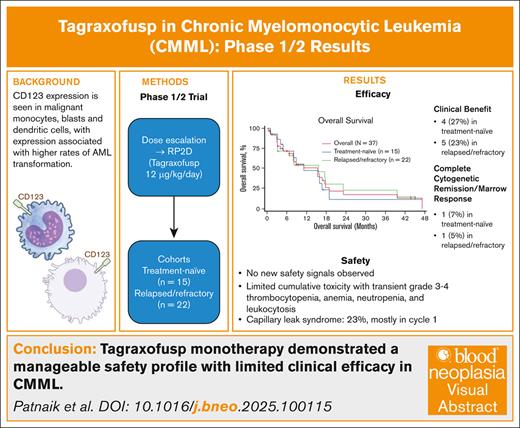
Key Points
In older patients with CMML, tagraxofusp monotherapy is tolerable without new safety signals, with modest clinical efficacy.
Although CD123-targeting monotherapy had limited efficacy, some patients derived benefit, and combination therapies are being explored.
Visual Abstract
Chronic myelomonocytic leukemia (CMML) is an aggressive hematologic neoplasm characterized by an expansion of CD123+ monocytes and plasmacytoid dendritic cells (pDCs). pDC bone marrow clusters in CMML have been associated with higher rates of acute myeloid leukemia transformation. We evaluated tagraxofusp, a CD123-targeted therapy, in a phase 1/2 trial for patients with CMML. There were no dose-limiting toxicities. At the recommended phase 2 dose of 12 μg/kg per day, 37 patients were treated: 15 were treatment naïve; 22 had relapsed/refractory disease (median number of previous therapies, 1 [range, 1-7]). Common nonhematologic treatment-emergent adverse events (AEs) included fatigue (49%), hypoalbuminemia (46%), nausea, hypokalemia, and decreased appetite (44% each). Capillary leak syndrome occurred in 9 patients (23%; grade 3-4, 13%), whereas tumor lysis syndrome was seen in 13%. Hematologic grade 3/4 treatment-related AEs included thrombocytopenia (28%), anemia (13%), leukocytosis (15%), and neutropenia (13%). No complete or partial responses were observed. One patient each in the treatment-naïve and relapsed/refractory groups achieved complete cytogenetic remission with marrow response. Stable disease and clinical benefit were achieved by 40% and 27% of treatment-naïve patients and by 59% and 23% of relapsed/refractory patients, respectively. After a median follow-up of 43.7 months, overall survival was 11.2 months in treatment-naïve and 15.6 months in relapsed/refractory patients. Exploratory analysis showed stable CD123+ blast frequency, mutational variant allele frequencies, and monocyte subsets with treatment. Tagraxofusp demonstrated a manageable safety profile with limited clinical efficacy in CMML. This trial was registered at www.ClinicalTrials.gov as #NCT02268253.
Introduction
Chronic myelomonocytic leukemia (CMML) is a disorder of clonal hematopoietic stem cells that exhibit overlapping characteristics of myelodysplastic syndromes (MDS) and myeloproliferative neoplasms (MPN).1 Patients with myelodysplastic CMML present with cytopenia(s), with symptoms such as fatigue, increased bleeding/bruising, and transfusion dependence, whereas those with myeloproliferative CMML (MP-CMML) present with leukocytosis, splenomegaly, and constitutional symptoms.1 The prognosis of CMML is poor, with a median overall survival (OS) of 15 to 32 months, with 15% to 30% transforming to acute myeloid leukemia (AML) in 3 to 5 years.1-3
Allogeneic hematopoietic stem cell transplant (allo-SCT) remains the only curative option for affected patients but is associated with significant morbidity and mortality, given that most patients with CMML are older (median age at diagnosis, 73 years) and have comorbidities.1,4 Hypomethylating agents (HMAs), such as azacitidine and decitabine, have been evaluated for CMML. These agents achieve a modest overall response rate ranging from 39% to 60% and complete response (CR) rates between 9% to 16%; however, their impact on mutational allele burdens is minimal.5-9 Cytoreductive agents such as hydroxyurea or cytarabine are used for palliation of symptoms (eg, leukocytosis and organomegaly) but they do not alter disease pathogenesis.7,10 Therefore, therapeutic strategies that selectively target mutant clones and offer sustained clinical benefit are much needed for this aggressive neoplasm.
Several lines of evidence support CD123 as a potential therapeutic target for patients with CMML, including CD123 expression on CMML leukemic stem cells.11 In a study of patients with CMML, CD123+ mononucleated cells infiltrating the bone marrow (BM) were identified as lineage-negative plasmacytoid dendritic cells (pDCs), an aberrant increase that was associated with regulatory T-cell accumulation and a greater risk of acute leukemic transformation.12 Clusters of cells with DC morphology have also been identified in 33% of patients with CMML, 25% at diagnosis and among patients who were negative at initial diagnosis, 14% occurred after progression while on HMAs, and 29% occurred at the time of AML transformation.13
CD123 expression in AML stem cells is associated with increased chemoresistance and has been linked to a lower likelihood of achieving a CR after induction chemotherapy.14,15 In high-risk MDS, CD123-targeted chimeric antigen receptor T-cell therapy eliminated MDS stem cells in vitro as well as in patient-derived xenografts.16 Tagraxofusp, a first-in-class CD123-targeted therapy, is a recombinant fusion protein consisting of human interleukin-3 conjugated to a truncated diphtheria toxin payload.17 Tagraxofusp is the only drug approved in the United States and the European Union to treat blastic plasmacytoid DC neoplasm,18 an aggressive hematologic malignancy characterized by cells expressing CD123 and other markers.
Because CD123 is overexpressed by CMML cells (clonal monocytes, blasts, and pDCs),19 there is a potential therapeutic window to target these clonal cells with a CD123-targeting agent, while minimizing toxicity to normal BM, including normal hematopoietic stem cells. Herein, we report results for patients with CMML within a phase 1/2 trial that aimed to determine the safety and antitumor activity of tagraxofusp.
Methods
Study design
This nonrandomized, multicenter, open-label trial evaluated tagraxofusp monotherapy in patients with myelofibrosis or CMML in 3 stages. Stage 1 followed a conventional 3+3 design, with escalating doses of tagraxofusp administered to identify the recommended phase 2 dose (RP2D). After RP2D selection, stage 2 dose expansion was undertaken. During the course of stage 2, the MDS/MPN 2015 criteria became available and represented the updated consensus criteria for evaluating clinical benefit in patients with MDS/MPN.20 Stage 3A was thus initiated to evaluate individual components of the MDS/MPN 2015 criteria in patients with CMML to enable a prospective definition of a primary efficacy end point specific to CMML. Results from the myelofibrosis population are reported separately.
The study was conducted according to the Declaration of Helsinki and good clinical practice guidelines. The institutional review board of each participating center approved the protocol. All patients provided written informed consent before study entry. The trial was registered at ClinicalTrials.gov (identifier: NCT02268253).
Participants
Eligible patients were adults (≥18 years of age) with CMML-1 or CMML-2 as defined by the World Health Organization 2016 classification (revised fourth edition)21 and were either (1) treatment-naïve with molecular features associated with a poor prognosis; or (2) refractory/resistant/intolerant to HMAs, hydroxyurea, or intensive chemotherapy. In the relapsed/refractory group, resistance/intolerance to hydroxyurea was defined as uncontrolled myeloproliferation (platelets >400 × 109/L and white blood count [WBC] of >10 × 109/L after 3 months of at least 2 g/d of hydroxyurea); myelosuppression at a clinically relevant dose; or presence of unacceptable hydroxyurea-related nonhematologic toxicities. HMA failure was defined as disease progression after at least 4 to 6 cycles of azacitidine or decitabine; relapse after achieving response; or intolerance to azacitidine or decitabine at the prescribed dose. Patients who were treatment naïve were eligible if high risk based on the presence of morphological features (2016 World Health Organization prognostic system),21 and the clinical and molecular features described by Groupe Français des Myélodysplasies, the Mayo Molecular Model, or the CMML-specific prognostic scoring model (CPSS-Mol).22,23 Key exclusion criteria included persistent and unmitigated grade ≥2 toxicities from previous therapies, disease-related therapy or other investigational therapy within 14 days of study entry, allo-SCT within 3 months of study entry, and immunosuppressive therapy. Full eligibility is included in the supplemental Appendix.
Treatment
In stage 1, patients received 7, 9, or 12 μg/kg tagraxofusp via IV infusion on days 1 through 3 every 21 days for cycles 1 through 4, every 28 days for cycles 5 through 7, and every 42 days for cycles 8 and beyond. After the dose escalation phase, all patients were treated at the RP2D (12 μg/kg) via IV infusion on days 1 to 3 of a 21-day cycle for cycles 1 through 4, and then on days 1 through 3 of a 28-day cycle for cycle 5. Dose delays of up to day 10 of each cycle were allowed. Hospitalization was required for the first cycle of tagraxofusp starting on the day of infusion (or the previous day) and ending ∼24 hours after the last infusion. Subsequent cycles could be administered in outpatient settings. Treatment was continued in patients with evidence of ongoing disease control without intolerable toxicity even if an overall response was not attained.
Outcomes
The primary objectives of stage 1 were to determine the maximum tolerated dose or the maximum tested dose at which multiple dose-limiting toxicities (DLTs) were not observed for tagraxofusp monotherapy and to characterize tagraxofusp’s safety profile. See supplemental Appendix for DLT definitions. The objectives of stage 2 were to assess preliminary evidence of efficacy and to further characterize the safety profile of tagraxofusp at the selected dose. The objectives of stage 3A were to characterize the safety profile of tagraxofusp and obtain preliminary evidence of response. The primary efficacy end point was investigator-assessed overall response rate; secondary efficacy end points included OS and duration of follow-up. An additional objective of stage 3A was to evaluate molecular mechanisms and the relationship between CD123 expression and clinical response (methods of the exploratory translational analysis are in the supplemental Appendix).
Stages 1 and 2 of the study used the 2006 International Working Group MDS criteria24 for response assessment, and stage 3A used the 2015 MDS/MPN response criteria.20 As a result, different response criteria were used within the study for patients receiving tagraxofusp at the RP2D. Hence, an algorithmically derived response assessment was performed for consistency for all patients who received the RP2D by using the modified 2015 MDS/MPN response criteria.
A limitation of the 2015 MDS/MPN response criteria is the absence of a stable disease (SD) category. For the purpose of this trial, and as has been used previously in other CMML studies,5 SD was included when assessing responses and was defined as patients who did not meet any criteria, including CR, complete cytogenetic remission (CCyR), partial remission, marrow response, or clinical benefit, and did not meet the criteria for disease progression. Details on response assessment and criteria are provided in the supplemental Appendix.
Statistical analysis
All patients who received ≥1 dose of tagraxofusp were included in the safety analysis. Efficacy-evaluable patients included a modified intent-to-treat population consisting of patients who received ≥1 dose of tagraxofusp 12 μg/kg per day and had an efficacy assessment after the first treatment or discontinued due to death or progressive disease. Patient characteristics and responses were summarized using frequency and percentage for categorical data and median and range for quantitative data. Time-to-event outcomes were summarized using Kaplan-Meier analysis and reported as median with 95% confidence intervals (CIs). The sample size ensured a power of at least 80% for clinical activity assessment. Statistical analyses were performed using SAS statistical software, version 9.4.
Results
Patient disposition
A total of 42 patients received at least 1 dose of tagraxofusp (1 at 7 μg/kg per day, 2 at 9 μg/kg per day, and 39 at 12 μg/kg per day). Stage 1 dose escalation did not reveal any DLTs at any dose; hence, 12 μg/kg was the maximum tested dose and selected as the RP2D. All 39 patients who received 12 μg/kg per day were included in the safety population, and 37 of whom were efficacy evaluable.
The median duration of therapy was 75 days (range, 2-464), with a median of 4 cycles (range, 1-15) in patients treated at 12 μg/kg (supplemental Figure 1). As of the data cutoff, all patients have discontinued treatment. The primary reasons for treatment discontinuation were disease progression (59%) and adverse events (AEs; 21%).
Patient characteristics
Fifteen patients were treatment naïve, with a median age of 64 years (range, 48-82), 20% were female, and 8 had MP-CMML. Among 22 patients with relapsed/refractory disease, the median age was 70.5 years (range, 60-87), 32% were female, and 10 had MP-CMML (Table 1). Compared to the relapsed/refractory patients, more treatment-naïve patients had high (47% vs 18%) and intermediate (27% vs 14%) cytogenetic risk, as well as high CPSS-Mol risk (33% vs 18%). Relapsed/refractory patients were treated with a median of 1 (range, 1-7) previous line of therapy, most of them having received HMA (73%) or hydroxycarbamide/hydroxyurea (32%).
Safety
Commonly reported treatment-emergent AEs (TEAEs) in patients in the safety population who received tagraxofusp 12 μg/kg per day (n = 39) are summarized in Table 2. Of the hematologic TEAEs, treatment-related AEs (TRAEs) included thrombocytopenia in 12 patients (31%; grade 3-4, 11 [28%]), anemia in 9 (23%; grade 3-4, 5 [13%]), leukocytosis in 5 (13%; grade 3-4, 2 [5%]), and neutropenia in 4 (10%; grade 3-4, 4 [10%]). An additional patient had neutropenia, not considered treatment related. There were 5 grade 5 TEAEs (none related to tagraxofusp), including lung infection (2 patients), and acute respiratory failure, cerebral infarction, and cerebrovascular accident (1 patient each).
Capillary leak syndrome (CLS) was reported in 9 patients (23%); 4 (10%) had a grade 1/2 event, 5 (13%) had a grade 3/4 event, and no grade 5 events occurred. Seven of these patients had CLS events during cycle 1 (grade 1/2, n = 4; grade 3, n = 3) at a median of 4 days (range, 2-7). Two patients had CLS after cycle 1, with a median time to onset of 58 days (range, 26-126): 1 had grade 3 CLS at cycle 2, and another had recurring grade 4 CLS (1 event at cycle 3 and 1 event at cycle 4). CLS was managed with supportive care as previously described.25 CLS events resolved in 7 patients, with a median time to resolution of 8 days (range, 2-11) or 12.5 days (range, 4-21), when CLS onset was at cycle 1 or after cycle 1, respectively. Four patients with CLS required dose interruption and were able to restart tagraxofusp at the same dose after a median of 6.5 days (range, 2-11). In 2 patients, CLS led to treatment discontinuation (1 grade 3 and 1 grade 4, each).
Tumor lysis syndrome (TLS) occurred in 5 patients (13%); of these, 1 (3%) had a grade 1/2 event, and 4 (10%) had a grade 3/4 event. All these patients had hyperleukocytosis at TLS onset. Ten patients (26%) had acute kidney injury, with grade 3/4 in 3 (7.7%) patients. Notably, 2 of these patients had a history of chronic kidney disease and had creatinine levels increased at study entry. Acute kidney injury considered treatment-related was reported in 4 (10.3%) patients (all grade 1-2, except 1 grade 3); of these, 2 had concomitant serum creatinine increases. Serum creatinine increase was reported in 9 patients (23% of all patients) and was considered a TRAE in 4, including 1 patient whose AE was grade 3.
Importantly, TEAEs led to treatment discontinuation in 9 patients (23%); 3 TRAEs lead to discontinuation: CLS (n = 2; 1 with concomitant TLS) and pancreatitis (n = 1). Sixteen patients (41%) required dose interruption; TRAEs were attributable in 13. Most common TRAEs leading to treatment discontinuation were hypoalbuminemia (5 patients), CLS (4 patients), and weight increase and pyrexia (3 patients each). One patient required dose reduction because of dyspnea.
Efficacy
In the 15 treatment-naïve patients, the duration of follow-up for response assessment ranged from 1 to 47.2 months (median, not estimable). No patients achieved complete or partial response; 1 patient achieved CCyR and marrow response after 1 cycle of tagraxofusp; and 6 (40%) had SD (all lasting for >8 weeks) as best response (Table 3). Additionally, 4 patients (27%) achieved any clinical benefit according to the 2015 MDS/MPN criteria,20 including 3 hematologic responses and 1 symptom response (supplemental Figure 1).
In 22 patients with relapsed/refractory disease, after a median follow-up of 43.7 months (range, 0.4-45), no patients achieved complete or partial response; 1 patient achieved CCyR and marrow response after 1 cycle of tagraxofusp; and 13 (59%) achieved SD as best response (lasting >8 weeks in 7 patients; Table 3). Two patients (9%) were bridged to allo-SCT after achieving SD. An additional 5 patients (23%) achieved any clinical benefit, including 4 hematologic responses (1 had platelet and spleen response) and 1 spleen response (supplemental Figure 1).
The 2 efficacy-evaluable patients with MP-CMML had a total symptom score reduction, and 1 (25%) of 4 patients with splenomegaly at baseline had a spleen response (see supplemental Appendix).
No patient was red blood cell (RBC) or platelet transfusion-dependent at baseline. For treatment-naïve patients, 11 (73%) became RBC transfusion-dependent, and 7 (47%) became platelet-transfusion dependent (for any 8-week period). Eleven (73%) patients achieved a ≥50% reduction in absolute monocyte count, including 4 (27%) who achieved normalization. Five patients (33%) achieved a ≥50% reduction in WBCs, including 6 (40%) who achieved normalization. Six patients (40%) achieved a ≥50% reduction in BM blasts. Among 10 patients who presented with ≥5% blasts at baseline, 3 (30%) achieved a reduction to <5% with treatment.
One treatment naïve (7%) and 4 (18%) relapsed/refractory patients had AML transformation during tagraxofusp treatment.
Among patients with relapsed/refractory disease, 16 (73%) became RBC transfusion-dependent and 15 (68%) became platelet transfusion-dependent (for any 8-week period). Thirteen patients (59%) achieved a ≥50% reduction in absolute monocyte count, including 4 (18%) who achieved normalization. Nine patients (41%) had a ≥50% reduction in WBCs, including 2 (9%) who achieved normalization. Two (9%) patients had a ≥50% reduction in BM blasts. Among 9 patients with >5% blasts at baseline, 2 (22%) achieved a reduction to <5% with treatment.
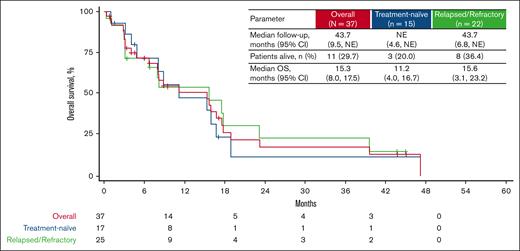
The median OS in those with treatment-naïve disease was 11.2 months (95% CI, 4.0-16.7) after a follow-up ranging from 1 to 47 months. In patients with relapsed/refractory disease, after a median follow-up of 43.7 months (range, 0.4-45.0), the median OS was 15.6 months (95% CI, 3.1-23.2; Figure 1). Twelve (80%) treatment-naïve and 14 (64%) relapsed/refractory patients died while on study (supplemental Table 1).
Kaplan-Meier analysis of OS for treatment-naïve and relapsed/refractory patients with CMML receiving tagraxofusp 12 μg/kg per day. NE, not estimable.
Kaplan-Meier analysis of OS for treatment-naïve and relapsed/refractory patients with CMML receiving tagraxofusp 12 μg/kg per day. NE, not estimable.
Exploratory translational analysis
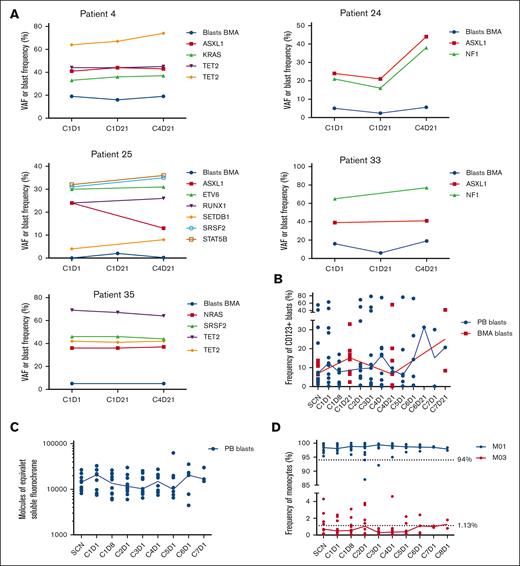
Variant allele frequencies (VAFs) of specific gene mutations associated with CMML were monitored in 5 patients. During 4 treatment cycles, VAFs remained stable in 4 of 5 patients, with a median VAF of all mutations of 34.5% (range, 4%-69%) on cycle 1 day 1, and 39.5% (range, 8%-77%) on cycle 4 day 21 (Figure 2A). Conversely, 1 patient exhibited an approximately twofold increase in VAF for 2 mutations (ASXL1, 21%-44%; and NF1, 21%-38%).
Longitudinal analysis of mutational variant allele frequencies, proportions of CD123+ blasts, expression patterns of CD123 blasts and proportions of monocyte subsets in CMML patients enrolled on the clinical trial. (A) Frequency of BMA blasts and VAF of specific gene mutations associated with CMML in 5 patients receiving tagraxofusp 12 μg/kg over 4 treatment cycles. (B) Median (represented by solid line) frequency of CD123+ blasts assessed by flow cytometry in the PB and BMAs of 13 patients with CMML receiving tagraxofusp 12 μg/kg per day. (C) Relative expression of CD123 on the surface of blasts in the PB during treatment (line represents median, n = 13). (D) Frequency of monocyte subsets (MO1 and MO3 monocytes) over the course of treatment (solid line represents median, n = 13). BMA, BM aspirate; C1D1, cycle 1, day 1; PB, peripheral blood; VAF, variant allele frequency.
Longitudinal analysis of mutational variant allele frequencies, proportions of CD123+ blasts, expression patterns of CD123 blasts and proportions of monocyte subsets in CMML patients enrolled on the clinical trial. (A) Frequency of BMA blasts and VAF of specific gene mutations associated with CMML in 5 patients receiving tagraxofusp 12 μg/kg over 4 treatment cycles. (B) Median (represented by solid line) frequency of CD123+ blasts assessed by flow cytometry in the PB and BMAs of 13 patients with CMML receiving tagraxofusp 12 μg/kg per day. (C) Relative expression of CD123 on the surface of blasts in the PB during treatment (line represents median, n = 13). (D) Frequency of monocyte subsets (MO1 and MO3 monocytes) over the course of treatment (solid line represents median, n = 13). BMA, BM aspirate; C1D1, cycle 1, day 1; PB, peripheral blood; VAF, variant allele frequency.
The median frequency of CD123+ blasts in the peripheral blood and BM aspirate as well as monocyte subsets were monitored in 13 patients. Although the frequency of CD123+ blasts varied over the course of treatment, minimal reduction in the frequency of CD123+ blasts was observed over time, with limited data beyond cycle 6 (Figure 2B). In addition, there was no observed loss of CD123 expression on the surface of peripheral blood blasts during treatment (Figure 2C). Frequencies of classical (MO1) and nonclassical (MO3) monocytes were consistent with CMML disease and did not appear to change over the course of treatment (Figure 2D).
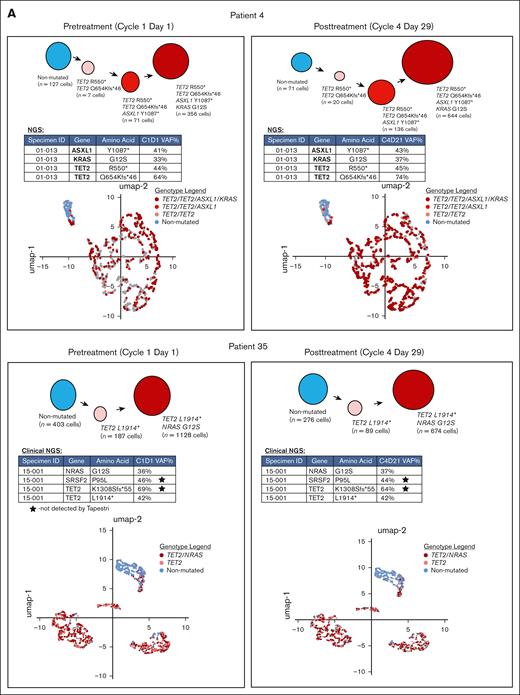
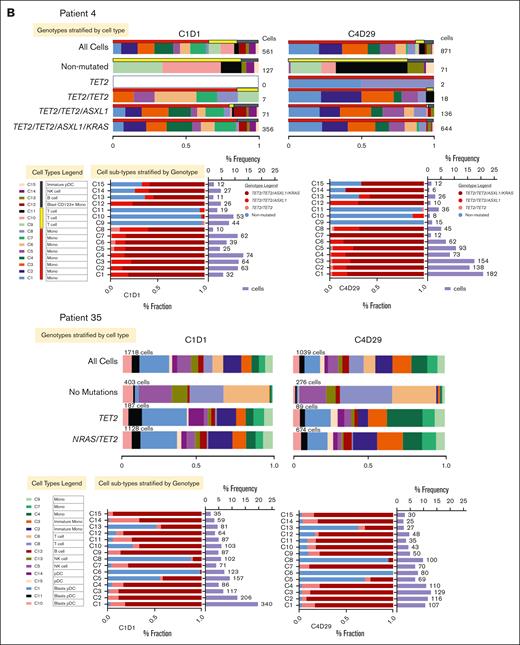
Two patients whose best response was SD were selected for single-cell proteogenomic analysis (Mission Bio Tapestri platform).25 Single-cell assessments were carried out on peripheral blood samples obtained before therapy on cycle 1 day 1 and cycle 4 day 29. The clinical information of these patients is summarized in supplemental Table 2. There was no change in the mutational spectrum or VAFs in either patient when comparing pretherapy with posttherapy samples. The clonal architecture was also not affected by tagraxofusp treatment (Figure 3A).
We also assessed the distribution of individual cell types in the 2 patients with SD as best response by surface marker expression. Overall, there was an increase in the percentage of T cells in the unmutated cells from 1 patient but not the other (Figure 3B). Otherwise, there were no significant differences in the phenotypes of cell types across the 2 time points. Individual cell subtype surface marker expression distribution (normalized using the centered log ratio) for CD123 across the different cell types was also not significantly different (supplemental Figure 2). When CD123+ cell types were assessed in detail, the only significant difference was the decrease in CD56 expression in one of the patients (supplemental Figure 2). Expression of CD303, a more specific marker for pDCs,26 was also not significantly different at either time point (supplemental Figure 3). Similarly, when CD3+ T-cell types were assessed in detail, there were no significant differences between pretherapy and posttherapy in either patient (supplemental Figure 4).
Discussion
In this study of patients with CMML, no DLTs were observed at the 3 dose levels tested. Tagraxofusp demonstrated a manageable safety profile at the selected dose of 12 μg/kg, with no new safety signals observed, establishing the safety of tagraxofusp in an older patient population in which comorbidities are common. Despite the incidence of transient grade 3/4 thrombocytopenia, anemia, neutropenia, and leukocytosis, cumulative hematologic toxicity was limited. CLS, an AE of special interest, was reported in 23% of patients, with most events occurring in cycle 1 and most recovering rapidly, with a median time to resolution of 8 to 12.5 days after early intervention strategies, as previously described.27
The incidence of TLS (13%) warrants TL prophylaxis in patients with hyperleukocytosis. Moreover, patients with preexisting kidney disease would benefit from close monitoring and renal risk factor management.
Overall, the clinical activity of tagraxofusp in CMML was modest. Among treatment-naïve patients, optimal marrow response with CCyR was observed in 1 patient (7%), with any clinical benefit in 4 patients (27%). Similarly, among relapsed/refractory patients, optimal marrow response with CCyR was observed in 1 patient (5%), with any clinical benefit in 5 patients (23%). Despite the limited number of patients with MP-CMML evaluable for symptoms and spleen response, tagraxofusp monotherapy led to total symptom score reduction in both evaluable patients and to spleen response in 1 (25%) of 4 patients with splenomegaly at baseline. This suggests a potential benefit in MP-CMML, a CMML population with characteristics more similar to MPN than MDS, higher symptom burdens, higher CPSS risk scores, and shorter OS.28
The median OS was 15.3 months in the total population (95% CI, 8.0-17.5), and 11.2 months (95% CI, 4.0-16.7) in treatment-naïve patients, and 15.6 months (95% CI, 3.1-23.2) in relapsed/refractory patients. The longer OS in patients with relapsed/refractory disease than those with treatment-naïve patients may be, in part, because of the eligibility criteria and demographics of the populations. Treatment-naïve patients had molecular features associated with a poor prognosis (based on study inclusion criteria). Indeed, a higher frequency of patients with high or intermediate-2 CPSS-Mol was found in the treatment-naïve vs the relapsed/refractory group (80% vs 59%). Furthermore, ASXL1, NRAS, NF1, and TET2 mutations were observed in 4 (27%), 2 (13%), 1 (7%), and 1 (7%) treatment-naïve patients, respectively, vs 5 (23%), 2 (9%), 0 (0%), and 4 (18%) patients with relapsed/refractory disease, respectively.
Because CMML has overlapping features with MDS and MPN, few clinical trials include a CMML-only population, and treatment paradigms are adopted from studies in which most patients had MDS.29 This trial was designed to evaluate tagraxofusp specifically in patients with CMML. Although our sample size was 39 patients, it was sufficient to achieve meaningful results in this rare, orphan disease. Indeed, trials of single-agent HMAs have consistently shown benefits in mixed populations of treatment-naïve and relapsed/refractory CMML, with CR rates of 9% to 16%, marrow response rates of 27% to 37%, CCyR of up to 25%, and a median OS of 18 to 37 months.5,7,8 Notably, although symptoms and spleen responses were not prospectively evaluated in these trials, rates of responses after single-agent HMAs were reported to be between 15% and 33%, which compare similarly to response rates reported herein with tagraxofusp in patients with MP-CMML. Thus, MP-CMML may represent a specific target population in which tagraxofusp may be used in combinatorial regimens in the future.
Tagraxofusp did not reduce the CD123+ BM blast percentages in treated patients. We also did not observe changes in mutational allele burdens; clonal architecture; and MO1 (classical) and MO3 (nonclassical) monocyte partitions by flow cytometry, with the latter test having been shown to be a potential biomarker of HMA response in CMML. CD123+ pDCs have been associated with disease development in CMML.12,13 In our analysis, CD123-targeted monotherapy did not demonstrate any significant differences in clonal or cellular architecture in CMML. Because only a small subset of patients (those in stage 3A of enrollment) were included in the assessment of CD123+ blasts, monocyte partition, mutational burden, and clonal architecture, findings of these translational analyses should be interpreted with caution. However, translational studies conducted to investigate molecular mechanisms of response or nonresponse may serve as proof of principle that this approach can be applied more widely in clinical trials of rare cancers, in which developing effective therapies has traditionally been difficult.
Previously, only pDC aggregates were thought to alter disease biology. However, recent data suggest that these cell aggregates are heterogeneous and include other cell types (eg, myeloid DCs, monocytes, and myeloid-derived suppressor cells, among others).13 The mechanism through which DC aggregates alter disease biology is still unclear, but several mechanisms appear to be involved. Among these, DC aggregates have been shown to be associated with an immune checkpoint (IDO [indoleamine 2,3-dioxygenase]) and changes in the T-cell compartment such as regulatory T-cell expansion, potentially indicating a critical role in disease progression through immune evasion.13,30 This complex interaction among various cell types and mechanisms may explain why targeting CD123+ components alone is insufficient to change disease biology, underscoring the need for combination therapies.
Studies are underway to evaluate various combination regimens, and preliminary evidence suggests improved clinical response with novel regimens (44%-55% CR, and 51% marrow response in trials of lenzilumab + azacitidine31 and venetoclax + decitabine + cedazuridine).32 It is also possible that combination therapies targeting both pDCs and IDO could be synergistic in altering CMML disease biology by halting critical DC–T-cell interactions. Tagraxofusp may represent a rational backbone on which to develop combination regimens, and the combination of tagraxofusp with the HMA decitabine is currently under investigation in a phase 1/2 trial in patients with CMML (ClinicalTrials.gov identifier: NCT05038592).33
In conclusion, we demonstrated that tagraxofusp has a tolerable safety profile in an older patient population with CMML without new safety signals. Tagraxofusp monotherapy has modest clinical activity in CMML. We observed a limited impact on clonal architecture and cellular microenvironment with tagraxofusp monotherapy in CMML. Future research should further characterize the complex DC aggregate architecture and immune cell-cell interactions within the CMML BM microenvironment at various disease stages.
Acknowledgments
The authors thank the study participants, investigators, and site staff for making this study possible. Medical writing and editing assistance were provided by Annie Cheang and Claire Gilmore of Phillips Group Oncology Communications, Inc, under the direction of the authors, and was funded by Menarini Group, New York, NY.
The study was funded by Menarini Group.
Authorship
Contribution: M.M.P. and G.G.-M. contributed to the conception and design of the study; M.M.P. and H.A. led the study; R.L. and A.G. analyzed the data; and all authors acquired the data, contributed to data interpretation, manuscript writing, editing, and content review, and approved the final draft of the manuscript.
Conflicts-of-interest disclosure: M.M.P. reports research funding from Epigenetix, Kura Oncology, Polaris, Solu Therapeutics, and Stemline Therapeutics; and a consulting/advisory role with CTI Pharmaceuticals and AstraZeneca. H.A. reports research funding from Incyte; consulting/advisory roles with GlaxoSmithKline (GSK), Incyte, Karyopharm, and PharmaEssentia; honoraria from GSK, Incyte, PharmaEssentia, and Sobi; and travel accommodations/expenses from Incyte and Karyopharm. E.S.W. reports advisory/consulting roles with AbbVie, Blueprint, Daiichi Sankyo, Immunogen, Kite, Kura Oncology, Novartis, Qiagen, Rigel, Ryvu, Schrödinger, Servier, Stemline Therapeutics, Syndax, and Takeda; speaking role with Astellas Pharma, Dava, and Pfizer; participation on data and safety monitoring board (DSMB) with AbbVie and Gilead; and serving as a section editor for UpToDate. A.Y. reports consulting/advisory roles with AbbVie, Acceleron Pharma, Apellis, Blueprint Medicines, CTI Pharmaceuticals, Gilead, Incyte, Karyopharm, Notable Labs, Novartis, Pfizer, PharmaEssentia, Protagonist, Therapeutics Inc, and Servier. J.M.F. reports research funding from Actinium, Astellas Pharma, Celgene, Chorida, Kura Oncology, Novartis, Pfizer, Roivant, Sellas, Servier, Stemline Therapeutics, and Takeda; consulting/advisory roles with Autolus, Bristol Myers Squibb (BMS), Lava Therapeutics, Remix, and Syndax; and honoraria for educational events from Aptitude Health, IntrinsiQ Specialty Solutions, and MJH Life Sciences. V.G. reports research funding from AbbVie (payments made to the institution); consulting/advisory roles with AbbVie, BMS Celgene, Daiichi Sankyo, GSK, and Pfizer; honoraria from BMS Celgene, GSK, and Novartis; participation on DSMB or advisory board for Daiichi Sankyo and GSK; and reports stock ownership with Syndax. G.J.S. reports research funding from AbbVie, Actinium, Actuate, Agios, Arog, Astellas Pharma, AlloVir, Amgen, Aptevo, AltruBio, AVM Biotechnology, BMS/Celgene, BioPath, Biomea, Biosight, Cellularity, Celator, Constellation, Cogent, Cellectis, Cullinan, Daiichi Sankyo, Deciphera, Delta-Fly, Fate, Forma, Fujifilm, Gamida Cell, Genentech/Roche, GlycoMimetics, Geron, Gilead, Incyte, Janssen, Jazz, Karyopharm, Kite/Gilead, Kronos Bio, Kura Oncology, Immunogen, lmmune-Onc, Loxo, Marker, Mateon, Novartis, Onconova, Ono Pharma UK, Orca, Pfizer, PrECOG, REGiMMUNE, Rigel, Samus, Sangamo, Sellas, Stemline Therapeutics, Syros, Takeda, Tolero, and Trovagene; reports consulting/advisory roles from BMS, Curios, Daiichi Sankyo, and Novartis; reports honoraria on the speakers bureau for AbbVie, Agios, Amgen, Astellas Pharma, Blueprint Medicines, BMS Celgene, Karyopharm, GSK, Kite (Gilead), Jazz, Rigel, Seattle Genetics, and Stemline Therapeutics; serves as a board or advisory committee member for Agios, Autolus, AVM Biotechnology, BMS, Gamida Cell, Gilead, GSK, Incyte, Novartis, Orca, Rigel, and Stemline Therapeutics; and holds stock in Amgen, BMS, and Janssen/Johnson & Johnson. M.T. reports participation on the Sumitomo advisory board and research grant from BMS. S.W. reports a spouse's employment and stock ownership with Cardinal Health; reports consulting/advisory roles with AbbVie, BMS, and MorphoSys; received honoraria from CTI BioPharma/Sobi; and reports travel accommodations/expenses from Sobi. T.L.L. reports travel accommodations/expenses from Agilent Technologies. A.A.M. reports research funding from BMS, Novartis, and Stemline Therapeutics; and participation on a DSMB for Mayo Clinic Cancer Center. T.B. reports consulting/advisory roles with MorphoSys, Pfizer, and Takeda; honoraria from Takeda; and research funding from MorphoSys. R.L., A.G., and I.G. report employment with Menarini Group. N.P. reports research grant from the US Department of Defense and the National Institutes of Health/National Cancer Institute; serves as a consultant/scientific adviser board member/speaker for AbbVie, Aplastic Anemia and MDS International Foundation, Aptitude Health, Astellas Pharma US, Blueprint Medicines, BMS, Cancer.Net, CareDx, Celgene, Cimeio Therapeutics AG, ClearView Healthcare Partners, CTI BioPharma, Curio Science, Dava Oncology, EUSA Pharma, Harborside Press, Imedex, Immunogen, Intellisphere, Karyopharm, Magdalen Medical Publishing, Medscape, Menarini Group, MorphoSys, Neopharm, Novartis Pharmaceuticals, OncLive, Pacylex, Patient Power, PeerView Institute for Medical Education, PharmaEssentia, and Physician Education Resource; serves on the board of directors/management with Dan's House of Hope; reports a leadership role with the American Society of Hematology committee on communications and the American Society of Clinical Oncology Cancer.Net editorial board; and reports licenses with Karger Publishers. The remaining authors declare no competing financial interests.
Correspondence: Mrinal M. Patnaik, Hematology, Mayo Clinic, 2100 First St SW, Rochester, MN 55905; email: patnaik.mrinal@mayo.edu.
References
Author notes
M.M.P. and H.A. contributed equally to this study.
The data that support the results of this study may be requested for products and the relevant indications that have been authorized by the regulatory authorities in Europe/the United States (or, if not, after 2 years have elapsed since the study completion). The Menarini Group will review requests individually to determine whether (1) the requests are legitimate and relevant and meet sound scientific research principles, (2) the requests are within the scope of the participants’ informed consent, and (3) the request is compliant with any applicable law and regulation and with any contractual relationship that Menarini Group and its affiliates and partners have in place with respect to the study and/or the relevant product. Before making data available, requestors will be required to agree in writing to certain obligations, including, without limitation, compliance with applicable privacy and other laws and regulations. Proposals should be directed to medicalinformation@menarinistemline.com.
The full-text version of this article contains a data supplement.