 | .194325 | −1.3 |
| ENSG00000261713 | SSTR5-AS1 | .093192 | −1.7 | .481641 | −0.8 | .862472 | −0.1 | .790265 | −0.3 | .969677 | 0 | .301099 | −1.1 |
| ENSG00000078589 | P2RY10 | .299781 | −0.2 | .07939 | −0.4 | .736213 | 0.1 | .002469 | −0.9 | .002711 | −0.8 | .000308 | −0.8 |
| ENSG00000130222 | GADD45G | .081734 | 0.9 | .920215 | 0.1 | .190908 | −0.7 | .006607 | 1.8 | .014 | 1.1 | .3605 | −0.6 |
| ENSG00000162009 | SSTR5 | NA | −0.4 | .924947 | −0.2 | NA | −0.2 | NA | −0.2 | NA | −0.2 | NA | −0.3 |
| Gene ID . | Gene name . | Breast carcinoma . | Colorectal carcinoma . | Glioblastoma . | Hepatobiliary carcinoma . | NSC lung carcinoma . | Pancreatic adenocarcinoma . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | P value . | log2FC . | ||
| ENSG00000168398 | BDKRB2 | 8.34E−06 | −2.5 | 2.01E−10 | −3.4 | .017445 | −1.3 | .029873 | −1.5 | 7.62E−05 | −1.9 | 8.29E−14 | −4.6 |
| ENSG00000170989 | S1PR1 | 4.25E−16 | −3 | 5.66E−23 | −4.1 | .000261 | −1.6 | 1.33E−06 | −2.7 | 1.29E−11 | −2.8 | 6.49E−26 | −4.3 |
| ENSG00000173578 | XCR1 | 1.42E−10 | −3.1 | 2.88E−12 | −3.6 | .001756 | −1.4 | .000563 | −2 | 4.14E−07 | −2.3 | 3.56E−15 | −4 |
| ENSG00000179934 | CCR8 | 9.95E−20 | −5.1 | 2.56E−09 | −3.5 | 6.89E−05 | −2.2 | .00036 | −2.5 | 4.48E−11 | −3.3 | 3.38E−10 | −3.8 |
| ENSG00000182162 | P2RY8 | 5.20E−21 | −3.7 | 2.77E−31 | −4.8 | .067366 | −0.8 | 1.80E−05 | −2.2 | 1.85E−08 | −2.3 | 1.98E−15 | −3.6 |
| ENSG00000126266 | FFAR1 | 2.97E−11 | −4.3 | 9.82E−10 | −3.9 | .000244 | −2.2 | .001767 | −2.5 | 7.43E−07 | −2.7 | 1.85E−06 | −3.4 |
| ENSG00000176136 | MC5R | 1.71E−09 | −3.8 | 4.73E−13 | −4.3 | .004435 | −1.6 | .000332 | −2.9 | 3.65E−06 | −2.5 | 5.71E−08 | −3.4 |
| ENSG00000078549 | ADCYAP1R1 | 2.72E−17 | −4.4 | 1.43E−07 | −2.8 | .00033 | −1.8 | .001806 | −2 | 1.63E−06 | −2.3 | 4.25E−08 | −3.1 |
| ENSG00000130368 | MAS1 | 4.58E−12 | −3 | 2.72E−10 | −2.9 | 1.92E−06 | −2.1 | .000103 | −2.2 | 2.63E−11 | −2.7 | 9.52E−11 | −3.2 |
| ENSG00000134443 | GRP | 1.12E−05 | −2.8 | 1.37E−06 | −3 | .004968 | −1.7 | .003538 | −2.5 | .000561 | −1.9 | 2.46E−05 | −2.8 |
| ENSG00000230164 | MAS1LP1 | .0016 | −2.8 | .00586 | −2.4 | .244462 | −0.9 | .05131 | −2.1 | .019567 | −1.6 | .004013 | −2.6 |
| ENSG00000232168 | P2RY10BP | .088036 | −1.8 | .125546 | −1.8 | .159953 | −1.2 | .479725 | −0.8 | .066438 | −1.6 | .07665 | −2.1 |
| ENSG00000179271 | GADD45GIP1 | .03062 | −0.7 | 1.13E−06 | −1.8 | .009743 | −1 | .040748 | −0.8 | .002364 | −1 | 5.01E−08 | −1.8 |
| ENSG00000204687 | MAS1L | .085128 | −1.9 | .181923 | −1.4 | .408644 | −0.7 | .433884 | −0.9 | .943563 | −0.1 | .199627 | −1.5 |
| ENSG00000185899 | TAS2R60 | .285055 | −1 | .315573 | −1 | .617987 | −0.4 | .684782 | −0.4 | .281859 | 0.8 | .194325 | −1.3 |
| ENSG00000261713 | SSTR5-AS1 | .093192 | −1.7 | .481641 | −0.8 | .862472 | −0.1 | .790265 | −0.3 | .969677 | 0 | .301099 | −1.1 |
| ENSG00000078589 | P2RY10 | .299781 | −0.2 | .07939 | −0.4 | .736213 | 0.1 | .002469 | −0.9 | .002711 | −0.8 | .000308 | −0.8 |
| ENSG00000130222 | GADD45G | .081734 | 0.9 | .920215 | 0.1 | .190908 | −0.7 | .006607 | 1.8 | .014 | 1.1 | .3605 | −0.6 |
| ENSG00000162009 | SSTR5 | NA | −0.4 | .924947 | −0.2 | NA | −0.2 | NA | −0.2 | NA | −0.2 | NA | −0.3 |
Gene expression levels of non-OR proteins that interact with OR4N5 in tumor-educated blood platelets across 6 cancer types. The listed proteins are OR4N5-interacting non-ORs. Red text highlights the most downregulated proteins in each cancer type, with S1PR1, XCR1, CCR8, P2RY8, FFAR1, MC5R, ADCYAP1R1, and MAS1 being among the most significantly downregulated genes across all 6 cancer types.
FC, fold change; ID, identity; NA, Not Available; NSC, non-small cell.
Among 800 ORs analyzed, only OR2B6 and OR2W3 were consistently overexpressed in all 6 cancer types (P < .05). OR2B6, notably overexpressed in breast cancer, may act as a cancer driver27 (supplemental Table 4). Most ORs, including pseudogenes, exhibited reduced expression (P < .05), suggesting their roles as tumor suppressors. Approximately 20 ORs showed no significant difference in expression between tumor and normal samples, indicating a complex regulatory network. Reduced OR expression in cancers implies that these receptors may help inhibit tumor growth in healthy tissues.
Effects of point mutation in LBS of p38γ on cell proliferation and regulation of OR4N5 expression
Two dominant LBS mutants of p38γ significantly reduced p38γ protein levels after 18CN treatment, affecting Hut78 cell proliferation (Figure 7A-B). The lethality of the 3M_p38γ mutation underscores the critical role of the LBS in maintaining p38γ function. Although 18CN preferentially binds the LBS to promote cell proliferation, disruption of the LBS redirects binding to the ATP site, resulting in cell death (Figure 7C-D). Stable Hut78 cell lines expressing mild 3M_p38γ mutants showed that LBS mutations diminished endogenous p38γ levels, which in turn decreased OR4N5 expression upon 18CN treatment. This reduction contrasts with the increased OR4N5 expression observed in wild-type cells (Figure 2A), emphasizing the regulatory role of p38γ in OR4N5 expression and its importance in CTCL pathogenesis.
Evidence of specificity of 18CN to p38γ. (A) Six residues were identified using the ligand virtual screening strategy and the druggable site prediction method based on the hydrophobic interaction ranking of β-octyl glucoside with p38γ within a distance of <5 Å. They are Phe249, 3.56 Å; Leu294, 3.75 Å; Leu198, 3.80 Å; Pro246, 4.12 Å; and Val293, 4.91 Å. The 2 p38γ mutants were designed with the following amino acid substitutions: pLVXTP-3FLAG-p38γ mut_3M (3M-p38γ): contains 3-point mutations, Pro246Gly, Val 293Gly, and Leu 294Gly; pLVXTP-3FLAG-p38γ mut_3M-3m (3M-3m-p38γ): contains 6-point mutations: Pro246Gly, Val 293Gly, Leu 294Gly, Leu198Ala, Pro245Ala, and Phe249Ala. The mutant constructs were validated by Sanger sequencing (the supplemental Methods). (B) The WB analysis of 2 mutants targeting the lipid-binding domain of p38γ, which were designed based on the 6 amino acids depicted in panel A. The first mutant, 3M-p38γ, carries 3-point mutations (P246G-V293G-L294G) and functions as a dominant-negative p38γ in Hut 78 cells, referred to as Hut-3M. The second mutant, 3M-3m-p38γ, includes an additional 3 mutations (P246G-V293G-L294G) on top of the Hut-3M construct, creating a 6-site mutant expressed in Hut 78 cells. As a control, Hut-WT cells were derived from an empty lentiviral vector (pLVXTP) expressing WT p38γ – 3× Flag tags in Hut 78 cells. Flag indicates plasmid p38γ expression. Red brackets indicate reduced protein level upon treatment. (C) The 3 most druggable sites on p38γ were identified using the “all docking pose” method.1 Site 1: the LBS, in which CSH18/CN exhibits the highest binding affinity (MaxGScore of −10.9 kcal/mol). Site 2: the ATP-binding site, in which CSH18/CN also binds with a MaxGScore of −10.1 kcal/mol), as the second-preferentially site. Site 3: the N-terminal region above the ATP-binding site, with a MaxGScore of −9.2 kcal/mol. (D) Expression of LBS mutants of p38γ (3M-p38γ and 3M-3m-p38γ) affects cell proliferation. The p38γ-3M mutant is lethal upon transduction. However, a low-expression clone of 3M-p38γ, exhibiting reduced levels of both FLAG-tagged and endogenous p38γ, was successfully isolated. Cell proliferation was assessed using cell counting and trypan blue exclusion assays with an automated cell counter. The data indicate that disruption of the LBS on p38γ redirects CSH18CN to preferentially bind the ATP site, resulting in cell death. WT, wild-type.
Evidence of specificity of 18CN to p38γ. (A) Six residues were identified using the ligand virtual screening strategy and the druggable site prediction method based on the hydrophobic interaction ranking of β-octyl glucoside with p38γ within a distance of <5 Å. They are Phe249, 3.56 Å; Leu294, 3.75 Å; Leu198, 3.80 Å; Pro246, 4.12 Å; and Val293, 4.91 Å. The 2 p38γ mutants were designed with the following amino acid substitutions: pLVXTP-3FLAG-p38γ mut_3M (3M-p38γ): contains 3-point mutations, Pro246Gly, Val 293Gly, and Leu 294Gly; pLVXTP-3FLAG-p38γ mut_3M-3m (3M-3m-p38γ): contains 6-point mutations: Pro246Gly, Val 293Gly, Leu 294Gly, Leu198Ala, Pro245Ala, and Phe249Ala. The mutant constructs were validated by Sanger sequencing (the supplemental Methods). (B) The WB analysis of 2 mutants targeting the lipid-binding domain of p38γ, which were designed based on the 6 amino acids depicted in panel A. The first mutant, 3M-p38γ, carries 3-point mutations (P246G-V293G-L294G) and functions as a dominant-negative p38γ in Hut 78 cells, referred to as Hut-3M. The second mutant, 3M-3m-p38γ, includes an additional 3 mutations (P246G-V293G-L294G) on top of the Hut-3M construct, creating a 6-site mutant expressed in Hut 78 cells. As a control, Hut-WT cells were derived from an empty lentiviral vector (pLVXTP) expressing WT p38γ – 3× Flag tags in Hut 78 cells. Flag indicates plasmid p38γ expression. Red brackets indicate reduced protein level upon treatment. (C) The 3 most druggable sites on p38γ were identified using the “all docking pose” method.1 Site 1: the LBS, in which CSH18/CN exhibits the highest binding affinity (MaxGScore of −10.9 kcal/mol). Site 2: the ATP-binding site, in which CSH18/CN also binds with a MaxGScore of −10.1 kcal/mol), as the second-preferentially site. Site 3: the N-terminal region above the ATP-binding site, with a MaxGScore of −9.2 kcal/mol. (D) Expression of LBS mutants of p38γ (3M-p38γ and 3M-3m-p38γ) affects cell proliferation. The p38γ-3M mutant is lethal upon transduction. However, a low-expression clone of 3M-p38γ, exhibiting reduced levels of both FLAG-tagged and endogenous p38γ, was successfully isolated. Cell proliferation was assessed using cell counting and trypan blue exclusion assays with an automated cell counter. The data indicate that disruption of the LBS on p38γ redirects CSH18CN to preferentially bind the ATP site, resulting in cell death. WT, wild-type.
Discussion
In 1991, Buck and Axel discovered a family of intronless genes encoding ORs on olfactory sensory neurons in rats, revealing that odor detection relies on a diverse array of receptor proteins tuned to specific molecular features.28,29 OR proteins, conserved across vertebrates, contain 7 transmembrane domains, highlighting their evolutionary significance. Recent research shows the human olfactory system is more complex and sensitive than previously thought, with ORs functioning beyond olfaction in nonolfactory tissues across species.30-34
Growing evidence links ORs to cancer. For example, OR2B6 is a potential breast cancer biomarker,35 and OR51E2 activation inhibits prostate cancer growth but promotes invasiveness.36,37 Some ORs, such as OR51B4, suppress tumors by inhibiting cell proliferation and promoting apoptosis,38 whereas OR2J3 activation induces apoptosis in non–small-cell lung cancer.39,40 Other ORs, such as OR2AT4,41,42 OR2L13,43 OR4N2,44 OR7A5,45 OR7C1,46,47 OR10H1,48 OR51B5,49,50 and OR51E151,52 have also been implicated in cancer regulation.
This study is, to our knowledge, the first to report small chemical molecules stimulating T cells via olfactory receptors in relation to p38. We demonstrated that OR responses to chemical stimulation in Hut78 cells (malignant T cells) lead to the induction of a unique set of ORs, including OR4N5, OR2A7, and OR6C68, by CSH71 (Figures 1C and 2A-B; supplemental Figure 1A). These ORs are present in the blood but significantly reduced in cancers (Table 3). Gene silencing of OR4N5 in CTCL cells promotes proliferation, suggesting that CSH71 exerts anticancer effects by increasing tumor suppressing ORs and their networks.
Among 800 ORs analyzed, only 2 (and a few pseudogenes) act as tumor inducers in 6 cancer types, whereas ∼780 ORs, including pseudogenes, are reduced. OR4N5, its 21 OR-interacting proteins, and 19 non-OR proteins are significantly downregulated in CTCL and other cancers (Tables 4 and 5). This suggests a coordinated interplay between ORs and cancer biology. OR4N5 and its associated proteins likely form a large complex affecting signaling pathways via protein-protein interactions, inhibiting cancer progression.
To investigate how OR4N5 inhibits T-cell proliferation, we examined its role in TCR signaling within healthy PBMCs. OR4N5 was found in close proximity to the TCR complex via CD3E (Figure 6A-B), independent of CSH71 treatment. In contrast, p38γ specifically interacted with CD3Z within the inner plasma membrane only in CSH71-treated healthy PBMCs (supplemental Figure 5A). These interactions were notably absent in malignant CTCL Hut78 cells, in which aberrant CD3 localization was observed (supplemental Figure 5A-B).
CD3 comprises CD3D, CD3E, CD3G, and CD3Z,53 with the Z chain containing immunoreceptor tyrosine-based activation motifs that serve as phosphorylation sites for lymphocyte-specific protein tyrosine kinase (LCK) and Feline Yes-related Novel protein, proto-oncogene, a Src family tyrosine kinase (FYN). Phosphorylated CD3Z recruits ZAP70, initiating TCR signaling and immune responses.29,53,54 ZAP70 interacts with p38γ, leading to alternative p38 activation via Tyr326 phosphorylation rather than the classical TxY motif.8 OR4N5 likely interacts with CD3E on the plasma membrane, whereas elevated p38γ interacts with CD3Z on the inner membrane in PBMCs of patients with SS but not in healthy PBMCs, in which p38γ is undetectable. Overexpression of p38γ in SS PBMCs enhanced proximity to OR4N5, independent of CSH71 treatment (Figure 5).
With reduced express of OR4N5 in CTCL, the aberrant CD3E are scattered in the cytosol and surrounds the nuclear envelope. Membranous OR4N5 no longer interacts with CD3E in CTCL cells, which sets TCR signaling transduction on the loose via depending on other mechanisms such as the DLGH1-p38γ–nuclear factor of activated T-cells 1 axis.
GPCR dimerization, crucial for signal transduction, also applies to ORs,15, including OR4N5. Dimerization plays a pivotal role in olfactory biology, as demonstrated in insect models.55 This molecular configuration is essential for the proper signal transduction and sensitivity enhancement of the olfactory system, reflecting a fundamental aspect of olfactory biology across species.15,56 We are validating OR4N5 dimerization in CTCL, in which its interaction with CD3E may inhibit TCR activation and T-cell proliferation.
In conclusion, we propose a novel mechanism in which targeting p38γ’s LBS influences ZAP70-mediated alternative p38 phosphorylation, disrupting OR4N5-CD3E interaction and redirecting TCR signaling. This highlights the tumor-suppressive role of ORs in CTCL, emphasizing their potential as therapeutic targets. Selective small molecules could upregulate specific OR groups to suppress distinct cancer types, advancing personalized cancer treatments. Mycosis fungoides, being confined to the skin, presents an opportunity to explore topical applications as a potential treatment strategy. Future research should focus on SS, particularly its systemic blood involvement, to better address its unique pathophysiology and therapeutic needs.
Acknowledgments
Research reported in this publication included work performed in City of Hope Cores (Integrative Genomics and Bioinformatics Core, High Throughput Screening Core, Drug Discovery and Structural Biology Core, and Mass Spectrometry and Proteomics Core) supported by the National Cancer Institute (NCI) of the National Institutes of Health under award number P30CA033572 and the Light Microscopy Core at City of Hope. Other support included National Institutes of Health NCI 1R01CA233922-01 (S.T.R.) and the Leukemia and Lymphoma Society grant identity 6576-19 (S.T.R.). Institutional review board: 15328, IACUC17118 (S.T.R.).
Authorship
Contribution: X.H.Z. contributed to conceptualization, design and investigation, database mining, writing the original draft, the reviewing, editing, and corresponding, initializing several key experiments, and part of immunofluorescence staining and confocal microscopy experiments; H.L. contributed to conceptualization of docking/Glide score generating; J.H. contributed to cell viability assays, immunoprecipitation (IP) and western blot experiments, and most of the immunofluorescence staining and confocal microscopy experiments; H.L. contributed to conceptualization of docking/Glide score generating; S.N. contributed to kinase activity assays; H.W. contributed to providing samples from patients with cutaneous T-cell lymphoma Sézary syndrome; X.W. contributed to microarray analysis; W.H. and J.S. contributed to nuclear magnetic resonance 1-dimensional and saturation transfer difference experiments and data analysis; C.H.C. contributed to nuclear magnetic resonance 2-dimensional Chemical Shift Pertubation (CSP) experiments and data analysis; and S.T.R. contributed to overseeing the laboratory.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xu Hannah Zhang, Department of Hematology and Hematopoietic Cell Transplantation, Beckman Research Institute, City of Hope National Medical Center, 1500 Duarte Rd, Duarte, CA 91010; email: xuzhang@coh.org; and Steven T. Rosen, Department of Hematology and Hematopoietic Cell Transplantation, City of Hope National Medical Center, Beckman Research Institute, 1500 Duarte Rd, Duarte, CA 91010; email: srosen@coh.org.
References
Author notes
The data reported in this article have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus database (accession number GSE292987).
Original data analysis, confocal microscopy data, and cell viability assays are available on request from the corresponding author, Xu Hannah Zhang (xuzhang@coh.org); p38 kinase activity assays are available on request from Sangkil Nam (snam@coh.org); and original CSH18 RNA microarray data are available on request from Xiwei Wu (xwu@coh.org) at Integrative Genomics Core. Other raw data may be found in a data supplement available with the online version of this article.
The full-text version of this article contains a data supplement.


![OR4N5 is significantly reduced in CTCL. (A) Expression of OR4N5 (magenta), p38γ (red), and CD3 (green) was measured by immunofluorescence confocal microscopy in CTCL PBMC samples compared with normal healthy donors. Most CTCL cells showed weaker CD3 positivity (green) than control healthy PBMCs. (B) p38γ levels were measured by WB analysis (top). OR4N5 was significantly reduced in CTCL PBMC samples (patient [P1] with SS is shown), with statistical analysis of magenta expression presented (bottom) using Zen Software. GAPDH, glyceraldehyde-3-phosphate dehydrogenase, HD, healthy donor; P, patient.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodneoplasia/2/4/10.1016_j.bneo.2025.100101/3/m_bneo_neo-2024-000458-gr3.jpeg?Expires=1767712679&Signature=KdrkXk8aHC1K1EI6TvMbj-J2WNPyU~qv2oKS0y4LPE2VSEfFqHuiWvToPX~4u2XG5e4zqH~7rA90d~fpyGwFOoBfQPZ5G1znyGmMWmMl3rBWvRZloRlFHxwog7zp2fE84XAqnMxD9wyx8VumXnna-6P9SqIrQpxSmwarq6U9YUfMbCJjDeAoETFSIQg~35A5wCvpKFN~AaiS1VhCrfWE6DbC8kP1io5XJ0Ap~v7noSzYSWqRFUvN8YyK9rF8iLaTIV0v~xVrOVMOE2-4hb2CEyYIZfS~rj8VKKghXOSHBiGOLapMfi-a6di-hcJbRZzAZs7kYoxY7b5yB6BBtAInKA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)