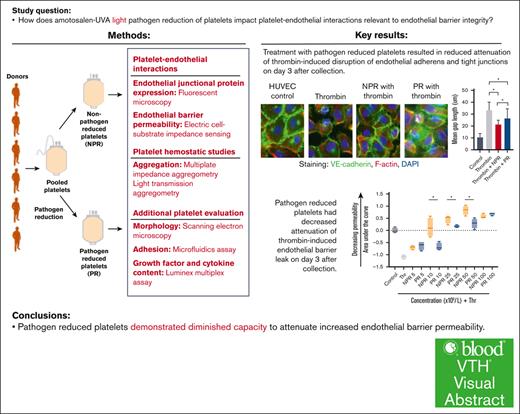
Key Points
PR platelets demonstrated diminished capacity to attenuate increased endothelial barrier permeability.
Visual Abstract
Platelet transfusion not only attenuates bleeding and promotes hemostasis but also plays a critical role in vascular stability and endothelial barrier integrity. Under amotosalen-UVA pathogen reduction of platelets, pathogen nucleic acids undergo adduction, which prevents their replication and greatly reduces the risk of transfusion-transmitted infections. Although pathogen-reduced (PR) platelets are increasing in clinical use, the physiologic effects of pathogen reduction on platelets, particularly its impact on platelet-endothelial interactions, have yet to be described. This study compared PR platelets with nonpathogen-reduced (NPR) platelets in measures of effect on endothelial permeability in vitro. We hypothesized that PR platelets would be similar to NPR platelets. However, in endothelial cell immunohistochemistry and impedance assays, PR platelets demonstrated a significantly diminished capacity to attenuate endothelial barrier permeability at early storage time points. This small but significant difference requires further mechanistic and clinical study to understand its implications, particularly in patients with bleeding with vascular fragility.
Introduction
In both health and disease, platelets are critical regulators of hemostasis and vascular stability.1,2 Platelet transfusion is important, not only to attenuate bleeding and promote clotting but also as prophylaxis against hemorrhage in patients with thrombocytopenia.2,3 Furthermore, through direct platelet-endothelial interactions and soluble mediators, platelets play an essential role in protecting patients from vascular fragility.3-8
Under standard blood bank practices, apheresis platelets are stored at room temperature (22°C) with gentle agitation for 5 days. Platelet storage may be extended to 7 days in specialized containers and with negative secondary testing for bacteria.9,10 Storage at room temperature optimizes yield and circulation time of transfused platelets compared with cold (4°C) storage.9-11 However, room temperature storage increases the risk of complications including bacterial infection from pathogen contamination.12 The risk of bacterial contamination under current practices is estimated at 1 per 1000 to 1 per 2500 units.13 Furthermore, because many patients requiring platelet transfusion receive multiple units, the average per-patient risk is estimated at 1 per 250 of receiving a contaminated platelet transfusion and 1 per 1000 of developing sepsis from a platelet transfusion.14
Pathogen reduction is 1 way to mitigate the risk of pathogen contamination. Under pathogen reduction, nucleic acids of bacteria, viruses, and parasites undergo irreversible modification, thereby preventing replication.11,15-17 Current systems developed for pathogen reduction of blood products include INTERCEPT (Cerus Corporation, Concord, CA), Mirasol (Terumo BCT Inc, Lakewood, CO), and THERAFLEX (MacoPharma, Mouveux, France). Although all 3 systems disrupt nucleic acids to prevent pathogen replication, the mechanisms vary. INTERCEPT generates nucleic acid adduct formation through amotosalen-UVA light, Mirasol uses riboflavin-UV light for nucleic acid disruption, and THERAFLEX uses UVC light without the addition of a photosensitizing agent.18 Although these technologies likely do not have equivalent impacts on platelets, all 3 have shown a significant reduction in bacteria in platelet units after treatment.19 As a result, pathogen reduction may also increase storage times, which could improve platelet availability and inventory management. Currently, INTERCEPT is the only technology licensed for platelets up to 5 days of storage in the United States and up to 7 days of storage in France and Switzerland. Studies are ongoing to determine the viability of further extended storage durations.20,21
Despite the clear benefits of pathogen reduction, there is still much to learn about its impact on platelets, particularly its effect on platelet–endothelial cell interactions. In this study, we aim to characterize the in vitro functional differences between amotosalen-UVA pathogen-reduced (PR) platelets and nonpathogen reduced (NPR) platelets. These studies focus on platelet impact on endothelial barrier integrity with additional investigation of hemostatic properties, morphology, and platelet granule content. We hypothesize that PR platelets will have similar protective effects on endothelial permeability, as well as similar hemostatic potential, similar morphology, and similar growth factor and cytokine content compared with NPR platelets.
Methods
Preparation of human NPR and PR platelets
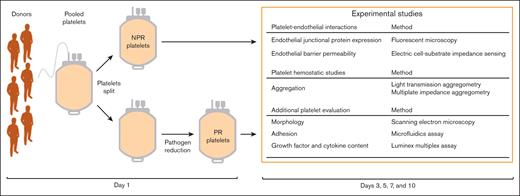
Leukoreduced apheresis platelets suspended in plasma were obtained from Vitalant (Denver, CO).22 For each trial (n = 3), platelets were collected from 6 donors, pooled, and then divided into 2 identical units on day 1 (Figure 1). One unit underwent pathogen reduction with the INTERCEPT Blood System, which uses amotosalen and UVA illumination for nucleic acid modification (PR platelets), and the other did not undergo any additional preparation (NPR platelets). This process produced 2 platelet units that were identical other than their pathogen reduction treatment. Platelets underwent standard donor screening and testing for pathogens through Vitalant as a part of their preparation. On day 2, both platelet units were shipped to the University of California, San Francisco for subsequent experiments. Platelet bags were then stored at room temperature (22°C) under gentle agitation (60 rpm), for the experiments which were conducted on days 3, 5, 7, and 10 after collection (Figure 1). Because pathogen reduction may lengthen shelf life, platelets were tested beyond their approved storage duration of 5 to 7 days. For all experiments, NPR and PR platelets were spun out of their anticoagulated plasma and adjusted to equal counts. Shipping, storage, and preparation for experiments were all conducted under identical conditions for each treatment group.
Preparation of NPR and PR platelets. In each trial (n = 3), platelets from multiple donors (n = 6) were pooled and then split into 2 identical units. Using the INTERCEPT blood system, PR platelets were treated with amotosalen, which, under UVA illumination, resulted in nucleic acid modification to prevent pathogen replication. NPR platelets were then compared with PR platelets from the same pooled donors in subsequent experiments. This procedure was conducted for each of the 3 trials used. Other than treatment with pathogen reduction, the NPR and PR platelets underwent identical preparation, storage, and experimental procedures.
Preparation of NPR and PR platelets. In each trial (n = 3), platelets from multiple donors (n = 6) were pooled and then split into 2 identical units. Using the INTERCEPT blood system, PR platelets were treated with amotosalen, which, under UVA illumination, resulted in nucleic acid modification to prevent pathogen replication. NPR platelets were then compared with PR platelets from the same pooled donors in subsequent experiments. This procedure was conducted for each of the 3 trials used. Other than treatment with pathogen reduction, the NPR and PR platelets underwent identical preparation, storage, and experimental procedures.
Platelet effect on endothelial junctional protein expression
Human umbilical vein endothelial cells (HUVECs) were obtained from PromoCell (Heidelberg, Germany) and maintained using growth medium (PromoCell, Heidelberg, Germany). For imaging experiments, the cells were grown to confluence on collagen-coated coverslips. NPR or PR platelets (trials 2 and 3,; days 3, 5, 7, and 10) at a concentration of 0.05 × 109 were added to the endothelial monolayer followed by coculture with thrombin (0.2 U/mL) to disrupt the monolayer. Cells were then fixed with 4% paraformaldehyde and stained with antibodies against vascular endothelial (VE)-cadherin (Cell Signaling Technologies, Danvers, MA), zonula occludens-1 (ZO-1; Invitrogen, Waltham, MA), and phalloidin (Cell Signaling Technologies, Danvers, MA). These antibodies were chosen to evaluate adherens junctions, tight junctions, and cell contraction, respectively.23,24 Fluorescently tagged secondary antibodies were used to detect the antigens. Four to 8 replicates of the treatment groups for each experimental day were performed. Images were captured at ×20 magnification using the Nikon Eclipse 80i microscope (Minato, Tokyo, Japan) with RT-sCMOS camera and SPOT imaging software (SPOT Imaging, Sterling Heights, MI). To quantify gaps between the endothelial monolayer, 5 nonoverlapping representative images per condition were captured. Gap measurements were manually counted at 20 representative points in each image and averaged for each treatment group.
Platelet effect on endothelial cell barrier permeability
HUVECs and pulmonary endothelial cells (PECs) were obtained from PromoCell (Heidelberg, Germany) and maintained using growth medium and MV, respectively (PromoCell, Heidelberg, Germany). To conduct electric cell–substrate impedance sensing (ECIS), the cells were then grown to confluence on L-cystine–reduced 96-well plates containing electrodes in each well. Two trials were performed using HUVECs (trials 1 and 2; days 3, 5, 7, and 10) and 1 trial was performed using PECs (trial 3; days 3, 5, 7, and 10). For each trial, the HUVEC or PEC monolayers were treated with NPR or PR platelets at different concentrations. Three to 8 replicates were performed for each group and time point. The cells were challenged after 30 minutes with thrombin (0.2 U/mL, Sigma, St Louis, MO). Monolayer integrity was measured using the ECIS system (ECIS 1600; Applied BioPhysics, Troy, NY). An increase or decrease in transendothelial electrical resistance across the monolayer indicated a decrease or increase in endothelial paracellular permeability, respectively. Monolayer resistance at 4, 16, or 64 kHz was analyzed in 8-minute intervals.
Platelet aggregation
Platelet aggregometry was interrogated in 2 ways. First, aggregation was assessed by light transmission aggregometry. NPR and PR platelets (trials 1 and 2; days 3, 5, 7, and 10) were analyzed on a Model 700 Whole Blood/Optical Lumi-Aggregometer (Chrono-log, Havertown, PA). NPR and PR platelets (0.2 × 109) were resuspended in Dulbecco's phosphate buffered saline and primed with CaCl2 (1 mM) for 1 minute before stimulation with thrombin (0.1 U/mL). The resulting aggregation was measured by the change in optical density over time for a total of 6 minutes. Aggregation was recorded as a percentage of the vehicle control.
Second, platelet aggregation of whole blood treated with NPR and PR platelets (0.2 × 109; trial 3; days 3, 5, 7, and 10) were measured by multiplate impedance aggregometry (Roche, Basel, Switzerland). Whole blood (0.3 mL) was diluted in warmed normal saline containing 3 mM of CaCl2 and treated with NPR or PR platelets. Collagen (3.23 μg/mL) or protease activated receptor-1–activating hexapeptide (thrombin analog, 0.0323 mM) was added (Hart Biologicals, Hartlepool, UK) based on the manufacturers’ recommendations, and stimulated impedance was measured for 6 minutes. Poststimulant addition change in impedance was followed over time.
Platelet morphology
To evaluate differences in morphology, NPR and PR platelets underwent scanning electron microscopy (SEM) imaging. In preparation, platelet samples (trial 3, day 7) were cooled to 2°C for 12 hours and then washed with Dulbecco's phosphate buffered saline buffer using serial centrifugation (2000 rpm × 10 minutes × 2) to remove the plasma. Primary fixation was carried out in 4% glutaraldehyde followed by secondary fixation in 1% osmium tetroxide. After a final rinse, 10 μL samples were drawn from the pellet and applied to a porous polymer membrane. This stub was immediately freeze-dried and then sputtered with ∼10 nm of gold. SEM study was carried out at 10 kV accelerating voltage using backscatter detection. Images were captured using a TESCAN Vega 3 microscope (TESCAN, Brno, Czech Republic) with digital image acquisition, a working distance of 10 mm and a temperature of 20°C. Four to 9 replicate images were captured from each treatment group at ×10 000, ×20 000, ×50 000, and ×100 000 magnification.
Platelet adhesion
To assess platelet adhesive properties, microfluidic assays under shear forces were performed. The flow chamber assays were run in parallel, so storage time, handling, coating, and all other conditions were the same. The microfluidic assays were conducted using Ibidi (Grafelfing, Germany) μ-Slide 6-channel parallel plate flow chambers (each measuring 0.1 mm × 1 mm × 17 mm) that had a withdrawing syringe pump (Kent Scientific, Torrington, CT) connected to the chamber outlet. To model prohemostatic surfaces exposed in the microvascular environment after barrier disruption, chambers were coated with rat tail collagen (100 μg/mL, Corning, Ithaca, NY) in 0.02 N acetic acid followed by tissue factor (EXTEM reagent; Werfen, Bedford, MA).25-27 NPR or PR platelet suspensions (trial 3; days 3, 5, 7, and 10) were perfused at approximate venous shear (100 s−1) for 10 minutes followed by a 20-minute wash at approximate arterial shear (1000 s−1).28 Adhered platelets were visualized with the Nikon Eclipse TE300 (Nikon Instruments Inc, Melville, NY) microscope with Plan Fluor ELWD (×60; numerical aperture, 0.7) at ambient temperature in air.29 Five ×60 phase-contrast images of each chamber were acquired with treatments run in triplicate on each experimental day.
Platelet growth factor and cytokine content
NPR and PR platelets (trials 2 and 3; days 3, 5, 7, and 10) were washed and lysed with radioimmunoprecipitation assay buffer and diluted using diluents supplied by the manufacturer. A Luminex panel was created targeting angiopoietin-1, angiopoietin-2, brain-derived neurotropic factor, CD40 ligand, epidermal growth-factor (EGF), fibroblast growth factor-2, interleukin (IL)-1β, IL-6, IL-8, IL-10, platelet-derived growth factor-BB (PDGF-BB), thrombospondin-2, tumor necrosis factor-alpha, vascular EGF (VEGF), and von Willebrand factor A2 (VWF-A2) (R&D Systems, Minneapolis, MN). The multiple analyte assay was performed using a MAGPIX instrument (Luminex Corporation, Austin, TX) with 4 replicates conducted for each group and time point. Data were analyzed using xPONENT software (Luminex Corporation, Austin, TX).
Statistical analysis
Platelet counts under different treatments and over time were considered as a percentage of the whole. Platelet microscopic gap length measurements, ECIS area under the curve (AUC) compared with controls and light transmission aggregation parameters were averaged for each treatment and day. For the microfluidics assay, replicate images from each group were captured and counts were averaged. Luminex analyte quantification was also averaged for each treatment and day. All studies were compared using a 2-way analysis of variance (ANOVA) with the exception of platelet light transmission, which was compared using the t test. A P value of <.05 was considered statistically significant and the Tukey test or Bonferroni correction was used for multiple comparisons. Statistical analyses were performed with R and Prism.30,31
Results
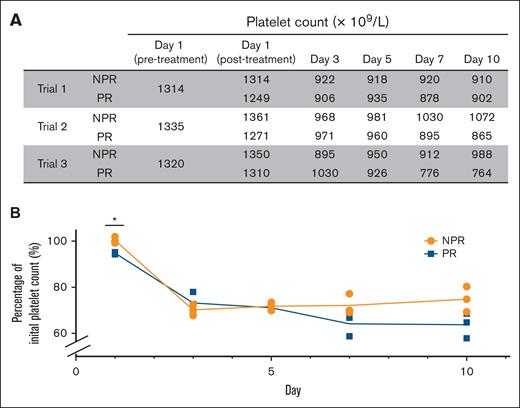
Platelets from 6 individual donors were collected and pooled together for each of the 3 trials (supplemental Table 1). Platelet counts were collected to determine whether platelet numbers were altered by pathogen reduction (Figure 2A-B). There was an expected statistically significant decrease in PR platelet counts after pathogen reduction treatment compared to NPR platelet counts attributable to the compound adsorption device. Loss of platelets was observed in both groups between day 1 and day 3, with transport between facilities. Between day 3 and day 10, platelet counts in both groups held steady, with an average attrition of 2.9% by day 10. There was a trend toward decreased counts in the PR group, although this did not reach statistical significance (Figure 2B).
NPR and PR platelet counts decrease with transport between facilities and storage. (A) NPR and PR platelet counts are shown on the day of collection before splitting (day 1, before treatment), after splitting and with either no additional preparation or pathogen reduction treatment (day 1 post-treatment NPR and PR, respectively), and on subsequent experimental days (days 3, 5, 7, and 10). (B) Percentage of initial platelet count in NPR (orange) and PR (blue) platelet groups is shown over time. Individual data points from each trial are shown at each time point. ∗P < .05 comparing the difference between median NPR and PR platelets by 2-way ANOVA with the Tukey honestly significant difference test post hoc at the given time point.
NPR and PR platelet counts decrease with transport between facilities and storage. (A) NPR and PR platelet counts are shown on the day of collection before splitting (day 1, before treatment), after splitting and with either no additional preparation or pathogen reduction treatment (day 1 post-treatment NPR and PR, respectively), and on subsequent experimental days (days 3, 5, 7, and 10). (B) Percentage of initial platelet count in NPR (orange) and PR (blue) platelet groups is shown over time. Individual data points from each trial are shown at each time point. ∗P < .05 comparing the difference between median NPR and PR platelets by 2-way ANOVA with the Tukey honestly significant difference test post hoc at the given time point.
Platelet-endothelial interactions
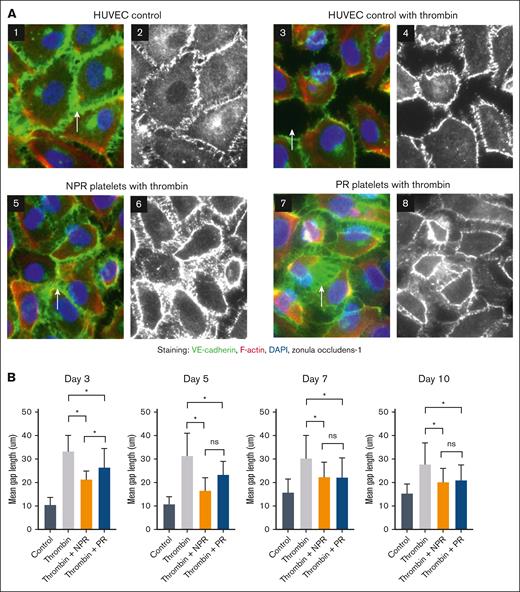
To understand the effects of NPR and PR platelets on endothelial junction proteins, HUVEC monolayers were stained for expression of the adherens junction protein VE-cadherin, the tight junction protein ZO-1, and the structural protein F-actin (Figure 3A).23,24 In the control group, the images show undisturbed membrane junctions of VE-cadherin (green) and ZO-1 (white), as well as a quiescent level of F-actin (red). After thrombin challenge, most cells are contracted with loss of VE-cadherin and ZO-1 expression, a subtle increase in F-actin expression, and gaps between cells. Pretreatment with NPR or PR platelets preserved VE-cadherin and ZO-1 surface expression, attenuated F-actin mobilization, and decreased cell gaps (Figure 3A). Average gap measurement between cells revealed a smaller gap length increase in NPR and PR platelets with thrombin compared with thrombin treatment alone. NPR platelets attenuated the thrombin response more than PR platelets on experimental day 3 but not on subsequent days (Figure 3B).
NPR and PR platelets attenuate thrombin-induced disruption of adherens and tight junctions. (A) Treatment with NPR or PR platelets lessened the loss of endothelial junctional proteins in response to thrombin challenge. HUVEC monolayers were stained for VE-cadherin (green), F-actin with phalloidin (red), and DAPI (4′,6-diamidino-2-phenylindole; blue) in images 1, 3, 5, and 7 as well as ZO-1 (white) in images 2, 4, 6, and 8. White arrows highlight gaps between endothelial cells. Platelets from trials 2 and 3 were tested on days 3, 5, 7, and 10, and representative images from trial 2, day 5 are shown. (B) Quantified gap measurement was significantly less in day 3 NPR platelets (orange) compared with PR platelets (blue), but that difference was not maintained as the platelets aged. In addition, there was an increase in gap size between cells with thrombin alone compared with control and a statistically significant decrease with both treatment of NPR and PR platelets. Differences were calculated with 2-way ANOVA. ∗P < .05. ns, nonsignificance.
NPR and PR platelets attenuate thrombin-induced disruption of adherens and tight junctions. (A) Treatment with NPR or PR platelets lessened the loss of endothelial junctional proteins in response to thrombin challenge. HUVEC monolayers were stained for VE-cadherin (green), F-actin with phalloidin (red), and DAPI (4′,6-diamidino-2-phenylindole; blue) in images 1, 3, 5, and 7 as well as ZO-1 (white) in images 2, 4, 6, and 8. White arrows highlight gaps between endothelial cells. Platelets from trials 2 and 3 were tested on days 3, 5, 7, and 10, and representative images from trial 2, day 5 are shown. (B) Quantified gap measurement was significantly less in day 3 NPR platelets (orange) compared with PR platelets (blue), but that difference was not maintained as the platelets aged. In addition, there was an increase in gap size between cells with thrombin alone compared with control and a statistically significant decrease with both treatment of NPR and PR platelets. Differences were calculated with 2-way ANOVA. ∗P < .05. ns, nonsignificance.
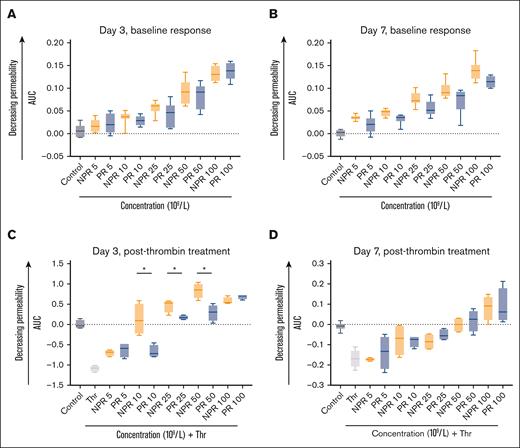
The effect of NPR and PR platelets on endothelial barrier permeability was assessed through ECIS. Endothelial cells (HUVECs) treated with NPR or PR platelets demonstrated an expected and similar dose-dependent increase in resistance (increase in AUC) compared with control (Figure 4A-B). This indicates decreasing endothelial barrier permeability with increasing platelet dose. Furthermore, the thrombin-induced increase in barrier permeability was attenuated by both NPR and PR platelets (Figure 4C-D). Notably, day 3 NPR platelets demonstrated a statistically significant increase in their attenuation of the thrombin response at lower concentrations compared with PR platelets (Figure 4C). This difference in protective effect was not maintained at later time points (Figure 4D). Because the endothelium is heterogeneous, we sought to determine whether a different cell type would differ in response to NPR and PR platelets.32 PECs treated with NPR or PR platelets also followed a dose-response pattern under baseline treatment and in response to thrombin (supplemental Figures 1 and 2).
NPR platelets (orange) and PR platelets (blue) have a differential response to thrombin challenge across an endothelial monolayer as measured by ECIS. (A-B) NPR and PR platelet treatment of HUVECs demonstrated a similar dose-response as measured by the AUC of transendothelial electrical resistance. This dose-response curve was preserved in both day 3 (A) and day 7 (B) platelets and there were no significant differences between NPR and PR response for a given concentration. (C-D) NPR platelets were more protective at lower concentrations against thrombin challenge (0.2 U/mL) as measured by AUC of transendothelial electrical resistance on day 3 (C) but this difference was not sustained to day 7 (D). For all studies, samples were performed with 3 to 8 replicates with means and standard deviations of representative samples shown. Analysis was conducted with 2-way ANOVA and the Tukey test post hoc. ∗P < .05. Thr, thrombin.
NPR platelets (orange) and PR platelets (blue) have a differential response to thrombin challenge across an endothelial monolayer as measured by ECIS. (A-B) NPR and PR platelet treatment of HUVECs demonstrated a similar dose-response as measured by the AUC of transendothelial electrical resistance. This dose-response curve was preserved in both day 3 (A) and day 7 (B) platelets and there were no significant differences between NPR and PR response for a given concentration. (C-D) NPR platelets were more protective at lower concentrations against thrombin challenge (0.2 U/mL) as measured by AUC of transendothelial electrical resistance on day 3 (C) but this difference was not sustained to day 7 (D). For all studies, samples were performed with 3 to 8 replicates with means and standard deviations of representative samples shown. Analysis was conducted with 2-way ANOVA and the Tukey test post hoc. ∗P < .05. Thr, thrombin.
Platelet hemostatic studies
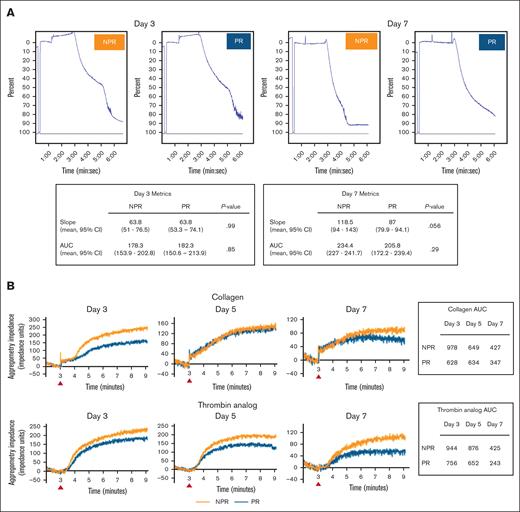
Under both light transmission and impedance aggregometry, NPR and PR platelets exhibited an aggregation response (Figure 5). With light transmission aggregometry, there were no qualitative differences and no statistically significant quantitative differences in slope or AUC between treatments (Figure 5A). With impedance aggregometry, NPR platelets had qualitatively stronger aggregation responses to both collagen and thrombin analog compared with PR platelets across days (Figure 5B).
NPR and PR platelets demonstrate variable but overall similar aggregation. (A) Under light transmission aggregometry induced by thrombin, aggregation of NPR and PR platelets had similar tracing morphology and no difference in slope or AUC across experimental days. Platelets from trials 1 and 2 were tested and representative curves of day 3 and day 7 platelets (trial 1) are shown. Both slope and AUC for replicates were compared using the t test and P < .05 was considered significant. (B) Under impedance aggregometry induced by collagen or thrombin analog, whereas both NPR (orange) and PR (blue) platelets (trial 3) demonstrated aggregation, NPR platelets exhibited a more robust response. Data are shown as impedance over time normalized to the baseline and as a calculated AUC. The red arrows indicate the time the agonist was administered. CI, confidence interval.
NPR and PR platelets demonstrate variable but overall similar aggregation. (A) Under light transmission aggregometry induced by thrombin, aggregation of NPR and PR platelets had similar tracing morphology and no difference in slope or AUC across experimental days. Platelets from trials 1 and 2 were tested and representative curves of day 3 and day 7 platelets (trial 1) are shown. Both slope and AUC for replicates were compared using the t test and P < .05 was considered significant. (B) Under impedance aggregometry induced by collagen or thrombin analog, whereas both NPR (orange) and PR (blue) platelets (trial 3) demonstrated aggregation, NPR platelets exhibited a more robust response. Data are shown as impedance over time normalized to the baseline and as a calculated AUC. The red arrows indicate the time the agonist was administered. CI, confidence interval.
Additional platelet evaluation
SEM was used to qualitatively investigate any differences in PR platelet morphology or structure. On review of representative images (day 7), both groups demonstrated a degree of aggregation and the presence of extracellular vesicles on their surfaces (supplemental Figure 3). The microfluidics assay allowed a limited look at morphology as well (supplemental Figure 4). In this assay, NPR platelets displayed more prominent processes starting on day 7 with the elongation of some platelets by day 10. In comparison, PR platelets had the earlier emergence of platelets displaying prominent processes on day 5. By day 10, the trend was toward poor attachment and round morphology (supplemental Figure 4). Furthermore, these morphologic changes were also reflected in platelet adherence properties. Specifically, PR platelets adhered to collagen in lower quantities starting 5 days after collection, with that decrease rising to statistical significance on day 10 (supplemental Figure 4).
Under Luminex multiplex assays, NPR and PR platelets demonstrated some differences in growth factor and cytokine content (supplemental Figure 5). Both NPR and PR platelets lost analyte content with time. PR platelets trended to a greater loss of content relative to NPR platelets although this difference was not seen across all analytes tested. CD-40L, VEGF, VWF-A2, and PDGF-BB were higher in NPR platelets at earlier time points, all of which have described roles in endothelial homeostasis. The quantification of analyte values is shown in supplemental Table 2.
Discussion
This study sought to elucidate the impact of pathogen reduction by amotosalen-UVA light on platelet-endothelial interactions, specifically the effect on endothelial junctional protein expression and endothelial barrier permeability in vitro. We additionally evaluated hemostatic differences by platelet aggregometry as well as morphology, adherence, and growth factor and cytokine content. While NPR and PR platelets behaved similarly across this series of experiments, we did observe key differences. Most notable is that, compared with PR platelets, NPR platelets were better able to attenuate increased endothelial barrier permeability on day 3 after collection.
In terms of platelet-endothelial interactions, this paper’s results explored the differential effects of NPR and PR platelets on endothelial monolayers. Previous studies have demonstrated the protective effect of standard issue platelets through the tightening of endothelial junctions and improved endothelial barrier function.7,8 Our results in NPR platelets are consistent with these previous studies. Specifically, treatment with NPR platelets preserved VE-cadherin and ZO-1 expression on the endothelial cell surface, decreased thrombin-induced gaps between endothelial cells, increased transendothelial electrical resistance across an endothelial monolayer, and attenuated thrombin’s increase in endothelial permeability. PR platelets performed similarly. However, on our earliest experimental day, PR platelets did not mitigate the thrombin response as well, resulting in larger gaps between endothelial cells compared with NPR platelets. Further, in response to thrombin, PR platelets attenuated resistance similar to NPR platelets only at the highest concentrations studied. This may suggest that pathogen reduction affects platelet impact on endothelial barrier permeability at an accelerated rate early on and above what is expected from the storage lesion alone. This finding may have important implications for PR platelet role in maintaining vascular stability in the clinical setting. To the authors’ knowledge, this is the first such study to examine the impact of pathogen reduction on endothelial barrier function.
In terms of other measures of platelet functionality, NPR and PR behaved similarly. NPR and PR platelets were similar in their aggregative properties, with no statistically significant difference between groups with light transmission aggregometry and a slight qualitatively weaker response with impedance aggregometry in PR platelets. Light transmission aggregometry may be considered a gold standard for platelet functional testing and has clinical applicability. Conversely, the impedance aggregometry assay, which was conducted in whole blood, may be a more holistic measure of platelet aggregation compared with light transmission aggregometry which looks at platelets alone. Prior studies may be more aligned with the impedance aggregometry data and have reported impaired aggregation in PR platelets under multiple types of stimulants.33-37 Additional studies are necessary to understand specific pathways that may drive these subtle potential differences in aggregation.
In terms of morphology, the SEM images appeared qualitatively similar between treatment groups. Visualization as part of the microfluidics assay demonstrated that PR platelets were more likely to become small and rounded with a decrease in adhesive properties at extended storage durations. We postulate that these changes in appearance may be due to apoptosis, and future directions will explore measures to evaluate this suspicion.
In the literature, morphologic characterization of amotosalen-UVA PR platelets is inconsistent, with studies reporting similar morphology scores and swirling in NPR and PR platelets, but some alterations in hypertonic shock resistance as PR platelets age.19,38-41 In addition, adhesive differences between NPR and amotosalen-UVA PR platelets are varied in the literature with mixed reports of increased or decreased coverage kinetics.42-44 Some of this variability is secondary to differences in experimental structure and further studies are necessary to interrogate specific pathways of adhesion.
Finally, our studies addressed platelet growth factor and cytokine content. As expected, both NPR and PR platelets tended to lose content over time. In addition, CD-40L, VEGF, and VWF-A2 were higher in NPR compared with PR platelets, particularly at the earlier time points. All 3 of these factors have been described as platelet-derived stabilizers of the endothelium.45 Furthermore, PDGF-BB, which is released, in part, to aid in vascular repair, also demonstrated statistically significant separation between NPR and PR platelets across time points and may be relevant to understanding the impact of PR platelets on vascular stability.46
Although data are mixed, pathogen reduction has also previously been reported to increase baseline platelet activation and accelerate the storage lesion.40,47 By flow cytometric analysis, PR platelets express more markers of activation such as P-selectin, annexin-V, and PAC-1 at baseline and as storage duration increases.19,34,48-52 These increases in activation are associated with decreased functionality and ultimately acceleration of the storage lesion. The changes in platelet-endothelial interactions and more subtle alterations in adhesion may be reflective of this accelerated storage lesion.
One important consideration in contextualizing the functional changes observed after pathogen reduction is the potential impact of pathogen reduction treatment on the transcriptome and resultant proteome. Pathogen reduction treatment has been described to deregulate 147 genes.53 However, despite these transcriptomic changes, pathogen reduction has a much lower impact on the platelet proteome.54 The functional correlatives of these changes are an active area of research.55,56 Relevant alterations to the aforementioned findings include decreased levels of the prosurvival protein B-cell lymphoma XL, which may result in apoptosis more quickly in PR platelets as well as G(i)α2 protein and platelet endothelial aggregation receptor 1, which both play a role in aggregation for hemostasis.53,56
This series of experiments has several important limitations. First, there were a limited number of trials and replicates conducted. Although experiments were conducted with pooled samples, these studies may not fully capture donor platelet heterogeneity. Second, the platelets underwent standard donor screening and testing as part of their initial preparation but did not undergo subsequent testing, particularly to look for bacteria. There is a small chance that any of the platelet aliquots could be contaminated. Third, in vitro experimental setups have limitations in their biological relevance. For example, although we endeavored to evaluate the effect of platelet adhesion through microfluidics, our system did not include endothelial cells, other blood cells, clotting factors, or other components important to hemostasis. Fourth, it follows that, despite finding subtle differences in these in vitro studies, it is unclear how or whether these differences may affect in vivo function or patients in the clinical setting. The complexity of model and human systems may lead to different results and implications when in vitro findings are applied to these systems. For example, prior work has demonstrated increased barrier protection by 4°C platelets in vitro, but this did not translate into in vivo vascular models of leak.7,57 Furthermore, temperature and pH, which were consistent across these in vitro studies, may alter platelet function, endothelial function, and platelet-endothelial interactions. As a result, it would be uncertain how these in vitro differences would manifest in clinical situations, particularly in acidotic or hypothermic conditions. Ultimately translational studies and clinical studies are needed to determine the implications of these findings.
Because of their importance in increasing transfusion safety, PR platelets are growing in their clinical use.58 Previous trials have demonstrated that not only are PR platelets effective in reducing bacteria, viruses, and a handful of parasites,40 but also that PR platelets can be safely administered without an increase in serious adverse events.15,17,59,60 However, a recent meta-analysis found patients transfused with PR platelets required a greater number of transfusions in a shorter timeframe and were at increased risk of platelet refractoriness.59 In addition, most clinical studies of PR platelets have focused on patients with thrombocytopenia in the oncology setting, and only a few have investigated the use of PR platelets in nononcologic hemorrhage.61 In particular, patients who have been transfused extensively, by definition, require large numbers of platelet unit transfusions and encompass a variety of diagnoses including trauma, liver disease, and postoperative bleeding. The hallmark of many of these diseases is an acquired fragility of the endothelial vasculature. These patients may significantly benefit from the vasculoprotective role of platelets and diminished function in this area could be deleterious for patient outcomes. It is imperative for further clinical study of PR platelets to focus on these types of patients as well as the implications of altered platelet-endothelial interactions. In addition to focused clinical study, the ability to ascertain endothelial dysfunction in patients with bleeding is needed to enable definitive evaluation of the differences between PR and NPR platelets in the clinical context.
In conclusion, higher concentrations of amotosalen-UVA PR platelets were required to attenuate increased endothelial barrier permeability compared with NPR platelets at early storage durations. Additional studies are needed to evaluate the impact of these findings, particularly in those receiving platelets for hemorrhage and shock, given this newly described effect on the vasculoprotective properties of PR platelets.
Acknowledgments
The authors acknowledge David Shimmin and Nanocraft (Menlo Park, CA) for their work in conducting scanning electron microscopy imaging.
This project was supported by the Pediatric Critical Care and Trauma Scientist Development Program K12 through the National Institute of Child Health and Human Development, the National Institutes of Health (NIH) (NIH 2K12 HD047349). This project was also supported by the National Heart, Lung, and Blood Institute, the NIH (NIH NHLBI R01HL147880).
Authorship
Contribution: A.B.N. designed the research, performed the experiments, analyzed the results, created the figures, and wrote and edited the manuscript; B.M., L.Z.K., and A.T. assisted with designing the research and edited the manuscript; L.V. assisted with the immunohistochemistry experiment and edited the manuscript; A.F. performed the impedance aggregometry and microfluidics experiments, and edited the manuscript; K.H. assisted with impedance aggregometry and edited the manuscript; J.C. edited the manuscript; S.P. designed the research, supervised the experiments and analysis, and edited the manuscript; and all authors approved the final draft of the manuscript before submission.
Conflict-of-interest disclosure: L.Z.K is a scientific consultant for Cerus and other companies that include Gamma Diagnostics, Coagulant Therapeutics, and Haemonetics. S.P is a consultant for Cellphire Inc, Velico Medical, and Octapharma. The remaining authors declare no competing financial interests.
Correspondence: Alison B. Nair, Department of Pediatrics, University of California, San Francisco, Mission Hall 550 16th St, San Francisco, CA 94158; email: alison.nair@ucsf.edu; and Shibani Pati, Department of Laboratory Medicine and Department of Surgery, University of California San Francisco, 513 Parnassus Ave, HSE-715, San Francisco, CA 94143; email: shibani.pati@ucsf.edu.
References
Author notes
Renewable materials, data sets, and protocols will be made available to other investigators via email to the corresponding author, Alison B. Nair (alison.nair@ucsf.edu).
The full-text version of this article contains a data supplement.